Introduction
High environmental temperatures may be fatal and are
associated with sudden death in animals and humans (1). The incidence of heat-associated
mortality is likely to increase with global warming, and with the
predicted increase in frequency and intensity of heat waves
(2,3). Scientific reports have indicated that
hyperthermia (40–45°C) is cytotoxic (2,4) and
prolonged exposure to temperatures >42°C causes cellular damage,
and protein denaturation and aggregation (5). However, the causes of the progression
from heat stress to lethal heat stroke and clinical hyperthermia,
and the mechanisms underlying hyperthermia-induced cytotoxicity,
remain poorly understood (6,7). A
previous study demonstrated that heart attacks caused by high
temperatures are associated with high mortality due to heart
diseases such as hypertension, coronary vessel occlusion, and
atherosclerosis (8).
Heat stress induces the expression of heat-shock
proteins (Hsps) (9,10). Hsps are usually divided into small
Hsps (sHsps) (molecular mass, <40 kDa), and Hsp40, Hsp60, Hsp70
(68–80 kDa), Hsp90 (83–99 kDa), and Hsp100 protein families. All
Hsp families share a common chaperoning function. It is generally
accepted that Hsps prevent cells from lethal thermal damage
(11). A lack of Hsp synthesis is
associated with exponential cell death (12). Furthermore, a previous study
reported that stretched and decreased myocyte shortening results in
an increase in the expression levels of Hsps in the isolated
perfused rabbit heart (13).
sHsps are ubiquitous components of protein quality
control cell networks, and can be induced by numerous events
including hypoxia, heat shock, ultraviolet light, and toxic free
radicals (9,14). As with other chaperones, sHsps have
a high capacity to bind unfolded proteins and facilitate substrate
refolding (15). Hsp27 and
αB-crystallin belong to the sHsp superfamily, and are functional,
stress-induced sHsps that are expressed in numerous tissues types,
notably in muscles (7). In humans,
there are 10 genes that encode sHsps (16); however, only Hsp27 and
αB-crystallin function as molecular chaperones (17,18).
The rapid upregulation of sHsps is regulated by transcriptional and
translational mechanisms (19).
Hsp27 and αB-crystallin possess a homologous α-crystallin domain,
which prevents actin microfilament disruption under stress
conditions (7). The effects of
sHsps on the cytoskeleton may be important not only in individual
cell tolerance to stress through cytoskeletal stabilization, but
may also be integral to the protection of the whole organism
through the maintenance of endothelial and epithelial barrier
functions (20).
Presently, it remains uncertain whether Hsp27 and
αB-crystallin proteins and genes induce similar changes in heart
cells and tissues in response to heat stress in vitro and
in vivo. Therefore, the present study investigated the
expression levels of Hsp27 and αB-crystallin, both in rats, and in
a myocardial cell line, following exposure to high temperature.
Materials and methods
Animals and experimental design
All experiments were performed in accordance with
the guidelines of the Animal Ethics Committee of Jiangsu province
(China) and were approved by the Institutional Animal Care and Use
Committee of Nanjing Agricultural University (Nanjing, China).
Sixty-day-old Sprague Dawley rats (n=60) were obtained from Qing
Long Shan Company (Nanjing (China) and housed at room temperature
(25°C) for 5 days. The rats were then randomly divided into six
groups (n=10): Five heat-stress exposure groups (for 20, 40, 60, 80
and 100 min, respectively) and a control group. All animals were
given ad libitum access to water and were fed the same feed
during the experiments. A controlled-climate chamber (New Jiangnan
Instrument Co., Ltd., Ningbo, Zhejiang) was pre-heated to 42°C with
circulating fresh air, and the relative humidity was kept between
55–65%. The control rats were maintained at room temperature. The
mental state and activities of the control and heat-stressed rats
were observed and recorded. Following each heat-stress period, the
rats were sacrificed within 3 min. Blood samples were then
collected, and the hearts were divided into two sections: The
ventriculus sinister, which was fixed in 4% paraformaldehyde for
pathologic observation, and the ventriculus dexter, which was
stored in liquid nitrogen until further experimentation.
Cell subculture and preparation
The H9c2 myocardial cells (American Type Culture
Collection, Manassas, VA, USA) were subcultured in Dulbecco's
modified Eagle's medium supplemented with 10% fetal calf serum
(Gibco Life Technologies, Carlsbad, CA, USA), and incubated at 37°C
in an atmosphere containing 5% CO2, until the fusion
rate of the H9c2 cells was >90%. The cells were then divided
into six groups: A control group (0 min), and five groups exposed
to heat stress for 20, 40, 60, 80 and 100 min. For prompt heat
exposure, the temperature in the incubator was raised from 37 to
42°C in a humidified atmosphere containing 5% CO2.
Western blot analysis
For protein extraction, all experimental rats were
humanely sacrificed by decapitation. A total of ~100 µg
heart tissue was collected and homogenized in 1 ml
phosphate-buffered saline (PBS) using a 623003 Fluko®
Super Fine Homogenizer (Fluko Equipment Shanghai Co. Ltd.,
Shanghai, China) and centrifuged at 2,000 × g. The cell pellets
were resuspended in 200 µl ice-cold radioimmunoprecipitation
assay lysis buffer containing 50 mM Tris, (pH 7.4), 150 mM NaCl, 1%
NP-40, 0.5% sodium deoxycholate, 0.1% SDS, and l ml
phenylmethylsulfonyl fluoride WB-0071 (Beijing Ding Guo Chang Sheng
Biotechnology Co. Ltd., Beijing, China). The homogenates were then
centrifuged at 14,000 × g for 5 min at 4°C, and the obtained
supernatants were used as total protein extracts. Following 0, 20,
40, 60, 80 and 100 min of heat stress treatment in an incubator at
42°C, H9c2 cells were washed twice with PBS and lysed in M-PERH
mammalian protein extraction reagent (28501; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with Halt™
protease inhibitor cocktail according to the manufacturer's
instructions (Thermo Fisher Scientific, Inc.). The cell homogenates
were then centrifuged at 14,000 × g for 5 min at 4°C and the
supernatants were used as total protein extracts. All protein
concentrations were measured using a Micro-Bicinchoninic Acid™
Protein Assay kit (Thermo Fisher Scientific, Inc.). A total of 20
µg H9c2 protein and heart sample protein (80 µg) were
loaded onto a 13% acrylamide gel with a 4% stacking acrylamide gel.
Migration was performed using a buffer containing 25 mM Tris, pH
7.6, 0.1% SDS, and 0.2 mg lysine. The proteins in the gels were
transferred onto a polyvinylidene difluoride membrane (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The membranes were then
blocked for 1 h at room temperature in Tris-buffered saline with
Tween 20 (TBST; Sigma-Aldrich, St. Louis, MO, USA) containing 5%
milk powder, and incubated overnight at room temperature with
αB-crystallin mouse immunoglobulin G monoclonal primary antibody
(cat. no. ADI-SPA-222-F) and GAPDH mouse IgG monoclonal primary
antibody (cat. no. ADI-CSA-335-E) (Enzo Life Sciences, Inc.,
Farmingdale, NY, USA). The blots were washed three times for 5 min
in TBST and 5% skim milk powder containing goat anti-mouse antibody
(cat. no. SN133; Sunshine Biotechnology Nanjing Co. Ltd., Nanjing,
China) at room temperature for 1 h. Following three 5 min washes
with TBST, the bands were revealed using diaminobenzidine
(Sigma-Aldrich) in a 30 ml buffer containing 60 mM Tris, pH 6.8,
0.2% hydrogen peroxide, and 200 ml 0.8% NiCI2. Following
staining, the membranes were washed in distilled water and dried at
37°C in an oven. The bands on the developed film were quantified
with Quantity One software, version 4.6.2 (Bio-Rad Laboratories,
Inc.). The density of each band was normalized to that of the GADPH
protein.
Detection of αB-crystallin and hsp27 mRNA
by fluorescence reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) in vivo and in vitro Isolation of total RNA and
reverse transcription
Total RNA was extracted from the cultured H9c2 cells
using RNAiso Plus reagent (Takara Biotechnology Co., Ltd., Dalian,
China), according to the manufacturer's instructions. Briefly,
after H9c2 myocytes were heat-stressed at 42°C for various time
periods, 1 ml RNAiso Plus reagent was added to 10 mm2
cell culture plates. For the rat tissue samples, 100 mg heart
tissue was homogenized with the 623003 Fluko® Super Fine
Homogenizer and 1 ml RNA extraction buffer (TRIzol; Takara
Biotechnology, Co., Ltd.) was added, according to the
manufacturer's instructions. RNA concentration was determined using
a M200PRO spectrophotometer (Tecan Austria GmbH, Grödig, Austria).
RNA samples were synthesized into cDNA using PrimeScript Reverse
Transcriptase Master Mix Perfect Real Time (cat. no. DRR036A;
Takara Biotechnology Co., Ltd.), according to the manufacturer's
instructions, and stored at −80°C for further analysis.
Design of PCR primers
Primer sets were specifically designed to anneal to
each target mRNA. The sequences for Hsp27, αB-crystallin and GAPDH
mRNA were obtained from the National Center for Biotechnology
Information Genbank (accession nos. NC_005111.4, NC_005107.4 and
NC_005103.4, respectively). The primers were designed using the
Primer Premier 5.0 software (Premier Biosoft, Palo Alto, CA, USA)
for conventional and RT-qPCR amplification. The primer sequences
for the genes were as follows: Hsp27, forward
5′-CGTGGTGGAGATCACTGGCAAGC-3′, and reverse
5′-CGGGCCTCGAAAGTGACCGG-3′; and αB-crystallin, forward
5′-CACGAAGAGCGCCAGGACGA-3′, and reverse
5′-CGTCGGCTGGGATCCGGTACT-3′; GAPDH, forward
5′-GGCTCTCTGCTCCTCCCTGTTCTAG-3′ and reverse
5′-GGCTCTCTGCTCCTCCCTGTTCTAG-3′. The expected sizes of the Hsp27
and αB-crystallin PCR products were 216 and 153 bp, respectively.
Primers were obtained from Invitrogen Life Technologies (Shanghai,
China).
RT-qPCR
Using the iQ5 Real-Time PCR Detection system
thermocycler (Bio-Rad Laboratories, Inc.), each cDNA sample (2
µl, 10X dilution) was suspended in 2X iQ™ SUPER®
Green Supermix (Bio-Rad Laboratories, Inc.) with 0.6 µl of
both sense and antisense primers, and double-distilled water to a
total volume of 20 µl. The PCR cycling conditions were as
follows: Enzyme activation was carried out at 95°C for 3 min
followed by 40 cycles of denaturation at 95°C for 20 sec, annealing
at 60°C for 30 sec and elongation at 72°C for 30 sec.
For each run, a negative control tube without cDNA
was analyzed alongside the experimental groups. A 4-fold dilution
series of the template was used in the PCR amplification reactions.
The data were analyzed using Bio-Rad iQ5 software (Bio-Rad
Laboratories, Inc.) and the αB-crystallin mRNA levels were
normalized using the following formula: Relative quantity of
αB-crystallin/hsp27 mRNA = 2−ΔΔCt ΔΔCt =
[(CtαB-crystallin/hsp27 mRNA − CtGAPDH mRNA)
control group − (CtαB-crystallin/hsp27 mRNA −
CtGAPDH mRNA) test group].
Analysis of the association between Hsp27
and αB-crystallin
To analyze the association between Hsp27 and
αB-crystallin, the STRING 9.1 database (http://string-db.rg/) was used, which aims to provide
a global perspective for as many organisms as possible. In the
STRING database, known and predicted associations are scored and
integrated, resulting in comprehensive protein networks covering
>1,100 organisms. This software extends the automated mining of
scientific texts for interaction information, and also includes
full-text articles (21).
Statistical analysis
Statistical differences between the heat-stressed
groups and the control group were analyzed by one-way analysis of
variance followed by a least significant difference multiple
comparison test, using the SPSS 20.0 for Windows (IBM SPSS, Armonk,
NY, USA). The results were expressed as the mean ± standard
deviation of at least three independent experiments. All
experiments were performed in triplicate. P<0.05 was considered
to indicate a statistically significant difference.
Results
Protein expression levels of Hsp27 and
αB-crystallin in vivo in response to heat stress
The protein expression levels of Hsp27 and
αB-crystallin were measured in vivo in response to various
durations of exposure to heat shock, and normalized to GAPDH
(Fig. 1). In the heat-stressed rat
heart, αB-crystallin decreased significantly (P<0.01) after 20
min exposure to heat, but increased by almost 3-fold after 40 min
(P<0.01), and decreased after 60, 80 and 100 min (P<0.01).
The protein expression levels of Hsp27 decreased significantly
(P<0.01) after all durations of exposure to heat stress;
however, after 40 min, the expression levels of Hsp27 were higher,
as compared with after 20 min.
Protein expression levels of Hsp27 and
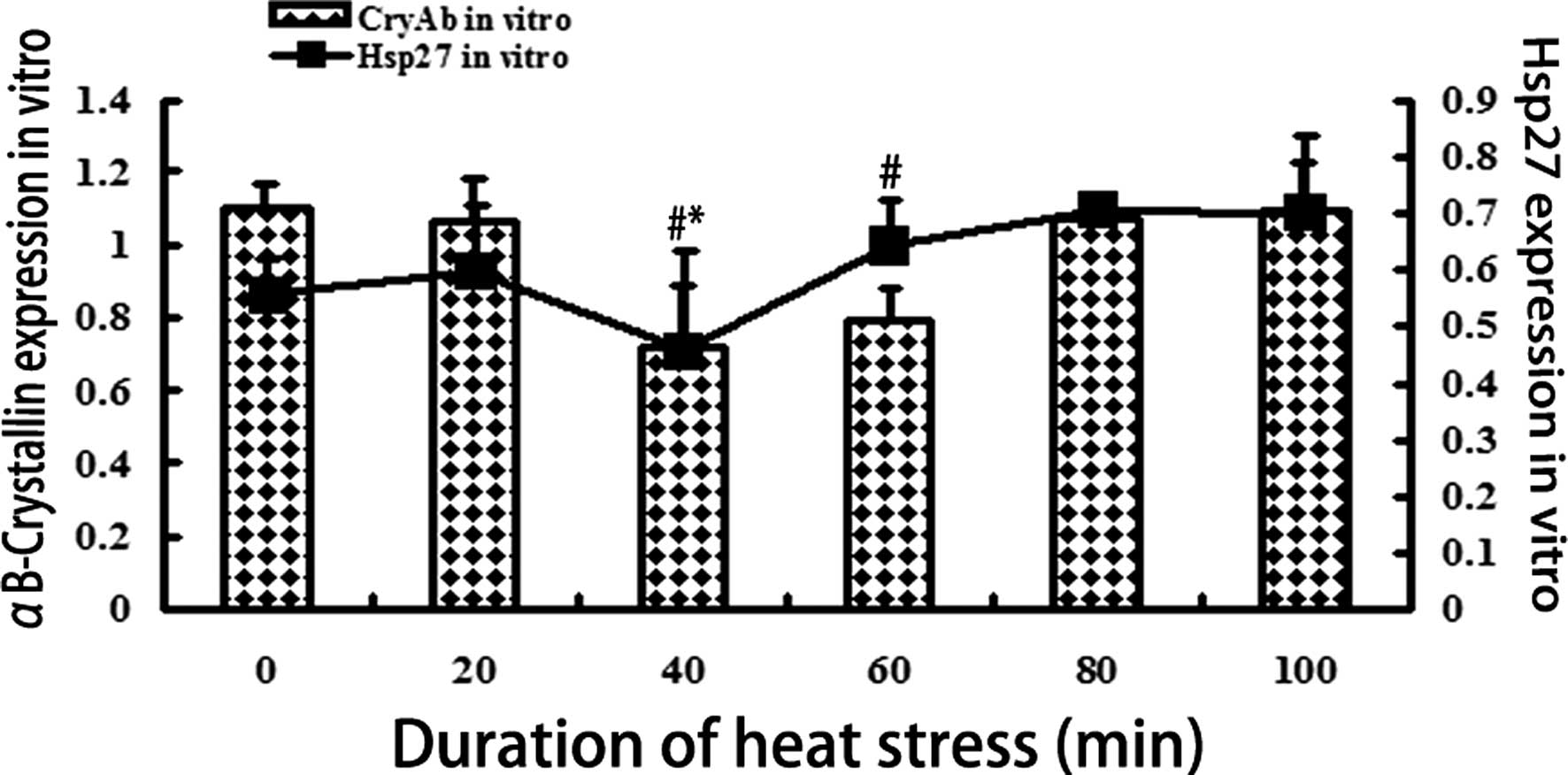
αB-crystallin in vitro in response to heat stress
The protein expression levels of Hsp27 and
αB-crystallin were measured in vitro in response to various
durations of exposure to heat shock, and normalized to GAPDH
(Fig. 2). The expression levels of
αB-crystallin decreased significantly (P<0.01) after 40 and 60
min of heat stress, as compared with the control group, but
increased after 80 and 100 min of exposure to heat stress
(P<0.01). However, no statistically significant difference in
the expression levels of αB-crystallin was observed between the 80
and 100 min groups. The expression levels of Hsp27 decreased after
40 min of heat exposure (P<0.01), but after 60, 80 and 100 min
the expression levels increased, as compared with the control
group.
mRNA expression levels of Hsp27 and
αB-crystallin in vivo in response to heat stress
The mRNA expression levels of Hsp27 and
αB-crystallin in response to heat shock exposure in vivo,
normalized to the GAPDH gene, are displayed in Fig 3. The RT-qPCR results demonstrated
that compared with the control group, the mRNA expression levels of
αB-crystallin and Hsp27 were significantly increased after 20, 40,
60, 80 and 100 min of heat stress in the rat heart (P<0.01).
mRNA expression levels of Hsp27 and
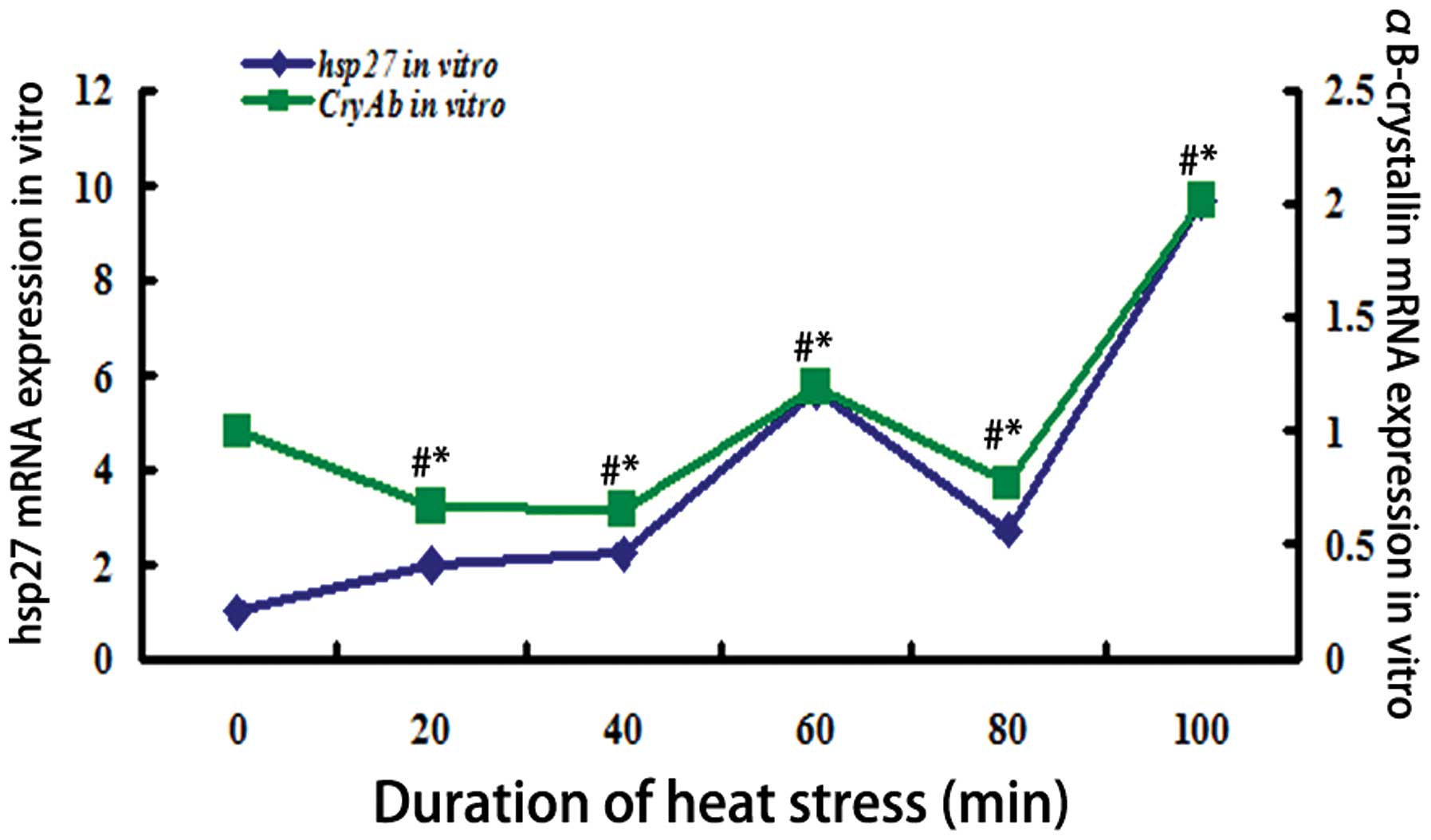
αB-crystallin in vitro in response to heat stress
The mRNA expression levels of hsp27 and
αB-crystallin in response to heat shock exposure in vitro,
normalized to the GAPDH gene, are displayed in Fig. 4. The mRNA expression levels of
αB-crystallin were significantly reduced (P<0.01) following 20,
40 and 80 min heat shock exposure, and those of Hsp27 were
significantly increased (P<0.01) following 20 and 40 min heat
shock exposure. However, following 60 min of heat shock exposure,
the levels of Hsp27 and αB-crystallin exhibited similar trends.
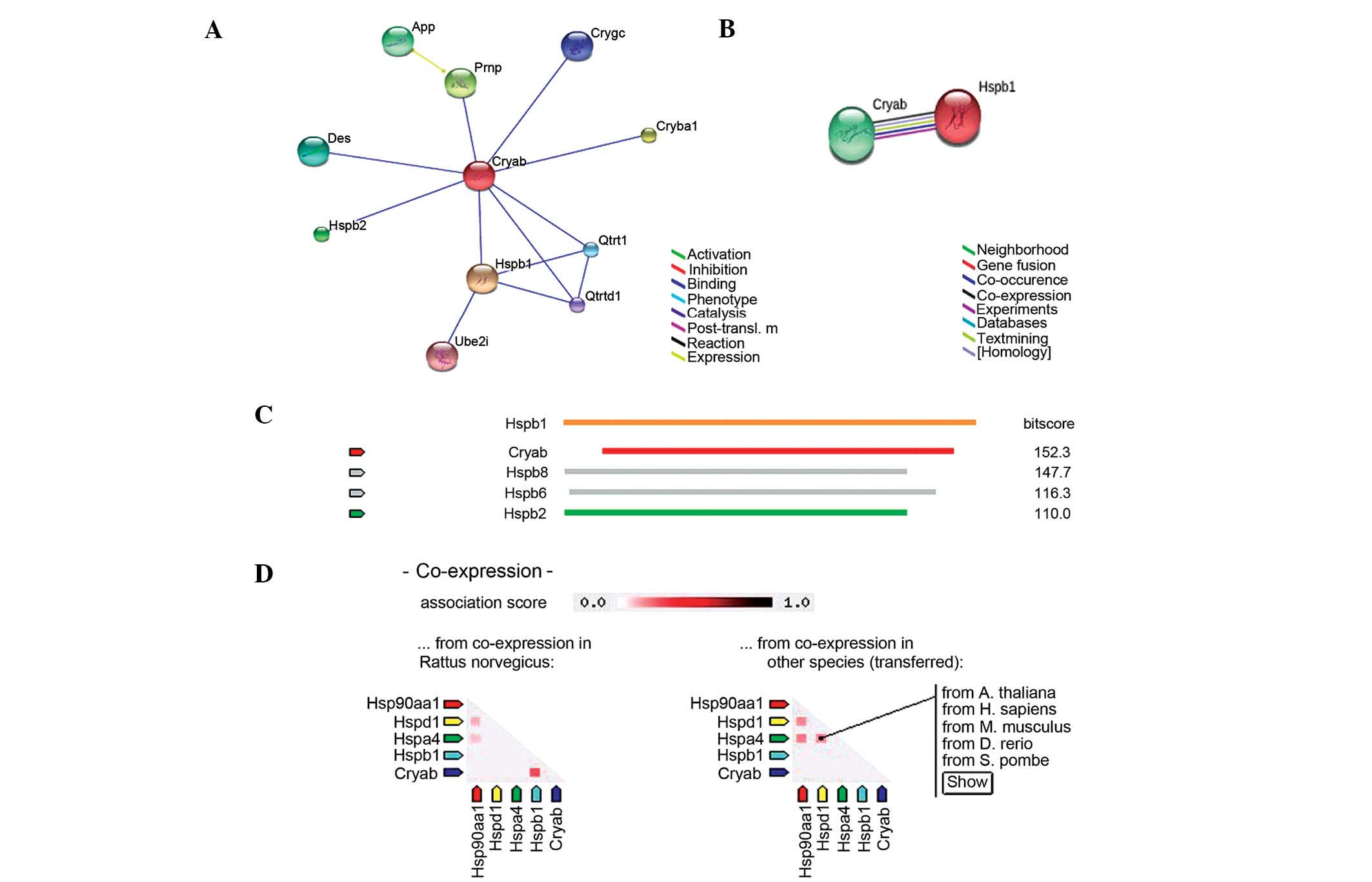
The association between Hsp27 and
αB-crystallin
These preliminary results suggest that the protein
and mRNA expression levels of Hsp27 and αB-crystallin exhibit a
similar trend in heat-stressed myocardial cells in vivo and
in vitro, respectively. According to the rat database in
STRING, αB-crystallin is able to bind Hsp27 in rat cells (Fig. 5A). In the evidence view in STRING
(Fig. 5B), the different colors
represent various sources of evidence from the database and
experiments, and confirmed the co-expression of αB-crystallin and
Hsp27. Compared with other proteins that are associated with
αB-crystallin, such as Hspb2, Hsp27 has the highest co-expression
score with αB-crystallin (Fig.
5C). The co-expression view (Fig.
5D) indicated that αB-crystallin and Hsp27 are co-expressed in
rats, but not in other species, such as Homo sapiens. In the
present study, both the protein and mRNA expression levels of Hsp27
and αB-crystallin exhibited similar trends in response to heat
shock in vivo and in vitro, respectively, thus
indicating that they may be co-expressed.
Discussion
Heat stress causes extensive cytoskeletal and
mitochondrial damage, as well as uncoupling of oxidative
phosphorylation. Hsps are thought to limit injury and accelerate
recovery by refolding disrupted proteins and preventing deleterious
peptide interactions (17,22). sHsps are a widespread and diverse
class of proteins. Of the major Hsps, Hsp27 and αB-crystallin have
recently been identified as molecular chaperones (9). Stressors that transiently induce
Hsp27 and αB-crystallin include heat shock, anticancer drugs,
radiation, and oxidative stress. As compared with other Hsps, Hsp27
and αB-crystallin bind to numerous non-native proteins via an
oligomeric complex, and thus represent the most efficient
chaperones in terms of quantity of substrate binding (14,23).
In the present study, the mRNA expression levels of αB-crystallin
were significantly reduced (P<0.01) following 20, 40 and 80 min
heat shock exposure, and those of Hsp27 were significantly
increased (P<0.01) following 20 and 40 min heat shock exposure.
However, following 60 min of heat shock exposure, the levels of
Hsp27 and αB-crystallin exhibited similar trends. The protein
expression levels of Hsp27 decreased after 40 min, as compared with
the other groups, whereas αB-crystallin decreased only after 40 and
60 min of heat exposure in vitro. After 60 min, both sHsps
exhibited an increase in expression levels. The protein expression
trends of both sHsps were not concordant with their mRNA expression
levels from the initiation of heat stress in vitro, however
at 100 min sharply increased to mRNA levels consistant with the
levels of protein expression. However, in heat-stressed rat heart
in vivo, both the mRNA expression levels of Hsp27 and
αB-crystallin significantly increased after all durations of heat
exposure. A previous study suggested that the expression levels of
Hsp27 and αB-crystallin may be regulated at the transcriptional
level (24). However, in rat
hearts in vivo, the protein expression levels of Hsp27 and
αB-crystallin decreased, except for αB-crystallin after 40 min of
heat exposure. Both sHsps exhibited decreased expression level
trends after 100 min of heat stress. A previous study demonstrated
that Hsp27 and αB-crystallin mRNA is overexpressed in the heart and
other smooth muscles following heat stress, but the expression of
their corresponding proteins does not follow the same trend
(25). The present study
investigated the mRNA and protein expression levels of Hsp27 and
αB-crystallin in response to heat stress. Hsp27 and αB-crystallin
may modulate interactions between cellular factors by forming small
or large oligomers, undergoing phosphorylation, and inducing
cell-cell contact in vivo (7,22,24).
A previous study confirmed that Hsp27 and αB-crystallin exhibited a
phosphorylation response in the atrial myocardium of patients
undergoing valve repair (22).
However, the specific mechanism underlying this phosphorylation
response remains to be investigated.
In response to stressful conditions, αB-crystallin
is able to bind to Hsp27, relocalize to the cytoskeleton, and binds
to microtubules via microtubule-associated proteins, which may
protect the cells from damaged intracellular proteins by
sequestering these proteins on the cytoskeleton (15,26).
The present study demonstrated that the expression levels of Hsp27
and αB-crystallin markedly differ in the rat heart in vivo,
as compared with the H9c2 cells in vitro. In the whole rat
body, there may be numerous factors that affect the heat-stressed
heart. The protein expression levels of Hsps in cardiomyocytes may
be regulated by hormones. Thyroid hormones may be involved in the
regulation of Hsp27 expression in the rat myocardium (27). However, in the H9c2 cell line,
after 60 min exposure to heat stress, the protein expression levels
of both sHsps increased to reach a baseline level, which was
different from their expression trends in vivo. The
comparative analysis of the present study suggests that the
mechanism underlying this protection is not the same between the
individual cell and the whole body. During the experiments, the
mortality rate of heat-stressed rats was 100% after 120 min. The
low protein expression levels of both Hsps after 80 min indicated
that the inherent expression levels of Hsp27 and αB-crystallin may
not be enough to resist acute heat stress (28). A previous study demonstrated that
overexpression of Hsp27 offers protection via an anti-apoptotic
mechanism (15). However, these
observations still require confirmation, and pre-induction of sHsps
prior to heat stress may be an effective way to reduce the
mortality rate.
In the present study, Hsp27 and αB-crystallin were
activated by acute heat stress at both the mRNA and protein level
in vivo and in vitro. These results were concordant
with those of previous studies that demonstrated that Hsp27 and
αB-crystallin have similar roles when subjected to various
stressors (29,30). Hsp27 and αB-crystallin are
characterized by a conserved C-terminal region, termed the
αB-crystallin domain, a variable N-terminal sequence, and in most
cases a short and variable C-terminal tail (16). The results of the present study
suggested that the protein and mRNA expression levels of both Hsp27
and αB-crystallin exhibit similar trends in the H9c2 rat cell line.
Furthermore, as confirmed by STRING, these results support the
evidence that αB-crystallin is able to bind to Hsp27 in rat cells
(Fig. 5B). The co-expression score
between αB-crystallin and Hsp27 was the highest in rats, but did
not occur in all the species analyzed (including Homo
sapiens). A previous study demonstrated that Hsp27 and
αB-crystallin proteins often accumulate as inclusion bodies in
numerous protein conformation diseases, and have a similar role to
molecular chaperones (19).
Investigating the specific mechanism underlying the co-expression
of Hsp27 and αB-crystallin will provide the basis for our future
experiments.
In conclusion, Hsp27 and αB-crystallin are activated
by acute heat stress both at the mRNA and protein level in
vivo and in vitro. However, the expression levels of
Hsp27 and αB-crystallin differed between the whole body system and
the cell line when subjected to heat stress. When co-expressed,
Hsp27 and αB-crystallin may have roles in rat myocardial cells
in vivo and in vitro.
Acknowledgments
The current study was supported by grants from the
National Key Basic Research Program of China (973 Program; grant
no. 2014CB138502), the National Natural Science Foundation of China
(grant no. 31372403), the National Department Public Benefit
Research Foundation (Agriculture) (grant no. 201003060-11), the
Priority Academic Program Development of Jiangsu Higher Education
Institutions, Graduate Research and Innovation Projects in Jiangsu
Province and the Sino-German Agricultural Cooperation Project of
the Federal Ministry of Food, Agriculture and Consumer Production,
Berlin, Germany.
References
|
1
|
Rohde MC, Corydon TJ, Hansen J, Pedersen
CB, Schmidt SP, Gregersen N and Banner J: Heat stress and sudden
infant death syndrome - stress gene expression after exposure to
moderate heat stress. Forensic Sci Int. 232:16–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feder ME and Hofmann GE: Heat-shock
proteins, molecular chaperones, and the stress response:
Evolutionary and ecological physiology. Annu Rev Physiol.
61:243–282. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Herbst J, Gilbert JD and Byard RW: Urinary
incontinence, hyperthermia, and sudden death. J Forensic Sci.
56:1062–1063. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu Y, Lu X, Wu D, Cai S, Li S and Teng X:
The effect of manganese-induced cytotoxicity on mRNA expressions of
HSP27, HSP40, HSP60, HSP70 and HSP90 in chicken spleen lymphocytes
in vitro. Biol Trace Elem Res. 156:144–152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martin J, Horwich AL and Hartl FU:
Prevention of protein denaturation under heat stress by the
chaperonin Hsp60. Science. 258:995–998. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bagatell R, Paine-Murrieta GD, Taylor CW,
Pulcini EJ, Akinaga S, Benjamin IJ and Whitesell L: Induction of a
heat shock factor 1-dependent stress response alters the cytotoxic
activity of hsp90-binding agents. Clin Cancer Res. 6:3312–3318.
2000.PubMed/NCBI
|
|
7
|
Garrido C, Paul C, Seigneuric R and
Kampinga HH: The small heat shock proteins family: The long
forgotten chaperones. Int J Biochem Cell Biol. 44:1588–1592. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singh IS and Hasday JD: Fever,
hyperthermia and the heat shock response. Int J Hyperthermia.
29:423–435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Adhikari AS, Sridhar Rao K, Rangaraj N,
Parnaik VK and Mohan Rao Ch: Heat stress-induced localization of
small heat shock proteins in mouse myoblasts: Intranuclear lamin
A/C speckles as target for alphaB-crystallin and Hsp25. Exp Cell
Res. 299:393–403. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ananthan J, Goldberg AL and Voellmy R:
Abnormal proteins serve as eukaryotic stress signals and trigger
the activation of heat shock genes. Science. 232:522–524. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beere HM: “The stress of dying”: The role
of heat shock proteins in the regulation of apoptosis. J Cell Sci.
117:2641–2651. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arya R, Mallik M and Lakhotia SC: Heat
shock genes - integrating cell survival and death. J Biosci.
32:595–610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Knowlton AA, Brecher P and Apstein CS:
Rapid expression of heat shock protein in the rabbit after brief
cardiac ischemia. J Clin Invest. 87:139–147. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Acunzo J, Katsogiannou M and Rocchi P:
Small heat shock proteins HSP27 (HspB1), αB-crystallin (HspB5) and
HSP22 (HspB8) as regulators of cell death. Int J Biochem Cell Biol.
44:1622–1631. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Concannon CG, Gorman AM and Samali A: On
the role of Hsp27 in regulating apoptosis. Apoptosis. 8:61–70.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kappé G, Franck E, Verschuure P, Boelens
WC, Leunissen JA and de Jong WW: The human genome encodes 10
alpha-crystallin-related small heat shock proteins: HspB1-10. Cell
Stress Chaperones. 8:53–61. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lanneau D, Wettstein G, Bonniaud P and
Garrido C: Heat shock proteins: Cell protection through protein
triage. ScientificWorldJournal. 10:1543–1552. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wettstein G, Bellaye PS, Micheau O and
Bonniaud P: Small heat shock proteins and the cytoskeleton: An
essential interplay for cell integrity? Int J Biochem Cell Biol.
44:1680–1686. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zoubeidi A and Gleave M: Small heat shock
proteins in cancer therapy and prognosis. Int J Biochem Cell Biol.
44:1646–1656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moseley PL: Heat shock proteins and heat
adaptation of the whole organism. J Appl Physiol (1985).
83:1413–1417. 1997.
|
|
21
|
Franceschini A, Szklarczyk D, Frankild S,
Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C
and Jensen LJ: STRING v9.1: Protein-protein interaction networks,
with increased coverage and integration. Nucleic Acids Res.
41:D808–D815. 2013. View Article : Google Scholar :
|
|
22
|
Clements RT, Feng J, Cordeiro B, Bianchi C
and Sellke FW: p38-MAPK-dependent small HSP27 and αB-crystallin
phosphorylation in regulation of myocardial function following
cardioplegic arrest. Am J Physiol Heart Circ Physiol.
300:H1669–H1677. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baler R, Dahl G and Voellmy R: Activation
of human heat shock genes is accompanied by oligomerization,
modification, and rapid translocation of heat shock transcription
factor HSF1. Mol Cell Biol. 13:2486–2496. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Parcellier A, Schmitt E, Brunet M, Hammann
A, Solary E and Garrido C: Small heat shock proteins HSP27 and
alphaB-crystallin: Cytoprotective and oncogenic functions. Antioxid
Redox Signal. 7:404–413. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sugiyama Y, Suzuki A, Kishikawa M, Akutsu
R, Hirose T, Waye MM, Tsui SK, Yoshida S and Ohno S: Muscle
develops a specific form of small heat shock protein complex
composed of MKBP/HSPB2 and HSPB3 during myogenic differentiation. J
Biol Chem. 275:1095–1104. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fostinis Y, Theodoropoulos PA, Gravanis A
and Stournaras C: Heat shock protein HSP90 and its association with
the cytoskeleton: A morphological study. Biochem Cell Biol.
70:779–786. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Knowlton AA and Sun L: Heat-shock
factor-1, steroid hormones, and regulation of heat-shock protein
expression in the heart. Am J Physiol Heart Circ Physiol.
280:H455–H464. 2001.
|
|
28
|
Tang S, Buriro R, Liu Z, Zhang M, Ali I,
Adam A, Hartung J and Bao E: Localization and expression of Hsp27
and αB-crystallin in rat primary myocardial cells during heat
stress in vitro. PloS One. 8:e690662013. View Article : Google Scholar
|
|
29
|
Ito H, Kamei K, Iwamoto I, Inaguma Y,
Tsuzuki M, Kishikawa M, Shimada A, Hosokawa M and Kato K: Hsp27
suppresses the formation of inclusion bodies induced by expression
of R120G α B-crystallin, a cause of desmin-related myopathy. Cell
Mol Life Sci. 60:1217–1223. 2003.PubMed/NCBI
|
|
30
|
Chávez Zobel AT, Loranger A, Marceau N,
Thériault JR, Lambert H and Landry J: Distinct chaperone mechanisms
can delay the formation of aggresomes by the myopathy-causing R120G
alphaB-crystallin mutant. Hum Mol Genet. 12:1609–1620. 2003.
View Article : Google Scholar : PubMed/NCBI
|