Introduction
Hepatocellular carcinoma (HCC) is a common type of
cancer worldwide, and is the third most common cause of
cancer-associated mortality (1).
The therapeutic effects of current treatment strategies for
patients with HCC remain unsatisfactory, thus it is important to
develop novel methods of treatment for this disease (2,3).
B cell lymphoma (Bcl)-2, Bcl-extra large (xL) and
Bcl-2-like-2 (w) are the anti-apoptotic protein members of the
Bcl-2 family in mammalian cells, which contain four BH domains
(BH1, BH2, BH3 and BH4) (4). The
Bcl-2 anti-apoptotic proteins have been reported to prevent
mitochondrial outer membrane permeabilization and repress apoptosis
under stress (5). The expression
of Bcl-2 anti-apoptotic proteins is often increased in numerous
types of tumor tissue, which is commonly associated with treatment
resistance (5,6). Previous studies have found that the
levels of Bcl-2 anti-apoptotic proteins are closely associated with
the pathological grade and survival rate of patients with HCC
(7–9). Therefore, the targeting of Bcl-2
anti-apoptotic proteins may be a candidate for the treatment of
patients with HCC. ABT-737 is an inhibitor of Bcl-2, Bcl-xL and
Bcl-w, which is currently in phase I clinical trials for patients
with leukemia (10–12). It has been previously reported that
ABT-737 also has antitumor effects in HCC cell lines (13–17).
However, certain pro-survival signaling pathways in HCC cells are
often activated upon ABT-737 treatment, which attenuates the
antitumor effect of ABT-737 (13,16,17).
Therefore, novel treatment strategies require investigation in
order to improve the efficacy of ABT-737.
Curcumin, also known as diferuloylmethane, is
obtained from Curcuma longa, and is regarded as a potent
anticancer drug in various types of tumor, including HCC (18–21).
Previous studies have demonstrated that curcumin is able to
effectively inhibit the proliferation of HCC cells in vitro
and in vivo (22–25). In addition, curcumin significantly
enhances the antitumor effects of certain traditional
chemotherapeutic drugs and molecular-targeted drugs (26–32).
However, the synergistic effect of curcumin and ABT-737 remains to
be fully elucidated.
The present study aimed to investigate the antitumor
effects of combination therapy of ABT-737 with curcumin on HepG2
cells. Whether curcumin enhances the antitumor effect of ABT-737
via the induction of apoptosis in HepG2 cells was investigated, and
the potential involvement of the reactive oxygen species
(ROS)-apoptosis signal-regulating kinase 1 (ASK1)-c-Jun N-terminal
kinase (JNK) pathway was examined.
Materials and methods
Reagents
HyClone Dulbecco's modified Eagle's medium (DMEM)
nutrient mixture and HyClone fetal bovine serum (FBS) were
purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
ABT-737, SP600125 and N-acetyl-l-cysteine (NAC) were purchased
from Sigma-Aldrich (St. Louis, MO, USA). An Annexin V-Fluorescein
Isothiocyanate (FITC)/Propidium Iodide (PI) Apoptosis Detection kit
was purchased from Beyotime Institute of Biotechnology (Beijing,
China). Cell Counting Kit-8 (CCK-8) reagent was obtained from
Dojindo Molecular Technologies, Inc. (Kumamoto, Japan). Invitrogen
Lipofectamine 2000 was obtained from Thermo Fisher Scientific, Inc.
Curcumin was purchased from Sigma-Aldrich and dissolved in
dimethylsulfoxide (Sigma-Aldrich) to prepare a stock solution,
which was used for treating HepG2 cells. Antibodies against Bcl-2
(cat. no. sc-25780), poly(ADP ribose) polymerase 1 (PARP-1; cat.
no. sc-25780) and caspase-3 (cat. no. sc-7148) were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Rabbit polyclonal
antibodies against myeloid cell leukemia-1 (Mcl-1; cat. no. 4572),
total-JNK (cat. no. 9252) and phosphorylated (p-)JNK (cat. no.
9255), and against glyceraldehyde 3-phosphate dehydrogenase (GAPDH;
cat. no. 2118) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Horseradish peroxidase-conjugated goat
anti-rabbit (cat. no. A0208) and anti-mouse IgG (cat. no. A0216)
antibodies were purchased from Beyotime Institute of
Biotechnology.
Cell culture
The HepG2 human HCC cell line was obtained from the
American Type Culture Collection (Manassas, VA, USA) and cultured
in DMEM supplemented with 10% FBS, streptomycin (100 µg/ml)
and penicillin (100 U/ml) (both from Sigma-Aldrich) at 37°C in a
humidified atmosphere containing 5% CO2. All seeded
cells were grown to 70–80% confluence prior to treatment. Cells
were treated with 2 µM curcumin, 10 µM ABT-737 or 10
mM NAC for 24 h and then subjected to further experiments.
Hoechst 33258 staining
The treated cells were fixed with 4%
paraformaldehyde (Sigma-Aldrich) for 10 min at room temperature and
then washed with phosphate-buffered saline (PBS) three times.
Subsequently, the cells were incubated with Hoechst 33258
(Sigma-Aldrich) according to the manufacturer's instructions. The
cells were then observed under a fluorescence microscope (IX81;
Olympus Corp., Tokyo, Japan) to identify apoptotic cells.
Trypan blue exclusion assay
Cells were treated with ABT-737 (0, 1, 5 or 10
µM) and/or curcumin (0 or 2 µM) for 36 h, harvested
and suspended in PBS. The cells were then added with an equal
volume of 0.08% Trypan blue solution (Sigma-Aldrich), and were
incubated for 5 min at room temperature. Subsequently, the living
and dead cells were counted using an optical microscope. The cells
failing to exclude the blue dye were defined as dead cells, and the
cell death rate was estimated as the percentage of dead cells.
Cytotoxicity assay
The cytotoxicity assay was performed using CCK-8
reagent. In brief, the HepG2 cells were seeded into 96-well plates
at a density of 1×103 cells/well. After 24 h, the cells
were incubated with ABT-737 (0, 1, 5 or 10 µM) and/or
curcumin (0 or 2 µM) for 36 h. Subsequently, CCK-8 reagent
was added to each well, according to the manufacturer's protocol.
Following incubation in the dark for 50 min at room temperature,
the absorption values of the cells were detected at a wavelength of
450 nm using a Multiskan Spectrum micro-plate reader (Thermo Fisher
Scientific, Inc.).
Western blot analysis
The total proteins of the treated cells were
extracted from the whole-cell protein lysates prepared with
radioimmunoprecipitation assay lysis and extraction buffer (Thermo
Fisher Scientific) according to manufacturer's instruction at room
temperature with centrifugation at 17,320 × g for 5 min at 4°C, and
the concentration of the protein was measured using a Bicinchoninic
Acid (BCA) Protein Assay kit (Sigma-Aldrich). Subsequently, 60
µg protein of each sample was loaded for 10% polyacrylamide
gel electrophoresis and then transferred onto polyvinylidene
difluoride (PVDF) membranes (Thermo Fisher Scientific). The PVDF
membranes were then incubated with 5% albumin in PBS with 1%
Tween-20 (TBST) for 2 h at 37°C. Subsequently, the membranes were
separately incubated with antibodies against Bcl-2 (1:1,000), Mcl-1
(1:2,000), PARP (1:1,000), cleaved caspase-3 (1:500), total-JNK
(1:1,000), phosphorylated (p)-JNK (1:200) and GAPDH (1:3,000) for
12 h at 4°C. Following incubation and washing with PBST three
times, the membranes were separately incubated with goat
anti-rabbit and anti-mouse IgG for 1 h at 37°C. Immunoreactivity
was then visualized by adding chemiluminescent peroxidase substrate
(Thermo Fisher Scientific) in the dark. The chemiluminescence
signal was recorded using the ChemiDoc XRS imaging system (Bio-Rad
Laboratories, Hercules, CA, USA). Data analysis and quantification
were performed using Quantity One software (version 4.6; Bio-Rad
Laboratories, Inc.)
ROS detection
The HepG2 cells were seeded into 12-well plates and
were cultured overnight, following which the cells were treated
with the different treatments. Following treatment, the cells were
washed with PBS three times, and the 2′,7′-dichlorofluorescin
diacetate fluorescent probe (Sigma-Aldrich) was added, according to
the manufacturer's protocol. Subsequently, the cells were washed
and harvested in PBS. The fluorescent value of each sample was then
detected at a 488 nm excitation wavelength and a 525 nm emission
wavelength using a fluorescent plate reader (VICTOR™ X5 Multilabel
Plate Reader; Perkin Elmer, Waltham, MA, USA), with the ROS levels
presented as the fold induction.
Transfection with small interfering RNA
(siRNA)
siRNA for ASK1 (5′-AAUUGCAGUCUGCACAGCCUUUCGG-3′) or
control siRNA were purchased from Shanghai GenePharma Co., Ltd.
(Shanghai, China). The HepG2 cells were seeded into six-well plates
and were cultured overnight. The cells at 70% confluence were then
transfected with siRNA (100 pmol/well) using Lipofectamine 2000,
according to the manufacturer's protocol.
Annexin V-FITC/PI staining
The treated cells, including the adherent and
floating cells were harvested in PBS. The cells were then incubated
with annexin V-FITC and PI for 15 min at room temperature. The
stained cells were then analyzed using flow cytometry (FACSCalibur;
BD Biosciences, San Jose, CA, USA).
Caspase-3 activity detection
Caspase-3 activity was detected by the specific
caspase-3 substrate, Ac-DEVD-AM C (Sigma-Aldrich). Briefly, the
treated cells were suspended in radioimmunoprecipitation assay cell
lysis buffer. The proteins were extracted according to the
abovementioned procedure and the concentration was measured using
the BCA kit. Subsequently, Ac-DEVD-AM C was added to each cell
lysate containing 200 µg protein, according to the
manufacturer's protocol. Following incubation at 37°C for 1 h, the
caspase-3 activity was measured by the fluorescence intensity at an
excitation wavelength at 365 nm and an emission wavelength at 435
nm using a Victor™ X5 Multilabel Plate Reader (Perkin Elmer,
Waltham, MA, USA).
Statistical analysis
All experiments in the present study were performed
a minimum of three times. The data are expressed as the mean ±
standard deviation. Statistical differences were analyzed using
two-way analysis of variance. Excel 2010 (Microsoft Corp., Redmond,
WA, USA) software was used for all statistical analyses. P<0.05
was considered to indicate a statistically significant
difference.
Results
Curcumin enhances the antitumor effect of
ABT-737 on HepG2 HCC cells
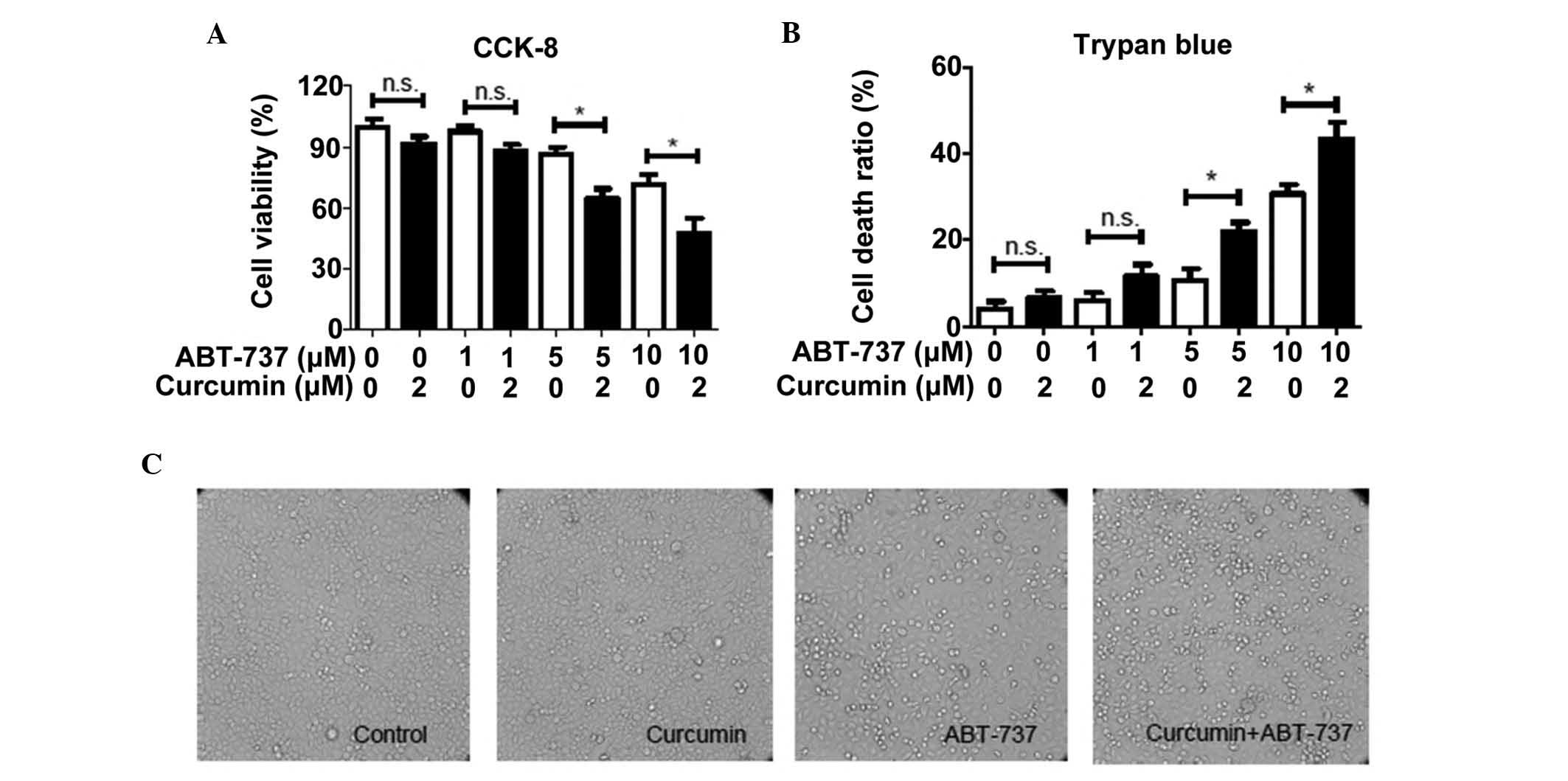
In the present study, a cytotoxicity assay was used
to evaluate the synergistic effect of curcumin and ABT-737 on HepG2
cells. As shown in Fig. 1A, no
significant alterations in cell viability were observed when the
cells were treated with 2 µM curcumin alone for 24 h,
whereas cell viability was observed to reduce in a dose-dependent
manner following co-treatment with ABT-737. In addition, 2
µM curcumin was observed to significantly enhance the
cytotoxic effects of ABT-737 (5 and 10 µM) on HepG2 cells
(Fig. 1A). To determine whether
the cytotoxic effects of ABT-737 and curcumin were associated with
the increase in the number of dead cells, a Trypan blue exclusion
assay was used to calculate the rate of cell death. As shown in
Fig. 1B, 5 or 10 µM
ABT-737-induced cell death was significantly increased by the
addition of 2 µM curcumin, which indicated that the increase
in cell death may be due to the synergistic effect of curcumin and
ABT-737 on HepG2 cells. Additionally, the number of floating cells,
defined as dead cells, in the DMEM of each well were markedly
increased in the curcumin and ABT-737 co-treatment group when
visually observed under a light microscope (Fig. 1C). These results further confirmed
the synergistic effect of curcumin and ABT-737 on HepG2 cells.
ABT-737 combined with curcumin
significantly induces apoptosis in HepG2 cells
As apoptosis is a key part of ABT-737-mediated cell
death (16,33,34),
whether curcumin promotes ABT-737-induced apoptosis in HepG2 cells
was further investigated in the present study. Hoechst 33258
staining demonstrated that the number of abnormal nuclei with the
typical characteristics of apoptosis was significantly increased in
the HepG2 cells co-treated with curcumin and ABT-737, compared with
the cells treated with ABT-737 alone (Fig. 2A). In addition, the activity of
caspase-3, which is a key executor of typical apoptotic pathway,
was markedly increased (Fig. 2B),
and the level of cleaved caspase-3 also increased accordingly
(Fig. 2C). These data suggested
that curcumin and ABT-737 synergistically induce apoptosis of HepG2
cells.
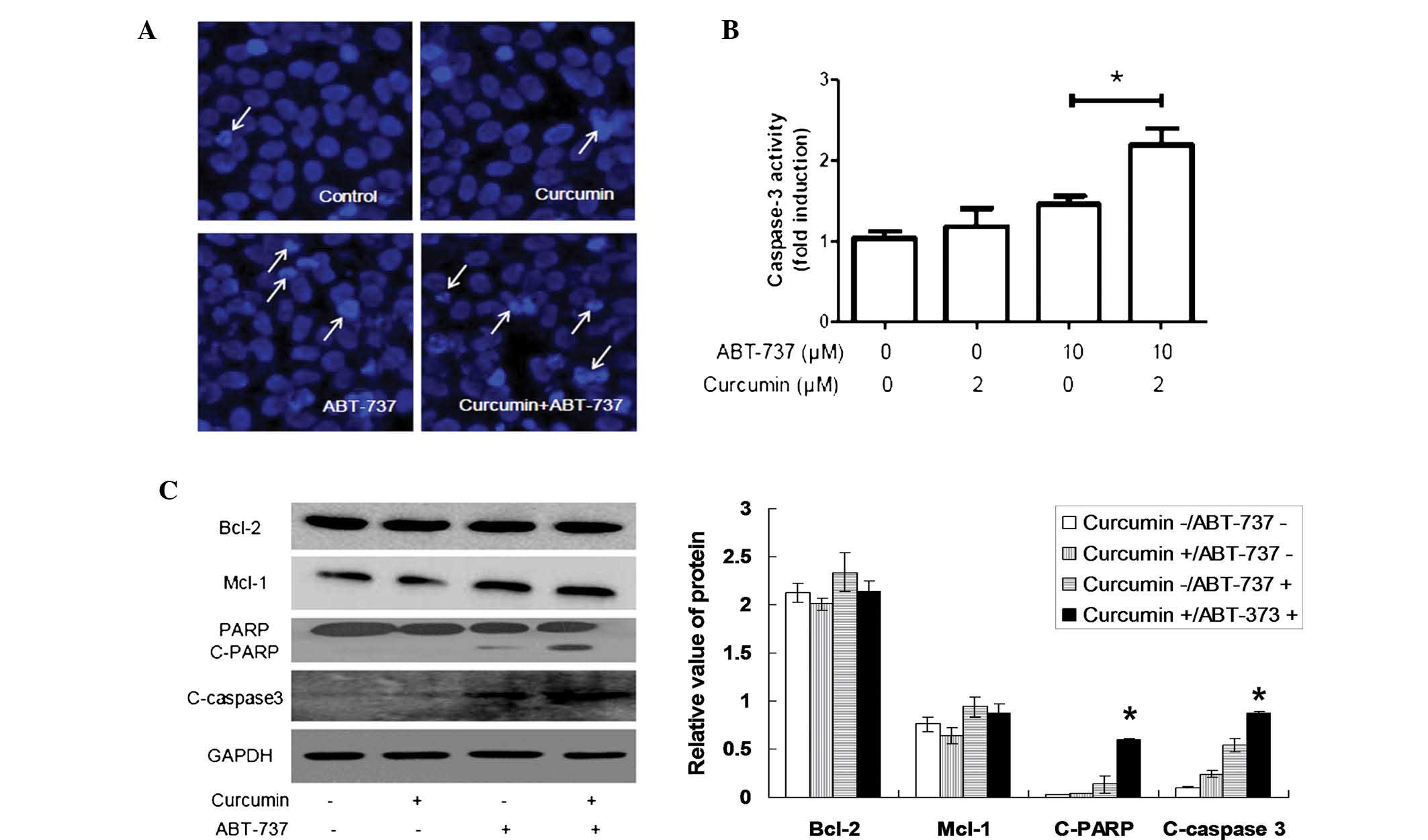 | Figure 2ABT-737 combined with curcumin
significantly induces apoptosis in HepG2 cells. (A) HepG2 cells
were separately treated with 1% dimethyl sulfoxide, 2 µM
curcumin, 10 µM ABT-737 and 2 µM curcumin+10
µM ABT-737 for 24 h, and were stained with Hoechst 33258.
Subsequently, the morphological characteristics of the nuclei were
observed under a fluorescence microscope (magnification, ×630). The
white arrows represent abnormal nuclei with typical characteristics
of apoptosis. (B) HepG2 cells were treated as in A, following which
caspase activity was detected and presented as fold induction. (C)
HepG2 cells were treated as in (A), following which the total
cellular proteins were extracted. Subsequently, the proteins were
loaded for western blotting and the levels of Bcl-2, Mcl-1,
PARP/C-PARP and C-caspase-3 were assessed. Data are expressed as
the mean ± standard deviation. *P<0.05 as indicated
or vs. all other groups. Bcl-2, B cell lymphoma-2; Mcl-1, myeloid
cell leukemia-1; PARP, poly adenosine diphosphate-ribose
polymerase; C-, cleaved; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase. |
ROS levels are increased in HepG2 cells
following co-treatment with ABT-737 and curcumin
A previous study demonstrated that ROS are important
in ABT-737-induced apoptosis (16), therefore, the present study further
investigated the role of ROS on the induction of apoptosis in HepG2
cells with the co-treatment of curcumin and ABT-737. As shown in
Fig. 3A, co-treatment with
curcumin and ABT-737 significantly increased the level of ROS in
HepG2 cells. In addition, the administration of the antioxidant NAC
attenuated the synergistic effect of curcumin and ABT-737 on the
induction of apoptosis (Fig.
3B–D), which indicated that the increase of ROS was an
important factor for the antitumor effect of curcumin and
ABT-737.
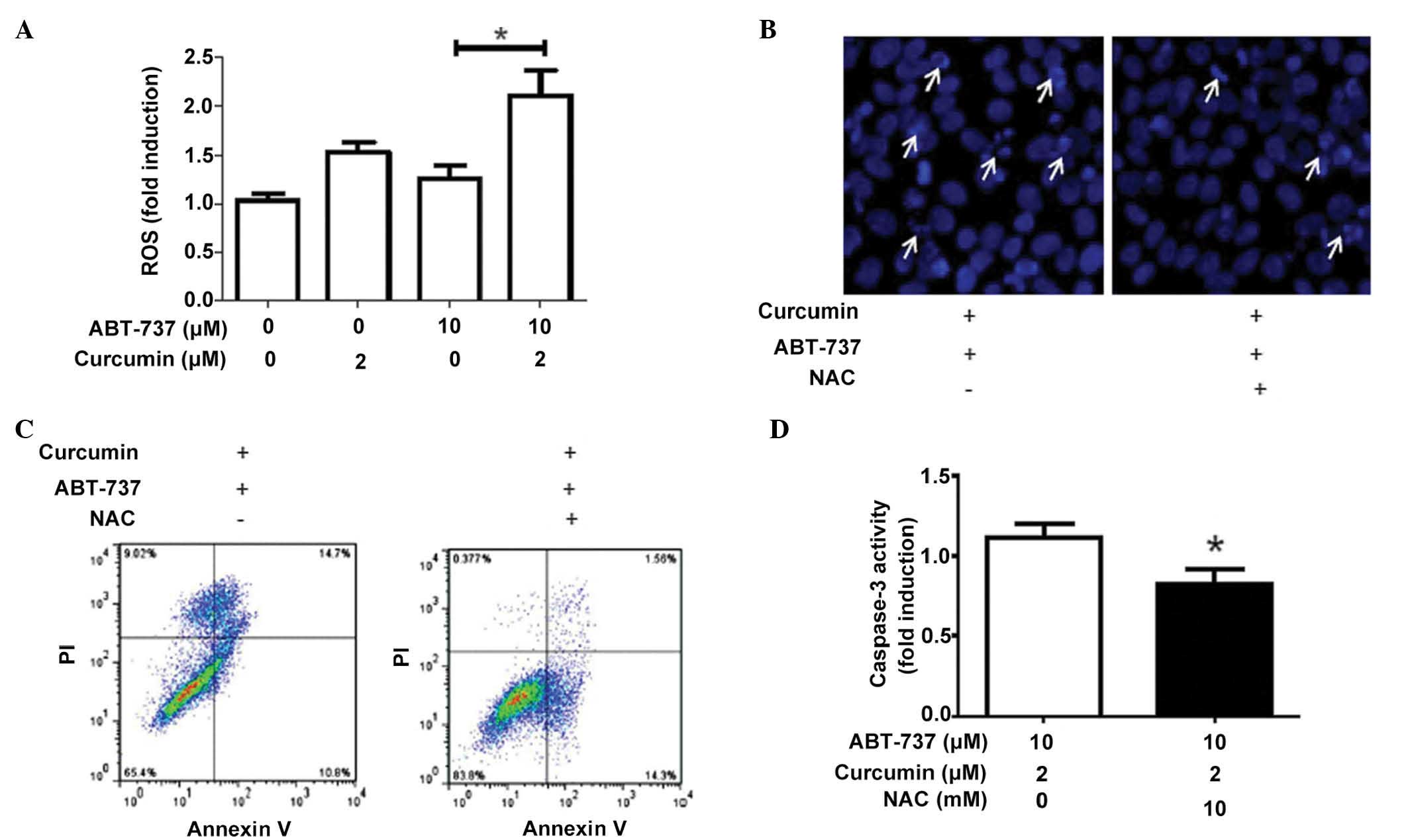 | Figure 3ROS levels are increased in HepG2
cells following co-treatment with ABT-737 and curcumin. (A) HepG2
cells were separately treated with 1% dimethyl sulfoxide, 2
µM curcumin, 10 µM ABT-737 and 2 µM
curcumin+10 µM ABT-737 for 24 h, following which a
2′,7′-dichlorofluorescin diacetate probe was used to measure the
levels of cellular ROS. HepG2 cells were treated with 2 µM
curcumin and 10 µM ABT-737 for 24 h in the presence or
absence of the antioxidant, NAC (10 mM). Subsequently, the levels
of HepG2 cell apoptosis were analyzed using (B) Hoechst 33258
staining (white arrows indicate cells undergoing apoptosis with
nuclear fragmentation visualized by Hoechst staining), (C) Annexin
V-fluorescein isothiocyanate/PI staining and (D) caspase-3 activity
detection. Data are expressed as the mean ± standard deviation.
*P<0.05. ROS, reactive oxygen species; PI, propidium
iodide; NAC, N-acetyl-L-cysteine. |
Increased ROS levels promote the
sustained activation of JNK, which leads to apoptosis in HepG2
cells
As increased levels of ROS have been reported to
activate JNK (35,36), and sustained activation of JNK has
been demonstrated as a mechanism for ABT-737-induced apoptosis
(16), changes in the activity of
JNK in curcumin and ABT-737 co-treated cells were further examined
in the present study. As shown in Fig.
4A, JNK was not activated at the 18 h time point in the
curcumin (2 µM)-treated cells or ABT-737 (10
µM)-treated cells, however, JNK was activated in the
curcumin and ABT-737 co-treated cells. In addition, the JNK
inhibitor, SP600125, significantly suppressed the apoptosis, which
was induced by the combination of curcumin and ABT-737 in the HepG2
cells (Fig. 4B). These results
indicated that curcumin may enhance the cytotoxicity of ABT-737 via
the sustained activation of JNK. Additionally, the antioxidant,
NAC, was able to reverse the activation of JNK induced by the
co-treatment of curcumin and ABT-737 (Fig. 4C), which suggested that the
increased ROS levels in the curcumin and ABT-737 co-treated cells
activated JNK. Downregulating the level of ASK1, which is often
important in the ROS-mediated activation of JNK (16), by siRNA attenuated the synergistic
cytotoxicity of curcumin and ABT-737 on the HepG2 cells (Fig. 4D). These data indicated that
curcumin enhanced the antitumor effect of ATB-737, which was
partially dependent on the ROS-ASK1-JNK pathway.
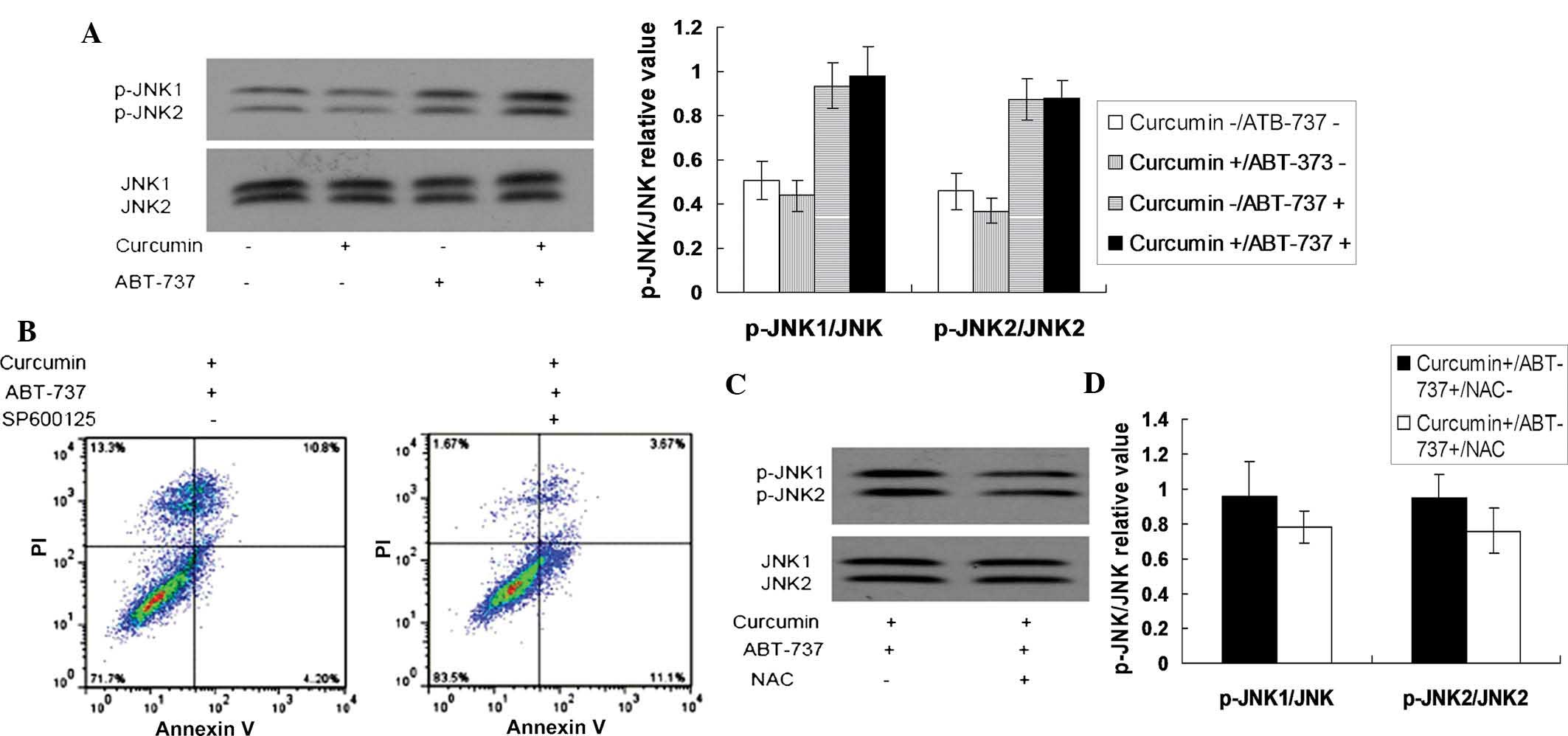 | Figure 4Increased levels of ROS promote the
sustained activation of JNK, which leads to the apoptosis of HepG2
cells. (A) HepG2 cells were separately treated with 1% dimethyl
sulfoxide, 2 µM curcumin, 10 µM ABT-737 and 2
µM curcumin+10 µM ABT-737 for 24 h, following which
the total cellular proteins were extracted. Subsequently, the
protein levels of p-JNK1/JNK2 and total JNK1/JNK2 were detected
using western blotting. (B) HepG2 cells were treated with 2
µM curcumin+10 µM ABT-737 for 24 h in the presence or
absence of 10 µM SP600125 (a JNK inhibitor). Following
treatment, apoptosis of the HepG2 cells was assessed using annexin
V-fluorescein isothiocyanate/PI staining. (C) HepG2 cells were
treated with 2 µM curcumin+10 µM ABT-737 for 24 h in
the presence or absence of antioxidant, NAC (10 mM). Subsequently,
the levels of p-JNK1/JNK2 and total JNK1/JNK2 were detected using
western blotting. (D) HepG2 cells were separately transfected with
control siRNA or ASK1 siRNA for 24 h, following which the cells
were treated with 2 µM curcumin+10 µM ABT-737 for 24
h. A Trypan blue exclusion assay was then used to measure the total
cell death ratio. *P<0.05 vs. control. ROS, reactive
oxygen species; JNK, c-Jun N-terminal kinase; p-, phosphorylated;
siRNA, small interfering RNA; PI, propidium iodide. |
Discussion
The pathology of HCC is complex, which presents a
challenge in the development of effective treatments for HCC
(37,38). Certain novel strategies of
combination therapy have been investigated previously, and have
shown potent antitumor activity in pre-clinical tests (39–44).
Combination therapy appears to be is a promising strategy for
patients with HCC, due to the fact that it can improve the
treatment effect and also alleviate the side effects of the drugs
(40). Previous studies have
confirmed that curcumin and ABT-737 are potential anticancer drugs,
and can induce cell death in HCC cells in a dose-dependent manner
(13,45,46).
In the present study, it was found that 2 µM curcumin alone
had no significant cytotoxic effects on the HepG2 cells, however it
enhanced the antitumor effect of ABT-737 on HepG2 cells. The
results indicated that curcumin markedly enhanced the level of ROS
in ABT-737-treated cells. In addition, the increased ROS levels
resulted in the promotion of HepG2 cell apoptosis, which was
partially dependent on the ASK1-mediated sustained activation of
JNK. Therefore, the combination of curcumin and ABT-737 may serve
as a potential therapeutic treatment strategy for patients with
HCC.
ROS are important in regulating cell survival and
cell death (47). It has been
reported that low levels of ROS promote cell survival via multiple
signaling pathways, whereas high levels of ROS can be ab important
mechanism of cell death (47). A
previous study confirmed that ABT-737 resulted in low levels of ROS
in HepG2 cells, which may have promoted cell survival via
cytoprotective autophagy (16). In
the present study, curcumin significantly increased the levels of
ROS in the ABT-737-treated HepG2 cells. Notably, the increased
levels of ROS did not further promote cell survival, however, it
markedly induced cell death. The different levels of ROS may have
determined the pro-survival or pro-apoptotic roles in HCC cells. In
addition, the reason for the marked increase in the levels of ROS
may be associated with mitochondrial stress-induced damage
following administration of the combination of the two drugs. The
associated mechanisms require further investigation.
JNK is a member of the mitogen-activated protein
kinase family and has three isoforms, including JNK1, JNK2 and JNK3
(48). JNK can be activated
through the phosphorylation of the Thr residue under diverse types
of stress, including ROS (35,49,50).
JNK is involved in the regulation of various cellular process,
including proliferation, differentiation, cell death and survival
(51). In addition, the activation
of JNK is closely associated with the carcinogenesis, development
and treatment of HCC (52).
Previous studies have demonstrated that ABT-737-mediated activation
of JNK can either promote cell survival or lead to cell death in
different types of HCC cell (13,16,17).
In the present study, it was found that JNK was not activated at
the 18 h time point in HepG2 cells with ABT-737 treatment alone,
however, it was activated when the cells were co-treated with
curcumin and ABT-737. In addition, the activation of JNK
significantly promoted cell death, which suggested the
pro-apoptotic role of JNK under these conditions. The
downregulation of ASK1, a key mediator of the ROS-JNK pathway
(53), was observed to markedly
reduce the total cell death rate in the curcumin and ABT-737
co-treated cells. However, the downstream signaling pathways of
activated JNK in the HepG2 cells with the co-treatment of curcumin
and ABT-737 are complex, therefore, the detailed mechanisms require
further investigation.
Collectively, the present study indicated that the
combination of curcumin and ABT-737 can efficaciously induce the
death of HCC cells, and may offer a potential treatment strategy
for patients with HCC.
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llovet JM: Updated treatment approach to
hepatocellular carcinoma. J Gastroenterol. 40:225–235. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peck-Radosavljevic M: Drug therapy for
advanced-stage liver cancer. Liver Cancer. 3:125–131. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levine B, Sinha S and Kroemer G: Bcl-2
family members: Dual regulators of apoptosis and autophagy.
Autophagy. 4:600–606. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View
Article : Google Scholar
|
|
6
|
Su J, Zhou L, Xia MH, Xu Y, Xiang XY and
Sun LK: Bcl-2 family proteins are involved in the signal crosstalk
between endoplasmic reticulum stress and mitochondrial dysfunction
in tumor chemotherapy resistance. Biomed Res Int. 2014:2343702014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo XZ, Shao XD, Liu MP, Xu JH, Ren LN,
Zhao JJ, Li HY and Wang D: Effect of bax, bcl-2 and bcl-xL on
regulating apoptosis in tissues of normal liver and hepatocellular
carcinoma. World J Gastroenterol. 8:1059–1062. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chun E and Lee KY: Bcl-2 and Bcl-xL are
important for the induction of paclitaxel resistance in human
hepatocellular carcinoma cells. Biochem Biophys Res Commun.
315:771–779. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schattenberg JM, Schuchmann M and Galle
PR: Cell death and hepatocarcinogenesis: Dysregulation of apoptosis
signaling pathways. J Gastroenterol Hepatol. 26(Suppl 1): 213–219.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Balakrishnan K and Gandhi V: Bcl-2
antagonists: A proof of concept for CLL therapy. Invest New Drugs.
31:1384–1394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Billard C: BH3 mimetics: Status of the
field and new developments. Mol Cancer Ther. 12:1691–1700. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu L and Liu S: Autophagy contributes to
modulating the cytotoxicities of Bcl-2 homology domain-3 mimetics.
Semin Cancer Biol. 23:553–560. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hikita H, Takehara T, Shimizu S, Kodama T,
Shigekawa M, Iwase K, Hosui A, Miyagi T, Tatsumi T and Ishida H:
The Bcl-xL inhibitor, ABT-737, efficiently induces apoptosis and
suppresses growth of hepatoma cells in combination with sorafenib.
Hepatology. 52:1310–1321. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang G, Zhan Y, Wang H and Li W: ABT-263
sensitizes TRAIL-resistant hepatocarcinoma cells by downregulating
the Bcl-2 family of anti-apoptotic protein. Cancer Chemother
Pharmacol. 69:799–805. 2012. View Article : Google Scholar
|
|
15
|
Zhang S, Li G, Ma X, Wang Y, Liu G, Feng
L, Zhao Y, Zhang G, Wu Y and Ye X: Norcantharidin enhances
ABT-737-induced apoptosis in hepatocellular carcinoma cells by
transcriptional repression of Mcl-1. Cell Signal. 24:1803–1809.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ni Z, Wang B, Dai X, Ding W, Yang T, Li X,
Lewin S, Xu L, Lian J and He F: HCC cells with high levels of Bcl-2
are resistant to ABT-737 via activation of the ROS-JNK-autophagy
pathway. Free Radic Biol Med. 70:194–203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang B, Ni Z, Dai X, Qin L, Li X, Xu L,
Lian J and He F: The Bcl-2/xL inhibitor ABT-263 increases the
stability of Mcl-1 mRNA and protein in hepatocellular carcinoma
cells. Mol Cancer. 13:982014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shehzad A, Lee J and Lee YS: Curcumin in
various cancers. Biofactors. 39:56–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Witkin JM and Li X: Curcumin, an active
constiuent of the ancient medicinal herb Curcuma longa L: Some uses
and the establishment and biological basis of medical efficacy. CNS
Neurol Disord Drug Targets. 12:487–497. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y and Zhang T: Targeting cancer stem
cells by curcumin and clinical applications. Cancer Lett.
346:197–205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Prasad S, Gupta SC, Tyagi AK and Aggarwal
BB: Curcumin, a component of golden spice: From bedside to bench
and back. Biotechnol Adv. 32:1053–1064. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chuang SE, Kuo ML, Hsu CH, Chen CR, Lin
JK, Lai GM, Hsieh CY and Cheng AL: Curcumin-containing diet
inhibits diethylnitrosamine-induced murine hepatocarcinogenesis.
Carcinogenesis. 21:331–335. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu H, Liang Y, Wang L, Tian L, Song R,
Han T, Pan S and Liu L: In vivo and in vitro suppression of
hepatocellular carcinoma by EF24, a curcumin analog. PLoS One.
7:e480752012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu MX, Zhao L, Deng C, Yang L, Wang Y, Guo
T, Li L, Lin J and Zhang L: Curcumin suppresses proliferation and
induces apoptosis of human hepatocellular carcinoma cells via the
wnt signaling pathway. Int J Oncol. 43:1951–1959. 2013.PubMed/NCBI
|
|
25
|
Zhang K, Rui X and Yan X: Curcumin
inhibits the proliferation and invasiveness of MHCC97-H cells via
p38 signaling pathway. Drug Dev Res. 75:463–468. 2014.PubMed/NCBI
|
|
26
|
Yin H, Guo R, Xu Y, Zheng Y, Hou Z, Dai X,
Zhang Z, Zheng D and Xu H: Synergistic antitumor efficiency of
docetaxel and curcumin against lung cancer. Acta Biochim Biophys
Sin (Shanghai). 44:147–153. 2012. View Article : Google Scholar
|
|
27
|
Boztas AO, Karakuzu O, Galante G, Ugur Z,
Kocabas F, Altuntas CZ and Yazaydin AO: Synergistic interaction of
paclitaxel and curcumin with cyclodextrin polymer complexation in
human cancer cells. Mol Pharm. 10:2676–2683. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Debata PR, Begum S, Mata A, Genzer O,
Kleiner MJ, Banerjee P and Castellanos MR: Curcumin potentiates the
ability of sunitinib to eliminate the VHL-lacking renal cancer
cells 786-O: Rapid inhibition of Rb phosphorylation as a preamble
to cyclin D1 inhibition. Anticancer Agents Med Chem. 13:1508–1513.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo J, Li W, Shi H, Xie X, Li L, Tang H,
Wu M, Kong Y, Yang L and Gao J: Synergistic effects of curcumin
with emodin against the proliferation and invasion of breast cancer
cells through upregulation of miR-34a. Mol Cell Biochem.
382:103–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meiyanto E, Putri DD, Susidarti RA,
Murwanti R, Sardjiman, Fitriasari A, Husnaa U, Purnomo H and
Kawaichi M: Curcumin and its analogues (PGV-0 and PGV-1) enhance
sensitivity of resistant MCF-7 cells to doxorubicin through
inhibition of HER2 and NF-kB activation. Asian Pac J Cancer Prev.
15:179–184. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ranjan K, Sharma A, Surolia A and Pathak
C: Regulation of HA14-1 mediated oxidative stress, toxic response
and autophagy by curcumin to enhance apoptotic activity in human
embryonic kidney cells. Biofactors. 40:157–169. 2014. View Article : Google Scholar
|
|
32
|
Zhou QM, Chen QL, Du J, Wang XF, Lu YY,
Zhang H and Su SB: Synergistic effect of combinatorial treatment
with curcumin and mitomycin C on the induction of apoptosis of
breast cancer cells: A cDNA microarray analysis. Int J Mol Sci.
15:16284–16301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Konopleva M, Contractor R, Tsao T, Samudio
I, Ruvolo PP, Kitada S, Deng X, Zhai D, Shi YX, Sneed T, et al:
Mechanisms of apoptosis sensitivity and resistance to the BH3
mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 10:375–388.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kline MP, Rajkumar SV, Timm MM, Kimlinger
TK, Haug JL, Lust JA, Greipp PR and Kumar S: ABT-737, an inhibitor
of Bcl-2 family proteins, is a potent inducer of apoptosis in
multiple myeloma cells. Leukemia. 21:1549–1560. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Biswas N, Mahato SK, Chowdhury AA,
Chaudhuri J, Manna A, Vinayagam J, Chatterjee S, Jaisankar P,
Chaudhuri U and Bandyopadhyay S: ICB3E induces iNOS expression by
ROS-dependent JNK and ERK activation for apoptosis of leukemic
cells. Apoptosis. 17:612–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shi Y, Nikulenkov F, Zawacka-Pankau J, Li
H, Gabdoulline R, Xu J, Eriksson S, Hedström E, Issaeva N, Kel A,
et al: ROS-dependent activation of JNK converts p53 into an
efficient inhibitor of oncogenes leading to robust apoptosis. Cell
Death Differ. 21:612–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kalinski T and Roessner A: Hepatocellular
carcinoma: Pathology and liver biopsy. Dig Dis. 27:102–108. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Effendi K and Sakamoto M: Molecular
pathology in early hepatocarcinogenesis. Oncology. 78:157–160.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ibrahim N, Yu Y, Walsh WR and Yang JL:
Molecular targeted therapies for cancer: Sorafenib mono-therapy and
its combination with other therapies (review). Oncol Rep.
27:1303–1311. 2012.PubMed/NCBI
|
|
40
|
Lachenmayer A, Toffanin S, Cabellos L,
Alsinet C, Hoshida Y, Villanueva A, Minguez B, Tsai HW, Ward SC,
Thung S, et al: Combination therapy for hepatocellular carcinoma:
Additive preclinical efficacy of the HDAC inhibitor panobinostat
with sorafenib. J Hepatol. 56:1343–1350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
He S, Wei YZ, Wang GL, Xu YY, Zhou JM,
Zhang YX and Chen L: Study of RNA interference targeting NET-1
combination with sorafenib for hepatocellular carcinoma therapy in
vitro and in vivo. Gastroenterol Res Pract. 2013:6851502013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Geng J, Li X, Lang X, Qiao C, Hu M, Yang
J, Feng J and Lv M: Combination of cetuximab and rapamycin enhances
the therapeutic efficacy in hepatocellular carcinoma. Technol
Cancer Res Treat. 13:377–385. 2014.
|
|
43
|
Nasr M, Selima E, Hamed O and Kazem A:
Targeting different angiogenic pathways with combination of
curcumin, leflunomide and perindopril inhibits
diethylnitrosamine-induced hepatocellular carcinoma in mice. Eur J
Pharmacol. 723:267–275. 2014. View Article : Google Scholar
|
|
44
|
Wang F, Dai W, Wang Y, Shen M, Chen K,
Cheng P, Zhang Y, Wang C, Li J, Zheng Y, et al: The synergistic in
vitro and in vivo antitumor effect of combination therapy with
salinomycin and 5-fluorouracil against hepatocellular carcinoma.
PLoS One. 9:e974142014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Darvesh AS, Aggarwal BB and Bishayee A:
Curcumin and liver cancer: A review. Curr Pharm Biotechnol.
13:218–228. 2012. View Article : Google Scholar
|
|
46
|
Wang WH, Chiang IT, Ding K, Chung JG, Lin
WJ, Lin SS and Hwang JJ: Curcumin-induced apoptosis in human
hepatocellular carcinoma j5 cells: Critical role of
Ca(+2)-dependent pathway Evid Based Complement. Alternat Med.
2012:5129072012.
|
|
47
|
Navarro-Yepes J, Burns M, Anandhan A,
Khalimonchuk O, del Razo LM, Quintanilla-Vega B, Pappa A,
Panayiotidis MI and Franco R: Oxidative stress, redox signaling and
autophagy: Cell death versus survival. Antioxid Redox Signal.
21:66–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sehgal V and Ram PT: Network motifs in JNK
signaling. Genes Cancer. 4:409–413. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Deng YT, Huang HC and Lin JK: Rotenone
induces apoptosis in MCF-7 human breast cancer cell-mediated ROS
through JNK and p38 signaling. Mol Carcinog. 49:141–151. 2010.
|
|
50
|
Bubici C and Papa S: JNK signalling in
cancer: In need of new, smarter therapeutic targets. Br J
Pharmacol. 171:24–37. 2014. View Article : Google Scholar :
|
|
51
|
Seki E, Brenner DA and Karin M: A liver
full of JNK: Signaling in regulation of cell function and disease
pathogenesis and clinical approaches. Gastroenterology.
143:307–320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yan F, Wang XM, Liu ZC, Pan C, Yuan SB and
Ma QM: JNK1, JNK2 and JNK3 are involved in P-glycoprotein-mediated
multidrug resistance of hepatocellular carcinoma cells.
Hepatobiliary Pancreat Dis Int. 9:287–295. 2010.PubMed/NCBI
|
|
53
|
Hayakawa R, Hayakawa T, Takeda K and
Ichijo H: Therapeutic targets in the ASK1-dependent stress
signaling pathways. Proc Jpn Acad Ser B Phys Biol Sci. 88:434–453.
2012. View Article : Google Scholar : PubMed/NCBI
|