Introduction
The skin can damaged due to burn injuries, chronic
wounds, skin excision, tumours or other dermatological conditions
(1). For the repair and
regeneration of damaged tissue, a continuous cascade of events
occurs, which involves the interaction of cellular components,
growth factors and cytokines within four sequential and overlapping
phases, including haemostasis, inflammation, proliferation and
tissue remodelling or maturation (2,3).
The Aloe vera plant has long been used in
medicine, as a dietary supplement and for cosmetic purposes
(4). Aloe vera extracts are
a rich source of polyphenols, including aloin and aloe emodin, and
exhibit a wide range of pharmacological properties, including
anti-inflammatory and anti-cancer properties (5). The claimed therapeutic uses of
Aloe vera range over a broad list of conditions, as do its
associated pharmacological activities. The majority of these claims
are based on historical use, rather than scientific evidence.
Different sections of the plant are used in the traditional
management of veterinary and human diseases (6). Aloe vera is the most
biologically active of the Aloe species, and is composed of
>70 active compounds, including vitamins, minerals, amino acids,
anthraquinones, enzymes and salicylic acids (7). The beneficial properties of the plant
are ascribed to the colourless leaf gel, which has been reported to
stimulate wound-healing and skin hydration, induce hematopoiesis,
antimicrobial and anti-inflammatory activities (8–10).
The pharmacological properties of Aloe vera appear to be
mediated predominantly by the activation of monocytes and
macrophages, and extracts of Aloe vera have been reported to
enhance the release of cytokines, including interleukin (IL)-1,
IL-2, IL-6, interferon, granulocyte/monocyte-colony stimulating
factor and tumor necrosis factor (TNF) in vitro (11). Oryan et al (12) reported that Aloe vera
aqueous extract may be used as a promising medication for wound
healing.
The common grape (Vitis vinifera L. Vitaceae)
is regarded as an important medicinal plant. European doctors have
suggested the use of grapevine sap, juice and whole grape in the
treatment of pain, allergic reactions, inflammation and to promote
wound healing (13,14). Resveratrol (trans-3,4′,
5-trihydroxystillbene), a phytoalexin that belongs to a group of
compounds termed stilbenes, is found in dietary items, including
red wine, grapes and peanuts (15). It has been demonstrated that
resveratrol confers several beneficial effects in human and animal
models, and is being investigated as a chemopreventative agent for
cancer and cardiovascular disease, most likely due to its
antioxidative and anti-proliferative activities (16,17).
IL-1β functions as a 'master' cytokine, which has an indispensable
role in orchestrating effective innate and adaptive immune
responses (18).
In the present study, the burn wound healing
properties of aloe emodin (Aloe vera) and resveratrol
(Vitis vinifera) were investigated using in vitro and
in vivo methods, in order to identify the clinical effects
of the two compounds.
Materials and methods
Materials
Resveratrol was isolated, as previously described by
Park et al (19). Aloe
emodin was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Dulbecco's modified Eagle's medium and RPMI-1640 medium were
purchased from Sigma-Aldrich and used as a culture media. A 100X
antibiotic and antimycotic solution containing 10,000 U/ml
penicillin, 10 mg/ml streptomycin and 25 µg/ml amphotericin
B in 0.9% NaCl, was purchased from Sigma-Aldrich. Multi-well plates
(6-, 12- or 48-well) were purchased from Corning Incorporated
(Corning, NY, USA). Human and murine vascular endothelial growth
factor (VEGF), murine IL-1β and murine monocyte chemoattractant
protein-1 (MCP-1) ELISA kits were obtained from Cusabio Biotech
Co., Ltd. (Wuhan, China). An ImmunoCruz™ staining kit for the
detection of murine VEGF was purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Rat monoclonal anti-mouse
MCP 1 (clone ECE.2; cat. no. CLYPAR-100-MCP1) (CCL2/MCP1 antibody
was raised against synthetic peptide corresponding to residues
102–130 of mouse MCP-1 specific to mouse CCR2), rat monoclonal
anti-mouse macrophage (clone BM8; cat. no. T-2028) (BM8 monoclonal
antibody reacts with mouse F4/80 antigen specific to mouse and rat)
and rabbit polyclonal anti-myeloperoxidase antibodies (cat. no.
P05164) (rabbit polyclonal to myeloperoxidase raised from human
granulocytes reacts specific to mouse, rat, human, pig and monkey)
were purchased from Sanbio BV (Uden, Netherlands), BMA Biomedicals
(Augst, Switzerland), and Thermo Fisher Scientific, Inc. (Waltham,
MA, USA), respectively.
Cells
The THP-1 human acute monocytic leukemia and HaCaT
human keratinocyte cell lines (American Type Culture Collection,
Rockville, MD, USA) were and maintained in RPMI-1640 medium
supplemented with 10% fetal bovine serum (Sigma-Aldrich),
penicillin (100 U/ml), streptomycin (100 µg/ml) and
amphotericin (0.25 µg/ml). The plates were incubated
overnight for 12 or 72 h.
Animals
Male BALB/c mice (5-week-old, n=30) were obtained
from ShanDong University (Jinan China) and housed one per cage (to
prevent attacks on wounds) for 1 week in a temperature-controlled
room at 25±1°C with 60% relative humidity and a 12-h light-dark
cycle, and provided with access to a standard laboratory diet and
water ad libitum prior to experimentation. The ethical
guidelines of the Animal Center of Yantai Yuhuangding Hospital,
Yantai, Shandong Province, China. The mice were treated according
to the ethical guidelines of the Animal Center of Yantai
Yuhuangding Hospital (Yantai, China), and the Animal Studies
Committee of the Shan Dong University approved all experimental
procedures.
Cell proliferation determination using
MTT assays
Following treatment with different concentrations of
aloe emodin (1, 100 and 500 ng/ml) for 24 h, the cells were washed
with phosphate-buffered saline (PBS; pH 7.2) and incubated in fresh
medium for 4 days. The number of surviving cells was then
indirectly determined using an MTT cytotoxicity assay
(Sigma-Aldrich), according to the manufacturer's instructions. The
absorbance was measured using a Multiskan EX ELISA plate reader
(MTX Lab Systems, Inc., Vienna, VA, USA) at a wavelength of 570 nm,
with a reference wavelength of 650 nm (20,21).
Measurement of burn wound healing
To examine the effects of aloe emodin and isolated
resveratrol on the burn wound healing process, burn wounds were
created on the backs of male BALB/c mice following anesthetization
with an intraperitoneal injection of pentobarbital (50 µg/g;
Hubei Nosk Chemical Co., Ltd., Hubei, China). Briefly, the hair on
the backs of the mice was removed using a hair remover (Xiantao
Topmed Nonwoven Protective Products, Co. Ltd., Hubei, China) under
anaesthesia with pentobarbital, and the back was subsequently wiped
with distilled water warmed to 37°C, followed by 70% ethanol. The
back skin was subjected with a 100°C custom-made soldering iron tip
(Shenzhen Kingdom Technology Co., Ltd. Guangdong, China) for 10
sec. A full-thickness excision of the burn skin wound was made in
the dorsal skin by lifting a fold of skin at the midline and
punching through two layers of skin using a sterile disposable
biopsy punch (diameter 8 mm) on the same day. The mice were housed
separately and provided with free access to a standard laboratory
diet and water following the completion of the surgical procedure.
Subsequently, the indicated quantities (100 mg ointment/mouse) of
resveratrol [2×10−4 and 5×10−4% (w/w)] and
aloe-emodin [1×10−8, 1×10−10 and
1×10−12% (w/w)] were layered on the burn wound surface
for 19 consecutive days and covered with Opsite Flexigrid film
dressing (Smith & Nephew, London, UK) to prevent infection or
licking of the ointment samples. Clear Vaseline alone was applied
to the control burn wound mice. On day 20, the mice were sacrificed
by cervical dislocation and the skin of the wound area was removed.
In the experiment of the effects of resveratrol on burn wound
healing, a vehicle control group, which received treatment with
clear Vaseline alone, and two treatment groups, treated with
ointment containing 2×10−4 or 5×10−4% (w/w)
aloe emodin (n=3 per group) were compared. In the experiments
examining the effects of resveratrol, the number of animals in each
treatment group were as follows: A vehicle control group treated
with clear Vaseline alone (n=11); a 1×10−8% (w/w) aloe
emodin-treated group (n=11); a 1×10−10 (w/w) aloe
emodin-treated group (n=7); and a 1×10−12% (w/w) aloe
emodin-treated group (n=7). The above topical applications were
performed under anaesthesia with pentobarbital.
Assessment of polymorphonuclear
neutrophil levels
The backs of the mice were subjected to burn wounds
using the same method as described above. A polyethylene filter
pellet (~6 mm diameter; 2 mm thickness; Hebei Yuyin Trade Co.,
Ltd., Hebei, China) containing the above-mentioned quantities of
aloe emodin and resveratrol was applied to the burn wound surface
and covered with Opsite Flexigrid film dressing to prevent
dislodging of the filter pellet. The filter pellets were removed
after 1, 3, 5 and 7 days and replaced with fresh filter pellets.
The control mice were treated with filter pellets containing 0.9%
(isotonic) NaCl solution alone at the same time-points. On day 9,
the mice were sacrificed and the filter pellets were removed prior
to the addition of 200 µl PBS (pH 7.0) to the filter
pellets. The solution was mixed for 10 min using a Vortex (vortex
mixer-V8, 200–3,000 rpm/min; Seoulin Bioscience, Seoul, Korea).
Following removal of the filter pellet, the mixture was centrifuged
at 1,000 × g for 10 min at 4°C. The obtained cell pellets were
resuspended in PBS.
The total number of leukocytes, including
polymorpho-nuclear leukocytes and macrophages, were measured using
a Vi-CELL XR Coulter cell counter (Beckman Coulter Inc., Brea, CA,
USA), and the ratio of polymorphonuclear leukocytes to macrophages
was determined using Giemsa-stained smear samples. Briefly, the
blood samples were placed on clean slides, air dried, fixed in
methanol and stained with Giemsa (Sigma- Aldrich). They were then
observed under ×100 magnification.
Measurement of cytokine levels in burn
wounds
For this assessment, seven mice were used for each
treatment group, which consisted of a vehicle control group treated
with 0.9% NaCl solution alone, and 1, 100, and 500 ng aloe emodin
and resveratrol-treated groups. These experiments were also
performed under anaesthesia with pentobarbital. Briefly, 100 mg
tissue was rinsed with 1X PBS, homogenized in 1 ml of 1X PBS and
stored overnight at −20°C. Two freeze-thaw cycles were then
performed to break the cell membranes, and the homogenates were
centrifuged for 5 min at 5,000 × g and 2–8°C. The supernatant was
removed and assayed immediately. The expression levels of MCP-1,
IL-1β and VEGF in the filter pellets were measured using mouse
MCP-1, IL-1β and VEGF ELISA kits (BD Biosciences, Franklin Lakes,
NJ, USA), according to the manufacturer's instructions. The
readings were taken at 450 nm using an ELISA reader (Thermo Fisher
Scientific, Inc.).
Wound analysis
Wound closure rate was assessed by tracing the wound
1, 2, 4, 8, 16 and 20 days post-wounding using transparency paper
and a permanent marker. The time to wound closure was defined as
the time-point at which the wound bed was completely
re-epithelialised with new tissue. The wound area was measured by
tracing the wound margin, and its surface area was calculated using
Image J software (National Institutes of Health, Bethesda, MA,
USA). The wound area was visually measured using an L×400
microscope (Labomed, Fremont, CA, USA) at ×10 magnification. The
wound healing rate was calculated using the following formula:
(Surface area of the original wound − surface area of the remaining
wound)/surface area of the original wound × 100.
Histopathological analysis
On days 4 and 10 of experimentation, seven mice were
sacrificed from each group were sacrificed and the wounds were
excised, together with the surrounding skin. The tissue samples
were fixed in 10% neutral-buffered formalin (Shijiazhuang
Xinlongwei Chemical Co., Ltd., Hebei, China) for at least 24 h,
progressively dehydrated in solutions containing an increasing
percentage of ethanol (70, 80, 95 and 100%), cleared in Histoclear
(AS ONE Corp., Tokyo, Japan), embedded in paraffin (Raylabel
Instrument Co., Ltd., Shanghai, China) under vacuum, sectioned (5
µm), de-paraffinised and stained with hematoxylin and eosin
(Beijing Tidybio Science & Technology Co., Ltd., Beijing,
China). The stained sections were examined for collagen,
inflammatory cells and blood vessel markers of healing.
Statistical analysis
The data are expressed as the mean ± standard error
of the mean. The results were analysed using one-way analysis of
variance and Dunnett's multiple comparison test. Statistical
analyses were performed using SPSS software version 16.0 (SPSS
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Cell proliferation assay
The molecular structures of aloe emodin and
resveratrol were similar to those reported previously (Fig. 1A and B). Treatment with either aloe
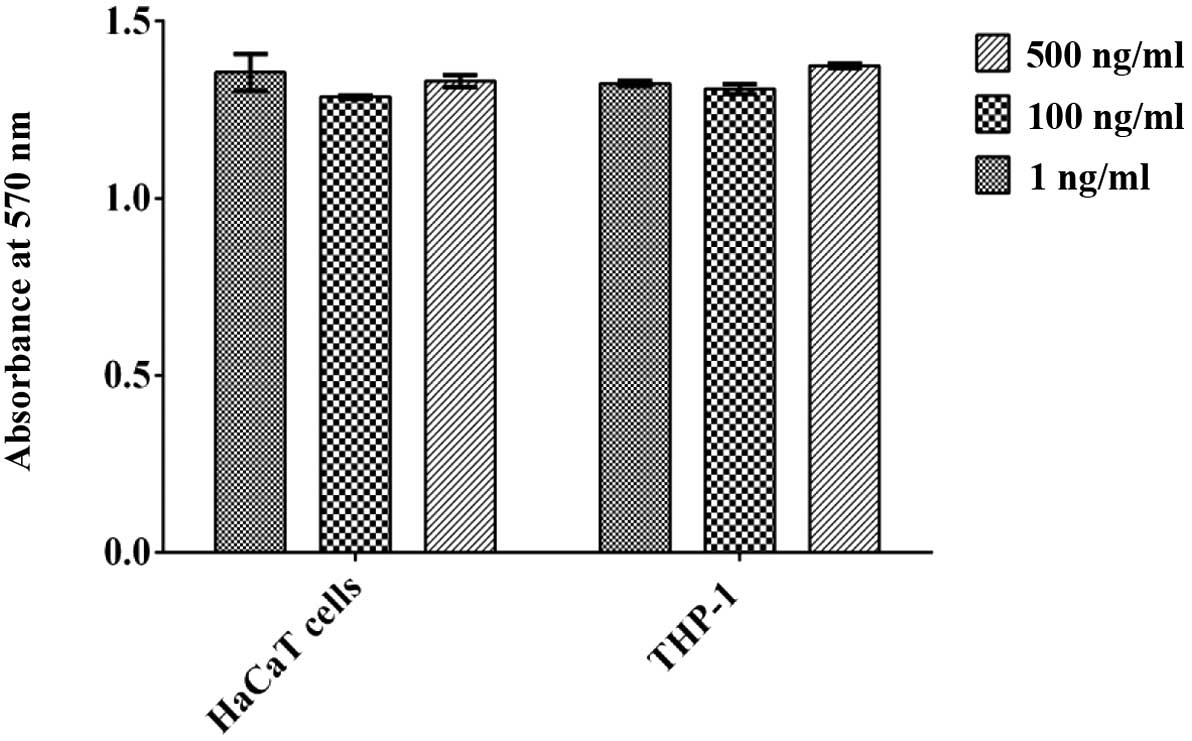
emodin or resveratrol did not result in cytotoxicity in either the
HaCaT cells or THP-1 macrophages at concentrations of 1, 100 and
500 ng/ml (Fig. 2). Furthermore,
aloe emodin and resveratrol had no effect on the proliferation of
the HaCaT cells or THP-1 macrophages.
Wound healing
Wound healing rates were measured on different days
following wounding. In all groups, wound healing rates increased
with increasing duration. Significant increases in wound-healing
activity were observed in the mice treated with aloe emodin and
resveratrol, compared with those that received control treatments.
The effects of the treatments on the wound-healing activity levels
in mice with excision wounds are presented in Tables I and II. In this model, the aloe
emodin-treated mice exhibited a significant increase in wound
healing rate (P<0.05), and a decreased time to
epithelialization, compared with the control mice.
 | Table IEffects of aloe emodin and resveratrol
on the levels of VEGF and MCP-1 in cultured HaCaT cells in
vitro. |
Table I
Effects of aloe emodin and resveratrol
on the levels of VEGF and MCP-1 in cultured HaCaT cells in
vitro.
| Treatment
(ng/ml) | VEGF
(ng/well)a | MCP-1
(ng/well)a |
|---|
| Medium alone | 87.32±7.47 | 2.471±0.241 |
| Aloe emodin | | |
| 1 | 96.18±8.46 | 3.341±0.345b |
| 100 | 92.26±6.25 | 3.333±0.431b |
| 500 | 97.40±6.21 | 3.697±0.143b |
| Medium alone | 84.62±2.67 | 2.562±0.533 |
| Resveratrol | | |
| 1 | 93.13±7.35 | 3.332±0.842b |
| 100 | 92.23±6.63 | 3.652±0.435b |
| 500 | 97.36±6.35 | 3.637±0.144b |
 | Table IIEffects of treatment with aloe emodin
and resveratrol on the epithelialization of the burn wounds in
mice. |
Table II
Effects of treatment with aloe emodin
and resveratrol on the epithelialization of the burn wounds in
mice.
| Group | Time to
decrustationa (days) | Healing
timeb (days) |
|---|
| Control | 9.80±1.56 | 21.89±2.36 |
| Aloe emodin | 7.24±1.45a | 17.20±4.35a |
| Resveratrol | 10.05±5.23 | 19.10±2.57a |
Effects of aloe emodin and resveratrol on
burn wound healing in mice
The wound injury area created by an iron bar heated
at 100°C reached a maximum on days 6–8, and wound repair was
subsequently observed. The burn wound area in the groups treated
with the topical application of resveratrol at doses of
2×10−4 and 5×10−4% (w/w) decreased on days 8
and 19, compared with those in the vehicle-treated control mice
(Fig. 3A–C). Previous studies have
investigated the effect of natural products isolated from medicinal
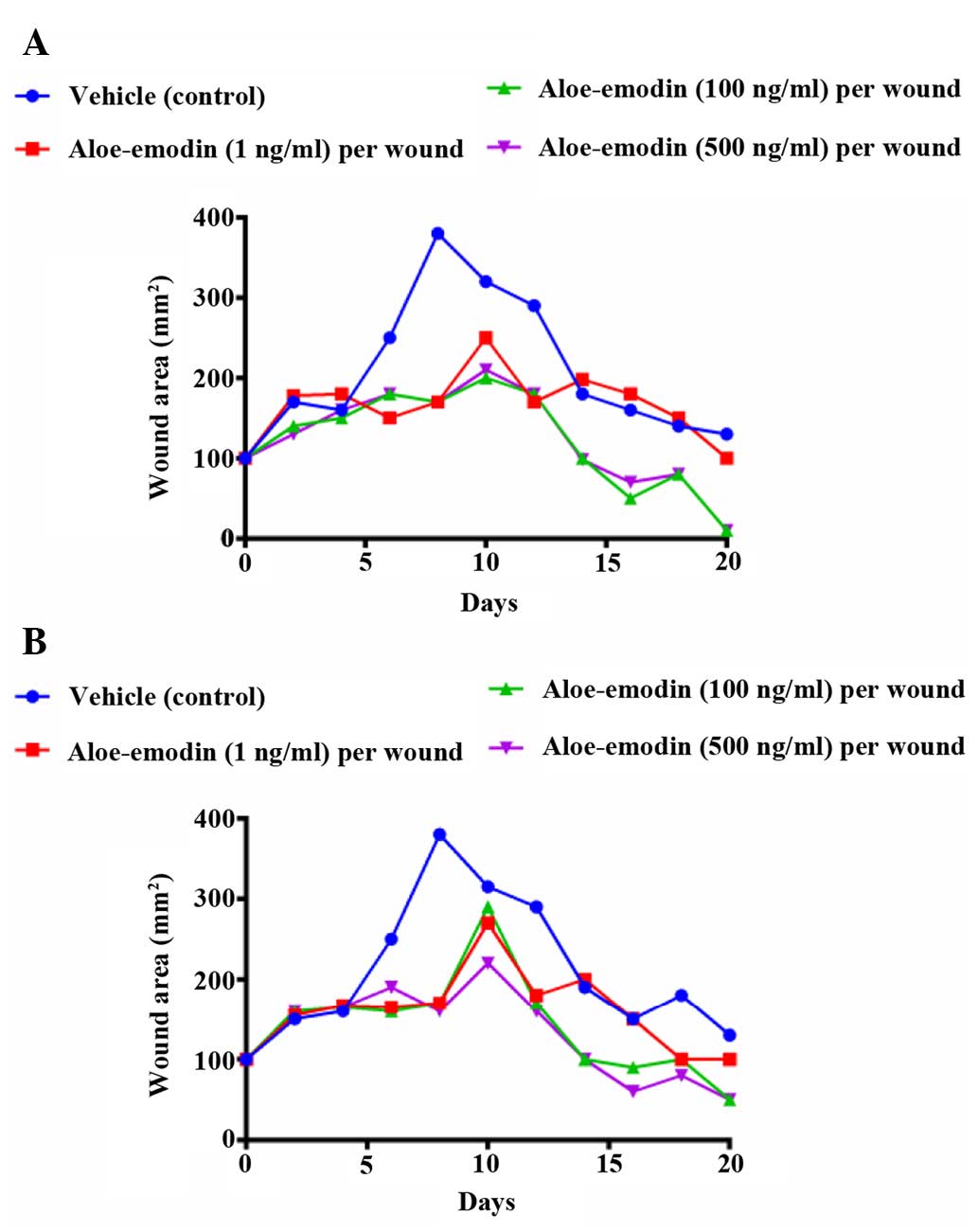
plants on skin regeneration in burn wound healing (22). The present study also demonstrated
that the burn wound areas in mice treated with aloe emodin and
resveratrol at doses of 1×10−8 and 1×10−12%
were significantly (P<0.05) reduced after 6–18 days, compared
with those of the control group, however, no significant difference
was observed between the non-control treatment groups (Fig. 4A and B).
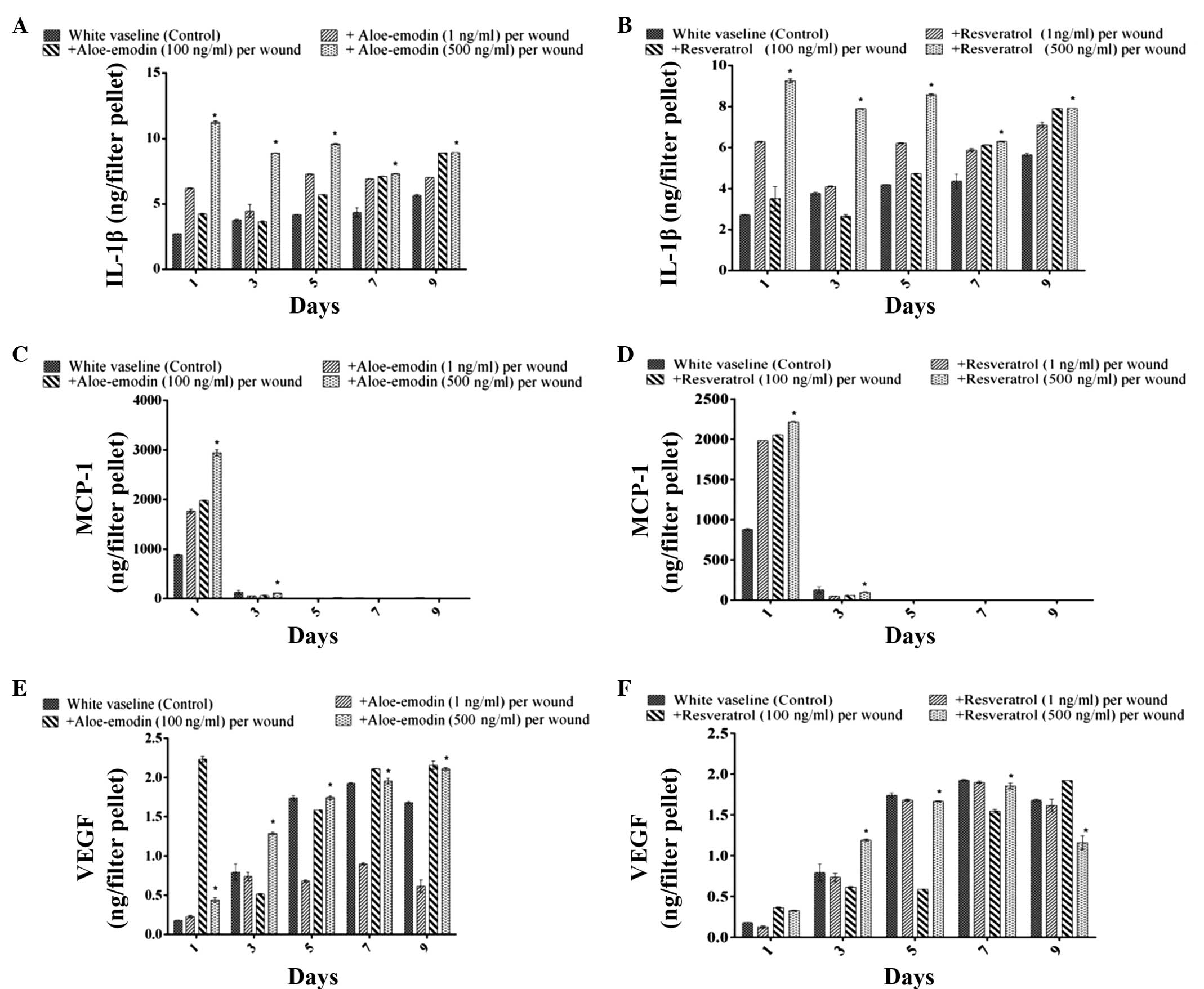
Measurement of the levels of IL-1β, MCP-1
and VEGF in the tissue samples
The levels of IL 1β, MCP-1 and VEGF in the exudates
of vehicle-treated burn wounded control mice were analysed. The
levels of IL-1β increased in a time-dependent manner over the 7
days following wound formation. It is well-known that the
production of IL-1β by macrophages is stimulated by
lipopolysaccharide. Aloe emodin (500 ng) significantly increased
the level of IL-1β on days 1, 3, 5 and 9, compared with the level
observed in the resveratrol (100 ng) and control groups (P<0.05)
(Figs. 5A and B). At a dose of 1
ng, aloe emodin also increased the level of IL-1β on day 6. The
migration of polymorphonuclear leukocytes was reduced by aloe
emodin and resveratrol (1, 100 and 500 ng/ml) 1 or 3 days after
treatment of the burn. The level of IL-1β production in the
exudates of the burn wound area of treated mice increased time
dependently 7 days after the tissue was wounded. The MCP-1 level in
the exudates of the wound area of control mice reached a maximum
level 1 day after burn treatment, and declined 3 days after burn
treatment (Figs. 5C and D). The
VEGF level in the exudates of the wound area of control mice
increased until day 7. The application of asiaticoside at a dose of
100 ng/filter pellet increased the VEGF level on days 1 and 5
compared with that in control mice, and at a dose of 1 ng/filter
pellet, it increased the VEGF level on day 9 (Figs. 5E and F).
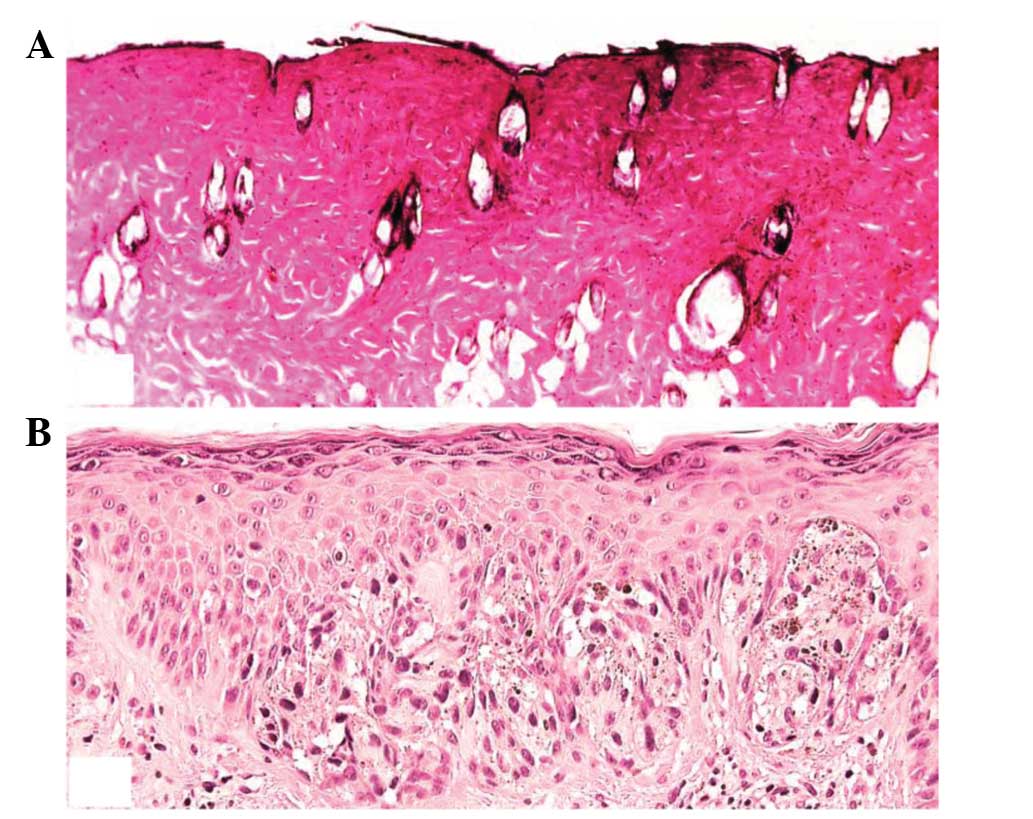
Histopathology
H&E-stained sections of granulation tissue
samples collected on days 4 and 10 following wound formation were
examined for cellular infiltration, epithelial regeneration and
matrix organization. Granulation tissue was collected on days 4 and
10 for H&E staining Histopathological analysis of the wounds on
day 4 indicated increased cellular infiltration in the treated
groups, compared with the control group, with no epidermal
regeneration observed. After 10 days, the wounds exhibited steady
and progressive wound healing in the control group. The eschar had
separated, leaving space for the epidermis to grow to complete
re-epithelisation. A reasonable level of collagen and numerous
inflammatory cells were observed in the corium. The regeneration,
stratification and polarity of the epithelial cells were markedly
higher in the aloe emodin-treated burn wound, compared with the
control. Fig. 6A and B shows the
appearance of the mouse burn wound skin tissues at day 10 in the
aloe emodin- and resveratrol-treated groups.
Discussion
Wound healing is a response to injury aimed at
reconstructing damaged tissue, and requires the precise
coordination of connective tissue repair, re-epithelialization and
angiogenesis for generation of new tissue and healing of the wound,
increase in fibroblast proliferation and the production of several
extracellular matrix proteins and growth factors (23,24).
Angiogenesis is required during wound healing, and supplies oxygen
and metabolites to new tissue, and disposes of metabolic waste
products during wound repair (25). Angiogenesis may also be a key
regulatory process in wound healing, as impairment of angiogenesis
leads to delayed or unsuccessful wound healing (26).
In the present study, the histological scores
demonstrated that the aloe emodin-treated group exhibited higher
levels of re-epithelialization and angiogenesis, compared with the
control group. Angiogenesis in granulation tissues improves
circulation in the wound site, thus providing oxygen and nutrients
that are essential for the healing process (27).
It was also observed that aloe emodin concentrations
between 10 and 100 ng per wound area The progressive changes in
wound area were measured in mm2 by tracing the wound
boundaries on a transparent paper every 2 days.
increased the production of VEGF, IL-1β and MCP-1
and the accumulation of macrophages and VEGF-positive cells in the
tissue surrounding the burn wound, compared with the control mice.
VEGF is a homodimeric glycoprotein, which is highly conserved and
shares structural homology with placental growth factor and
platelet-derived growth factor (28). Kitano et al (29) demonstrated the suppression of
TNF-α-induced fibroblast migration and fibronectin deposition in
vitro, and that VEGF induced neovascularization, but did not
affect cell proliferation or type 1 collagen production. MCP-1 is
one of the few chemo attractants expressed by cells, predominantly
fibroblasts (30). The recruitment
of macrophages occurs due to its upregulation, resulting in the
induction of fibrotic reactions through their expression of TNF-α.,
which is a proinflamatory cytokine expressed by epithelial cells
and macrophages (29). In the
present study, the direct stimulation of VEGF production in HaCaT
keratinocyte cell lines resulted in an increase in the healing
action of aloe emodin. Therefore, the results of the present study
suggested that aloe emodin promoted angiogenesis during skin wound
repair as a result of VEGF stimulation due to an increase in the
expression of IL-1β in macrophages. Further experiments are
required to elucidate the clinical implication of these findings
for burn wound healing.
In conclusion, the present study demonstrated that
the application of aloe emodin and resveratrol resulted in a
significant increase in healing activity when topically applied on
murine burn wounds. These results provide pharmacological evidence
supporting the potential use of Aloe vera and Vitis
vinifera for burn wound healing.
Acknowledgments
The authors would like to thank the Department of
Plastic of Aesthetic Center, Yantai Yuhuangding Hospital for
providing the animals and lab facilities for this study.
References
|
1
|
Reddy KK, Grossman L and Rogers GS: Common
complementary and alternative therapies with potential use in
dermatologic surgery: risks and benefits. J Am Acad Dermatol.
68:e127–e135. 2013. View Article : Google Scholar
|
|
2
|
Wild T, Rahbarnia A, Kellner M, Sobotka L
and Eberlein T: Basics in nutrition and wound healing. Nutrition.
26:862–866. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pereira RF, Carvalho A, Gil MH, Mendes A
and Bartolo PJ: Influence of Aloe vera on water absorption and
enzymatic in vitro degradation of alginate hydrogel films.
Carbohydr Polym. 98:311–320. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eshun K and He Q: Aloe vera: a valuable
ingredient for the food, pharmaceutical and cosmetic industries - a
review. Crit Rev Food Science Nutri. 44:91–96. 2004. View Article : Google Scholar
|
|
5
|
Naqvi S, Ullah MF and Hadi SM: DNA
degradation by aqueous extract of Aloe vera in the presence of
copper ions. Indian J Biochem Biophys. 47:161–165. 2010.PubMed/NCBI
|
|
6
|
Blumenhal M, Busse NR and Golddberg A: The
complete commission E. monographs therapeutic guide to herbal
medicine. Integrative Medicines Communications; Boston, MA: pp.
80–81. 1998
|
|
7
|
WHO Monographs on Selected Medicinal
Plants. 1. World Health Organization; Geneva, Switzerland: 1999
|
|
8
|
Dat AD, Poon F, Pham KB and Doust J: Aloe
vera for treating acute and chronic wounds. Cochrane Database Syst
Rev. 2:CD0087622012.PubMed/NCBI
|
|
9
|
Hamman JH: Composition and applications of
Aloe vera Leaf Gel. Molecules. 13:1599–1616. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Im SA, Lee YR, Lee YH, Lee MK, Park YI,
Lee S, Kim K and Lee CK: In vivo evidence of the immunomodulatory
activity of orally administered Aloe vera gel. Arch Pharm Res.
33:451–456. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Talmadge J, Chavez J, Jacobs L, Munger C,
Chinnah T, Chow JT, Williamson D and Yates K: Fractionation of Aloe
vera L. inner gel, purification and molecular profiling of
activity. Int Immunopharmacol. 4:1757–1773. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oryan A, Naeini AT, Nikahval B and Gorjian
E: Effect of aqueous extract of Aloe vera on experimental cutaneous
wound healing in rat. Veterinarski Arhiv. 80:509–522. 2010.
|
|
13
|
Nayak BS, Ramdath DD, Marshall JR, Isitor
GN, Eversley M, Xue S and Shi J: Wound-healing activity of the skin
of the common grape (Vitis Vinifera) variant, Cabernet Sauvignon.
Phytother Res. 24:1151–1157. 2010.PubMed/NCBI
|
|
14
|
Hemmati AA, Aghel N, Rashidi I and
Gholampur-Aghdami A: Topical grape (Vitis vinifera) seed extract
promotes repair of full thickness wound in rabbit. Int Wound J.
8:514–520. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chan MMY: Antimicrobial effect of
resveratrol on dermatophytes and bacterial pathogens of the skin.
Biochem Pharmacol. 63:99–104. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schneider Y, Vincent F, Duranton B, Badolo
L, Gossé F, Bergmann C, Seiler N and Raul F: Anti-proliferative
effect of resveratrol, a natural component of grapes and wine, on
human colonic cancer cells. Cancer Lett. 158:85–91. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Romero-Pérez AI, Ibern-Gómez M,
Lamuela-Raventós RM and de La Torre-Boronat MC: Piceid, the major
resveratrol derivative in grape juices. J Agric Food Chem.
47:1533–1536. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dinarello CA: Immunological and
inflammatory functions of the interleukin-1 family. Annu Rev
Immunol. 27:519–550. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park J and Boo YC: Isolation of
resveratrol from vitis viniferae caulis and its potent inhibition
of human tyrosinase. Evidence-Based Complement Alternat Med.
2013:6452572013. View Article : Google Scholar
|
|
20
|
Lee E and Surh YJ: Induction of apoptosis
in HL-60 cells by pungent vanilloids, (6)-gingerol and (6)-paradol.
Cancer Lett. 134:163–168. 1998. View Article : Google Scholar
|
|
21
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maenthaisong R, Chaiyakunapruk N,
Niruntraporn S and Kongkaew C: The efficacy of Aloe vera used for
burn wound healing: A systematic review. Burns. 33:713–718. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singer AJ and Clark RA: Cutaneous wound
healing. N Engl J Med. 341:738–746. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jettanacheawchankit S, Sasithanasate S,
Sangvanich P, Banlunara W and Thunyakitpisal P: Acemannan
stimulates gingival fibroblast proliferation; Expressions of
keratinocyte growth factor-1, vascular endothelial growth factor
and type I collagen; and wound healing. J Pharmacol Sci.
109:525–531. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
İnan A, Meral Ş, Cemile K, Metin E and
Cenap D: Effects of Aloe vera on colonic anastomoses of rats. Surg
Prac. 11:60–65. 2007. View Article : Google Scholar
|
|
26
|
Moon EJ, Lee YM, Lee OH, Lee MJ, Lee SK,
Chung MH, Park YI, Sung CK, Choi JS and Kim KW: A novel angiogenic
factor derived from Aloe vera gel: Beta-sitosterol, a plant sterol.
Angiogenesis. 3:117–123. 1999. View Article : Google Scholar
|
|
27
|
Szabo S, Kusstatscher S, Sakoulas G,
Sandor Z, Vincze A and Jadus M: Growth factors: New 'endogenous
drugs' for ulcer healing. Scand J Gastroenterol Suppl. 210:15–18.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tischer E, Gospodarowicz D, Mitchell R,
Silva M, Schilling J, Lau K, Crisp T, Fiddes JC and Abraham JA:
Vascular endothelial growth factor: A new member of the
platelet-derived growth factor gene family. Biochem Biophys Res
Commun. 165:1198–1206. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kitano A, Saika S, Yamanaka O, Ikeda K,
Okada Y, Shirai K and Reinach PS: Emodin suppression of ocular
surface inflammatory reaction. Invest Ophthalmol Vis Sci.
48:5013–5022. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Simpson JE, Newcombe J, Cuzner ML and
Woodroofe MN: Expression of monocyte chemoattractant protein-1 and
other β-chemokines by resident glia and inflammatory cells in
multiple sclerosis lesions. J Neuroimmunol. 84:238–249. 1998.
View Article : Google Scholar : PubMed/NCBI
|