Introduction
As the second most common type of genitourinary
malignancy along with urothelial cancer, bladder cancer comprises
90% of all primary bladder cancers (1). In spite of the advancements in
chemotherapy and surgical techniques for this cancer type, ~50% of
bladder cancer patients develop metastases and succumb to the
disease (2). As targeted agents
have had limited success in treating metastatic bladder cancer
(3–5), the development of treatments with an
enhanced ability to reduce the migratory capacity of bladder
transitional cell carcinoma (BTCC) it is urgently required.
fatty acid synthase complex (FASN), which was
discovered in 1994 by Kuhajda et al (6), is the only human protein with the
ability to catalyze a reductive de novo synthesis of
long-chain fatty acids from acetyl-coenzyme A (CoA) and malonyl-CoA
using nicotinamide adenine dinucleotide phosphate (6). In most normal tissue types, except
for adipose and liver tissues, FASN expression is low (6,7). In
addition, FASN is highly expressed in most types of human carcinoma
and its inhibition may therefore hold promise as a strategy for
cancer chemoprevention (8). Of
note, selective inhibition of FASN has been used for lung cancer
treatment (9). Thus, current
research on the discovery of novel diagnostic tools and treatment
methods for cancer focuses on fatty acid metabolism and the role of
FASN in cancer (10).
A previous study by our group has reported high
levels of FASN expression in BTCC, and revealed that inhibition of
FASN suppressed phosphorylated AKT (p-AKT) and induced apoptosis in
bladder cancer (11). However, to
the best of our knowledge, the possible role of FASN in the
migratory capacity of BTCC cells has not yet been assessed. The
present study therefore examined the effects of FASN-specific
small-interfering RNA (FASN-siRNA) and FASN inhibitor cerulenin
(Cer) on BTCC-cell migration. Furthermore, the effects of FASN
knockdown on the AKT pathway and the expression of matrix
metalloproteinase (MMP)-9 were investigated in order to assess the
molecular mechanism of the role of FASN in BTCC-cell migration. The
present study revealed the implication of FASN in the migratory
capacity of BTCC cells as well as an underlying mechanism, and
indicated that targeting of FASN may represent a novel therapeutic
strategy for BTCC.
Materials and methods
Cell culture and reagents
The 5637 and 253J bladder transitional cell
carcinoma lines were purchased from the cell bank of the Chinese
Academy of Sciences (Shanghai, China). The cells were cultured at
37°C in a humidified atmosphere containing 5% CO2 in
RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.), 100 IU/ml penicillin and 100 µg/ml
streptomycin (Gibco; Thermo Fisher Scientific, Inc.). Cells in the
logarithmic growth phase were used in all experiments. Cer was
obtained from Biomol (Enzo Life Sciences, Farmingdale, NY, USA) and
kept in a 10 mg/ml stock solution in dimethyl sulfoxide (Gibco;
Thermo Fisher Scientific, Inc.).
siRNA and siRNA transfection
Using the nucleotide sequence of the FASN gene from
GenBank (http://www.ncbi.nlm.nih.gov/genbank/; no. 004104 NM),
siRNA to target FASN was designed (5′-CCC AGG CUG AAG UUU ACA
ATT-3′). Furthermore, a negative control siRNA sequence (5′-UUC UCC
GAA CGU GUC ACG UTT-3′), named as Negative-siRNA, was designed. All
siRNAs were designed and synthesized by Shanghai GenePharma Co.,
Ltd. (Shanghai, China). The 5637 and 253J cells were plated in
six-well plates (2 ml medium/well) to reach ~70% confluency at the
time-point of transfection. Cells were transfected with FASN-siRNA
in the experimental group, and with Negative-siRNA in the negative
control group using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
After 12 h of transfection, cells were cultured in RPMI 1640
medium. To assess the transfection efficiency, FAM-siRNA (Shanghai
GenePharma Co., Ltd.) was analyzed by fluorescence microscopy
(BX50; Olympus Corporation, Tokyo, Japan).
Reverse transcription-quantitative
polymerase chain reaction
Total RNA from cells were prepared using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). RNA was
reverse-transcribed using an AMV Reverse Transcription System
(Promega Corporation, Madison, WI, USA) and was amplified RT-qPCR.
The sequences of the primers were as follows: FASN (sense, 5′-TAT
GCT TCT TCG TGC AGC AGT T-3′; antisense, 5′-GCT GCC ACA CGC TCC TCT
AG-3′). The Ct values were normalized to the reference gene β-actin
(sense, 5′-CGG GAA ATC GTG CGT GA-3′; antisense, 5′-TGC CCA GGA AGG
AAG GCT-3′) and amplification was performed using SYBR Green as the
fluorescent dye (Takara Biotechnology Co., Ltd., Dalian, China).
The specificity of the PCR products was assessed with melting-curve
analysis. The relative expression of mRNA was calculated using the
comparative delta ∆∆Cq. (quantification cycle) method (12) to compare the expression levels
among different samples.
Western blot analysis
Following transfection with FASN-siRNA for 48 h or
treatment with Cer (5 or 10 µg/ml) for 24 h in six-well
plates, cells were harvested and re-suspended in 60 µl lysis
buffer (Gibco; Thermo Fisher Scientific, Inc.). Following
determination of the protein concentration via the Bicinchoninic
Acid Protein kit (Beyotime Institute of Biotechnology, Inc.),
30-µg aliquots were separated by 8% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (Gibco; Thermo Fisher
Scientific, Inc.), followed by electro-transfer at 100 V onto a
polyvinylidene fluoride membrane (Roche, Basel, Switzerland).
Subsequent to blocking with 5% non-fat milk in Tris-buffered saline
containing 0.1% Tween 20 (TBST; Gibco; Thermo Fisher Scientific,
Inc.) for 2 h at room temperature, the membrane was incubated with
the following rabbit monoclonal primary antibodies at 4°C
overnight: Anti-FASN (cat. no. 3189; 1:1,000), total-AKT (cat. no.
4691; 1:1,000), p-AKT (cat. no. 4060; 1:1,000), MMP-9 (cat. no.
3852; 1:1,000) or β-actin (cat. no. 4970; 1:1,000); Cell Signaling
Technology, Danvers, MA, USA). Following three washes in TBST for
10 min each, the blots were incubated with horseradish
peroxidase-conjugated anti-rabbit immuno-globulin G at 1:2,000
dilution (cat. no. 7074; Cell Signaling Technology) for 2 h at room
temperature. Antigen was detected using standard chemical
luminescence methodology. Immune complexes were visualized using an
enhanced chemiluminescence kit (EMD Millipore, Billerica, MA, USA)
and exposure to Biomax film (Kodak, Rochester, NY, USA).
Transwell assay
Cell migration was assessed using Transwell pates
(pore size, 8 µm; Costar; Corning Inc., Corning, NY, USA).
Following transfection with FASN-siRNA for 48 h or treatment with 3
µg/ml Cer for 24 h, 5637 and 253J cells were washed once
with phosphate-buffered saline (PBS). 200 ml serum-free RPMI 1640
medium containing 1×105 cells and 3 µg/ml Cer in
the Cer treatment group was placed in the upper chamber, and the
lower chamber was filled with 500 ml of the same medium. Subsequent
to incubation of the plates for 24 h at 37°C, cells on the upper
side of the filters were removed with cotton-tipped swabs.
Following fixing in methanol (Gibco; Thermo Fisher Scientific,
Inc.), cells on the lower side of the filter were stained with 0.1%
of crystal violet in PBS (Gibco; Thermo Fisher Scientific, Inc.).
The number of migrated cells was quantified in six random
high-power microscopic fields per sample (BX50; Olympus
Corporation). Transwell assays were performed in triplicate.
Statistical analysis
SPSS 18.0 for Windows (SPSS, Inc., Chicago, IL, USA)
was used to analyze all data. Values are expressed as the mean ±
standard error of the mean. Comparisons between two groups were
performed using Student's t-test and the χ2-test was
utilized to assess statistical significance between categorical
data. P<0.05 was considered to indicate a statistically
significant difference between values.
Results
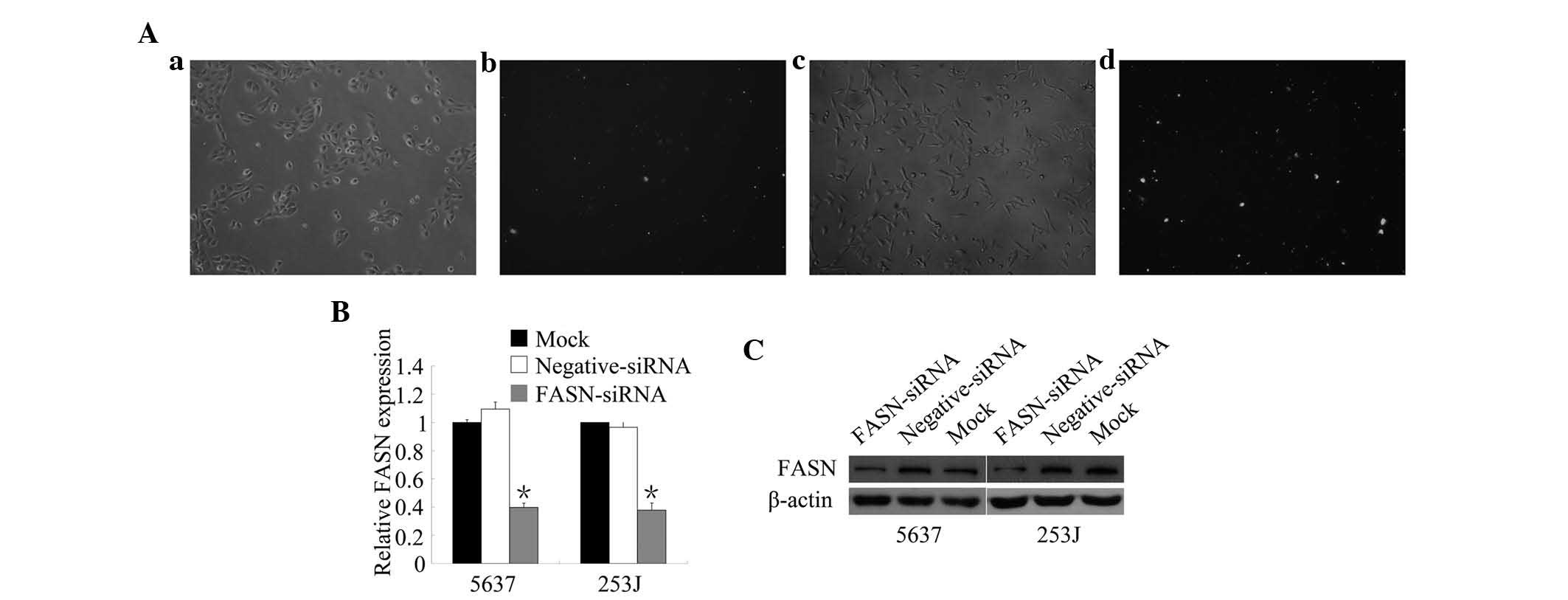
siRNA-mediated FASN knockdown
According to a previous study by our group
evaluating three different siRNA sequences targeting FASN (11), the most efficient of these siRNAs
was used in the present study to knockdown FASN expression in 5637
and 253J cells. To determine the transfection efficiency, 5637 and
253J cells were transfected with FAM-siRNA for 12 h respectively
and FAM fluorescence was detected by fluorescence microscopy
(Fig. 1A). The amount of
positively transfected cells observed visually to exceed 70%. To
assess the knockdown efficiency of FASN-siRNA in 5637 and 253J
cells, mRNA and protein expression were detected using RT-qPCR and
western blot analysis (Fig. 1B and
C). Transfection with FASN-siRNA resulted in a significant
reduction of FASN mRNA and protein expression in the BTCC cell
lines. These results indicated that FASN-siRNA was successfully
transfected into 5637 and 253J cells and efficiently knocked down
FASN.
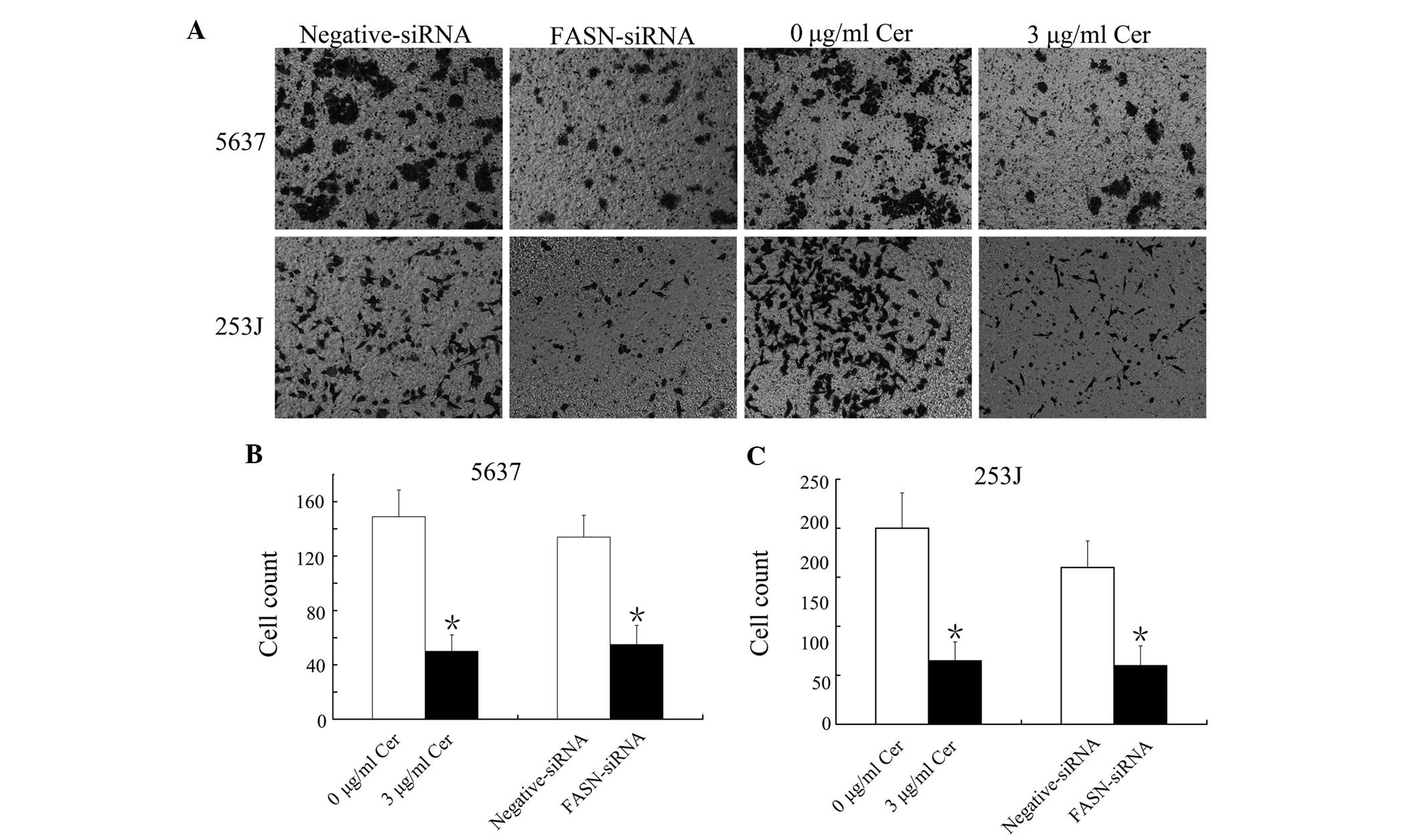
FASN inhibition by Cer and FASN-siRNA
suppresses the migratory capacity of BTCC cells
The present study assessed whether inhibition of
FASN expression affected the migration of 5637 and 253J cells using
a Transwell assay. Treatment with 3 µg/ml Cer for 24 h or
transfection with FASN-siRNA for 48 h significantly decreased the
percentage of 5637 and 253J cells that had transgressed through the
Transwell filter (Fig. 2A–C). The
results therefore indicated that FASN inhibition suppressed
BTCC-cell migration.
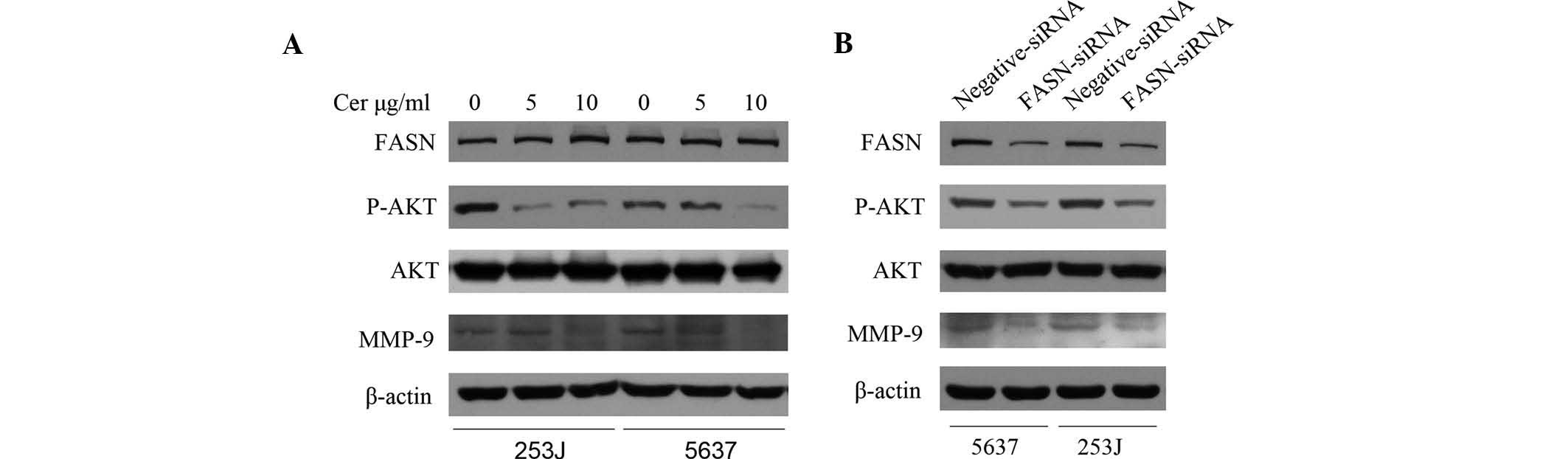
FASN inhibition de-activates AKT and
reduces MMP-9 expression
To identify the mechanism by which FASN inhibition
reduced the migratory capacity of BTCC cells, two key regulatory
factors of cell-migration, MMP-9 and AKT, were assessed. MMP-9, an
important indicator of tumor migration, has been shown to be
involved in the migratory capacity of bladder cancer cells
(13,14). Furthermore, the phosphoinositide-3
kinase (PI3K)/AKT pathway is one of the most important signaling
pathways and can regulate migration and invasion of bladder cancer
cells (15,16). Following transient transfection of
5637 and 253J cells with siRNA for 48 h or treatment with 5 and 10
µg/ml Cer for 24 h, the protein levels of MMP-9 and p-AKT
were found to be reduced (Fig. 3A and
B). However, Cer did not affect FASN expression in these cell
lines (Fig. 3A).
Discussion
The natural product Cer is a mycotoxin which was
initially developed as an anti-fungal agent and is now widely used
as a natural inhibitor of FASN activity (17,18).
Furthermore, trans-fection with siRNA is a commonly used method for
silencing the expression of specific genes (19). In the present study, FASN-siRNA and
FASN inhibitor Cer were used to inhibit FASN expression in the 5637
and 253J BTCC cell lines, which led to a decrease in the cells'
migratory potential. This was accompanied by decreases in MMP-9
expression and p-AKT levels, indicating that FASN inhibitor Cer and
FASN-siRNA reduced the migratory capacity of BTCC cells via
suppressing the activation of AKT, leading to a downregulation of
MMP-9 expression.
The role of FASN in cancer has become a focus of
current research on the discovery of novel diagnostic tools and
treatments for cancer. FASN expression and sensitivity to
FASN-targeting drugs are directly linked to cell growth, while only
being indirectly correlated with transformation, differentiation
and senescence in various cancer types, including breast (20), prostate (21), ovarian (22), lung (23) and liver cancer (24). In addition, FASN was also found to
be involved in cancer occurrence (25), progression (26), metastasis (27) and chemotherapeutic resistance
(28) in various types of cancer.
FASN is therefore a metabolic marker of cell proliferation as well
as a useful target for future drug development.
Previous studies indicated that FASN is correlated
with metastasis in several types of tumor cell (29,30).
However, in BTCC cells, the regulatory function of FASN on the
migratory capacity and the detailed underlying mechanisms have
remained elusive. The present study assessed the effects of FASN
inhibition on the expression of MMP-9, which is able to promote
cell migration, in human BTCC cells. The observed reduction of
MMP-9 expression was likely to be based on the simultaneous
suppression of p-AKT. Therefore, inhibition of FASN suppresses the
migratory capacity of BTCC cells, at least in part, by
de-activating p-AKT. These results indicated that FASN may be a
potential target for BTCC therapy.
Cancer metastasis, which is the main cause of
mortality in cancer patients, is a complex multi-step process
facilitated by several molecular key events. Identifying and
targeting various associated genes is the most promising strategy
to treat or prevent metastasis (2,31,32).
MMPs are a family of zinc-dependent endopeptidases which
participate in the proteolytic destruction of basement and
extracellular matrix membranes, therefore being essential for tumor
invasion and metastasis (33,34).
Among them, MMP-9 is one of the most important markers of cancer
invasion and metastasis (35). A
previous study has shown that MMP-9 is implicated in metastasis of
bladder cancer (36). In addition,
MMP-9 was shown to be associated with the pathological grade,
clinical stage and prognosis of tumors (37–39).
Therefore, the present study assessed whether FASN inhibition
regulates MMP-9 expression and found that the protein levels of
MMP-9 were significantly downregulated.
In recent years, the PI3K/AKT signaling pathway has
attracted broad scientific and clinical interest as a cancer drug
target (40,41). It is thought that activation of the
PI3K/AKT signaling pathway is associated with invasion and
metastasis of cancer cells (42,43).
The activated form of AKT, p-AKT, has a vital role in cell-cycle
progression, the apoptotic program and the migratory capacity of
cells via downregulating the expression of caspase 3 and
upregulating MMP-9 (44).
Therefore, inhibition of the PI3K/AKT pathway is regarded as a
potential strategy for the treatment of cancer (45,46).
In addition, FASN inhibition was shown to decrease AKT activity,
which was, at least in part, based on a reduction of membrane
phospholipid production (47). A
previous study by our group also supported the notion that
inhibition of FASN suppresses the AKT pathway (11). However, to date, the effects of
FASN on BTCC-cell migration and the possible involvement of the
PI3K/AKT pathway or MMPs has remained elusive. The present study,
showed that FASN inhibition downregulated MMP-9 expression by
targeting the AKT pathway in BTCC cells to reduce cell migration.
Thus, the present study not only provided further evidence for FASN
inhibition being a promising therapeutic approach to control
bladder-cancer invasion and metastasis, but also revealed the
underlying mechanism via the PI3K/AKT pathway and MMP-9.
The present study provided further evidence that
FASN has an important role in BTCC. FASN inhibition suppressed the
migratory capacity of BTCC cells through downregulating MMP-9
expression; furthermore, the PI3K/AKT signaling pathway was
indicated to be involved. These findings may enhance the
understanding of the underlying mechanisms of the inhibitory
effects of FASN knockdown on the migratory capacity of BTCC cells,
and suggested that targeting FASN may be a potential therapeutic
strategy for BTCC.
References
|
1
|
Shariat SF, Karakiewicz PI, Palapattu GS,
Lotan Y, Rogers CG, Amiel GE, Vazina A, Gupta A, Bastian PJ,
Sagalowsky AI, et al: Outcomes of radical cystectomy for
transitional cell carcinoma of the bladder: A contemporary series
from the Bladder Cancer Research Consortium. J Urol. 176:2414–2422.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Madersbacher S, Hochreiter W, Burkhard F,
Thalmann GN, Danuser H, Markwalder R and Studer UE: Radical
cystectomy for bladder cancer today-a homogeneous series without
neoad-juvant therapy. J Clin Oncol. 21:690–696. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Philips GK, Halabi S, Sanford BL, Bajorin
D and Small EJ; Cancer and Leukemia Group B: A phase II trial of
cisplatin (C), gemcitabine (G) and gefitinib for advanced
urothelial tract carcinoma: Results of Cancer and Leukemia Group B
(CALGB) 90102. Ann Oncol. 20:1074–1079. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu M, Dickinson SI, Wang X and Zhang J:
Expression and function of SIRT6 in muscle invasive urothelial
carcinoma of the bladder. Int J Clin Exp Pathol. 7:6504–6513.
2014.PubMed/NCBI
|
|
5
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
6
|
Kuhajda FP, Jenner K, Wood FD, Hennigar
RA, Jacobs LB, Dick JD and Pasternack GR: Fatty acid synthesis: A
potential selective target for antineoplastic therapy. Proc Natl
Acad Sci USA. 91:6379–6383. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wakil SJ: Fatty acid synthase, a
proficient multifunctional enzyme. Biochemistry. 28:4523–4530.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuhajda FP: Fatty acid synthase and
cancer: New application of an old pathway. Cancer Res.
66:5977–5980. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Orita H, Coulter J, Lemmon C, Tully E,
Vadlamudi A, Medghalchi SM, Kuhajda FP and Gabrielson E: Selective
inhibition of fatty acid synthase for lung cancer treatment. Clin
Cancer Res. 13:7139–7145. 2007.PubMed/NCBI
|
|
10
|
Abdel-Magid AF: Fatty acid synthase (FASN)
inhibitors as potential treatment for cancer, obesity, and liver
related disorders. ACS Med Chem Lett. 6:838–839. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang B, Li EH, Lu YY, Jiang Q, Cui D,
Jing YF and Xia SJ: Inhibition of fatty-acid synthase suppresses
P-AKT and induces apoptosis in bladder cancer. Urology.
80:484.e9–e15. 2012. View Article : Google Scholar
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
13
|
Saeb-Parsy K, Veerakumarasivam A, Wallard
MJ, Thorne N, Kawano Y, Murphy G, Neal DE, Mills IG and Kelly JD:
MT1-MMP regulates urothelial cell invasion via transcriptional
regulation of Dickkopf-3. Br J Cancer. 99:663–669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Di Carlo A, Terracciano D, Mariano A and
Macchia V: Urinary gelatinase activities (matrix metalloproteinases
2 and 9) in human bladder tumors. Oncol Rep. 15:1321–1326.
2006.PubMed/NCBI
|
|
15
|
Houédé N and Pourquier P: Targeting the
genetic alterations of the PI3K-AKT-mTOR pathway: Its potential use
in the treatment of bladder cancers. Pharmacol Ther. 145:1–18.
2015. View Article : Google Scholar
|
|
16
|
Chen M, Gu J, Delclos GL, Killary AM, Fan
Z, Hildebrandt MA, Chamberlain RM, Grossman HB, Dinney CP and Wu X:
Genetic variations of the PI3K-AKT-mTOR pathway and clinical
outcome in muscle invasive and metastatic bladder cancer patients.
Carcinogenesis. 31:1387–1391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng G, Palanisamy AP, Evans ZP, Sutter
AG, Jin L, Singh I, May H, Schmidt MG and Chavin KD: Cerulenin
blockade of fatty acid synthase reverses hepatic steatosis in ob/ob
mice. PLoS One. 8:e759802013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nomura S, Horiuchi T, Hata T and Omura S:
Inhibition of sterol and fatty acid biosyntheses by cerulenin in
cell-free systems of yeast. J Antibiot (Tokyo). 25:365–368. 1972.
View Article : Google Scholar
|
|
19
|
Valencia-Sanchez MA, Liu J, Hannon GJ and
Parker R: Control of translation and mRNA degradation by miRNAs and
siRNAs. Genes Dev. 20:515–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bessadóttir M, Skúladóttir EÁ, Gowan S,
Eccles S, Ögmundsdóttir S and Ogmundsdóttir HM: Effects of
anti-prolif-erative lichen metabolite, protolichesterinic acid on
fatty acid synthase, cell signalling and drug response in breast
cancer cells. Phytomedicine. 21:1717–1724. 2014. View Article : Google Scholar
|
|
21
|
Sadowski MC, Pouwer RH, Gunter JH, Lubik
AA, Quinn RJ and Nelson CC: The fatty acid synthase inhibitor
triclosan: Repurposing an anti-microbial agent for targeting
prostate cancer. Oncotarget. 5:9362–9381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Veigel D, Wagner R, Stübiger G, Wuczkowski
M, Filipits M, Horvat R, Benhamú B, López-Rodríguez ML, Leisser A,
Valent P, et al: Fatty acid synthase is a metabolic marker of cell
proliferation rather than malignancy in ovarian cancer and its
precursor cells. Int J Cancer. 136:2078–2090. 2015. View Article : Google Scholar
|
|
23
|
Jin X, Zhang KJ, Guo X, Myers R, Ye Z,
Zhang ZP, Li XF, Yang HS and Xing JL: Fatty acid synthesis pathway
genetic variants and clinical outcome of non-small cell lung cancer
patients after surgery. Asian Pac J Cancer Prev. 15:7097–7103.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Impheng H, Pongcharoen S, Richert L,
Pekthong D and Srisawang P: The selective target of capsaicin on
FASN expression and de novo fatty acid synthesis mediated through
ROS generation triggers apoptosis in HepG2 cells. PLoS One.
9:e1078422014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Migita T, Ruiz S, Fornari A, Fiorentino M,
Priolo C, Zadra G, Inazuka F, Grisanzio C, Palescandolo E, Shin E,
et al: Fatty acid synthase: A metabolic enzyme and candidate
oncogene in prostate cancer. J Natl Cancer Inst. 101:519–532. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Menendez JA, Vellon L, Mehmi I, Oza BP,
Ropero S, Colomer R and Lupu R: Inhibition of fatty acid synthase
(FAS) suppresses HER2/neu (erbB-2) oncogene overexpression in
cancer cells. Proc Natl Acad Sci USA. 101:10715–10720. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Murata S, Yanagisawa K, Fukunaga K, Oda T,
Kobayashi A, Sasaki R and Ohkohchi N: Fatty acid synthase inhibitor
cerulenin suppresses liver metastasis of colon cancer in mice.
Cancer Sci. 101:1861–1865. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeng L, Biernacka KM, Holly JM, Jarrett C,
Morrison AA, Morgan A, Winters ZE, Foulstone EJ, Shield JP and
Perks CM: Hyperglycaemia confers resistance to chemotherapy on
breast cancer cells: The role of fatty acid synthase. Endocr Relat
Cancer. 17:539–551. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Murata S, Yanagisawa K, Fukunaga K, Oda T,
Kobayashi A, Sasaki R and Ohkohchi N: Fatty acid synthase inhibitor
cerulenin suppresses liver metastasis of colon cancer in mice.
Cancer Sci. 101:1861–1865. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wellberg EA, Rudolph MC, Lewis AS,
Padilla-Just N, Jedlicka P and Anderson SM: Modulation of tumor
fatty acids, through overexpression or loss of thyroid hormone
responsive protein spot 14, is associated with altered growth and
metastasis. Breast Cancer Res. 16:4812014. View Article : Google Scholar
|
|
31
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nabeshima K, Inoue T, Shimao Y and
Sameshima T: Matrix metalloproteinases in tumor invasion: Role for
cell migration. Pathol Int. 52:255–264. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Overall CM and Kleifeld O: Tumour
microenvironment-opinion: Validating matrix metalloproteinases as
drug targets and anti-targets for cancer therapy. Nat Rev Cancer.
6:227–239. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Egeblad M and Werb Z: New functions for
the matrix metallopro-teinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kumar B, Koul S, Petersen J, Khandrika L,
Hwa JS, Meacham RB, Wilson S and Koul HK: p38 mitogen-activated
protein kinase-driven MAPKAPK2 regulates invasion of bladder cancer
by modulation of MMP-2 and MMP-9 activity. Cancer Res. 70:832–841.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Offersen BV, Knap MM, Horsman MR,
Verheijen J, Hanemaaijer R and Overgaard J: Matrix
metalloproteinase-9 measured in urine from bladder cancer patients
is an independent prognostic marker of poor survival. Acta Oncol.
49:1283–1287. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Donmez G, Sullu Y, Baris S, Yildiz L,
Aydin O, Karagoz F and Kandemir B: Vascular endothelial growth
factor (VEGF), matrix metalloproteinase-9 (MMP-9) and
thrombospondin-1 (TSP-1) expression in urothelial carcinomas.
Pathol Res Pract. 205:854–857. 2009. View Article : Google Scholar
|
|
39
|
Vasala K, Pääkko P and
Turpeenniemi-Hujanen T: Matrix metal-loproteinase-9 (MMP-9)
immunoreactive protein in urinary bladder cancer: A marker of
favorable prognosis. Anticancer Res. 28(3B): 1757–1761.
2008.PubMed/NCBI
|
|
40
|
Martelli AM, Tazzari PL, Evangelisti C,
Chiarini F, Blalock WL, Billi AM, Manzoli L, McCubrey JA and Cocco
L: Targeting the phosphatidylinositol 3-kinase/Akt/mammalian target
of rapamycin module for acute myelogenous leukemia therapy: From
bench to bedside. Curr Med Chem. 14:2009–2023. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Engelman JA, Luo J and Cantley LC: The
evolution of phospha-tidylinositol 3-kinases as regulators of
growth and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Carneiro BA, Meeks JJ, Kuzel TM, Scaranti
M, Abdulkadir SA and Giles FJ: Emerging therapeutic targets in
bladder cancer. Cancer Treat Rev. 41:170–178. 2015. View Article : Google Scholar
|
|
43
|
Paplomata E and O'Regan R: The
PI3K/AKT/mTOR pathway in breast cancer. Targets, trials and
biomarkers. 154–166. 2014.
|
|
44
|
Chen H, Yang X, Feng Z, Tang R, Ren F, Wei
K and Chen G: Prognostic value of caspase-3 expression in cancers
of digestive tract: A meta-analysis and systematic review. Int J
Clin Exp Med. 8:10225–10234. 2015.PubMed/NCBI
|
|
45
|
Wu S, Ju GQ, Du T, Zhu YJ and Liu GH:
Microvesicles derived from human umbilical cord Wharton's jelly
mesenchymal stem cells attenuate bladder tumor cell growth in vitro
and in vivo. PLoS One. 8:e613662013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jeong JW, Jin CY, Park C, Han MH, Kim GY,
Moon SK, Kim CG, Jeong YK, Kim WJ, Lee JD and Choi YH: Inhibition
of migration and invasion of LNCaP human prostate carcinoma cells
by cordycepin through inactivation of Akt. Int J Oncol.
40:1697–1704. 2012.PubMed/NCBI
|
|
47
|
Swinnen JV, Van Veldhoven PP, Timmermans
L, De Schrijver E, Brusselmans K, Vanderhoydonc F, Van de Sande T,
Heemers H, Heyns W and Verhoeven G: Fatty acid synthase drives the
synthesis of phospholipids partitioning into detergent-resistant
membrane microdomains. Biochem Biophys Res Commun. 302:898–903.
2003. View Article : Google Scholar : PubMed/NCBI
|