Introduction
In the history of tumor immunotherapy, dendritic
cell (DC) vaccines targeting cancer cells have been a significant
focus of investigation (1).
Abundant animal experiments, and phase I and II clinical trials
have demonstrated that DCs elicit specific antitumor immune
responses, which lead to tumor regression or stabilization
(2). DC vaccines have entered
phase I or II in clinical trials regarding the treatment of various
types of tumor (1).
Prostate cancer (PCa) is the second most common type
of malignancy in men in western countries (3) and the incidence of PCa in China has
recently risen (4). Treatment of
PCa predominantly comprises of surgery, radiotherapy, chemotherapy
and endocrine therapy, which exert certain therapeutic effects, yet
are accompanied by serious adverse reactions, particularly to
patients exhibiting sex hormone-independent PCa, and recurrence
and/or metastasis.
DC vaccine therapy has become a significant focus
for PCa immunotherapy and also in the field of biotherapy as a
whole. Studies in China and worldwide have evaluated DC vaccines
[loaded with prostate-associated antigen peptides, including
prostate-specific antigen (PSA), prostate-specific membrane antigen
(PSMA), prostatic acid phosphatase (PAP), prostate stem cell
antigen, related recombinant protein and tumor cell lysates] in
pre-clinical studies, and confirmed their safety and therapeutic
effect of stimulating specific immune responses against PCa
(5–7). Despite the progression of the DC
vaccine in basic and clinical research, various issues remain to be
clarified, such as the instability of the therapeutic effect, the
selection of an appropriate tumor antigen, the route and dose of
reinfused DCs, the selection of a gene transfection vector, and the
extension of the response time of the vaccine-induced cytotoxic T
lymphocyte (CTL). The present study hypothesizes that the DC
vaccine presents a novel therapeutic method for the treatment of
PCa, particularly for individuals with hormone resistance.
Currently, there are two strategies for constructing
DC vaccines (8): i) DCs loaded
with tumor antigen; and ii) DCs modified with cytokines. The former
aims to elevate the targeting effect of immunotherapy, and
predominantly consists of DCs pulsed with tumor antigens and DCs
pulsed with antigen coding genes. However, the latter focuses on
improving the efficacy of DC vaccines by introducing co-stimulatory
molecules, cytokines and chemokines into the DCs. Of these methods,
DCs modified by antigen coding genes effectively present tumor
antigens to T cells subsequent to stimulation and continue to
release endogenous tumor antigens. Therefore, DCs modified by
antigen coding genes are considered to be more effective in
stimulating T lymphocytes (in terms of a longer duration of antigen
presenting and an improved targeting effect) when compared with DCs
pulsed directly by tumor antigens or tumor cell debris. In
addition, numerous studies have identified the association between
DC function and a variety of cytokines, co-stimulatory molecules,
and chemokines (9–11). Therefore, introducing various
cytokines into DCs in order that they continue to express these
factors has become an additional method of promoting DC maturation,
improving DC activity, enhancing the antigen-presenting function
(by increasing the expression level of co-stimulatory molecules),
and strengthening the chemotaxis of DCs to T cells.
PSMA is a type of PCa marker, which is more
sensitive and more specific when compared with PSA and PAP. Its
highly specific expression in PCa tissues has rendered it a primary
target for targeted therapy and a specific focus within PCa
research (12). DCs are considered
to be the most potent antigen-presenting cells in current
knowledge. DCs stimulate and sensitize naive T cells in the
initiation of an early immune response. Furthermore, DCs elicit
immunization targeting PCa cells upon ingestion and processing of
tumor antigens, and presentation to T cells (13).
The selection of a vector is another key issue in
the construction of DC vaccines targeted to PCa cells. Currently,
gene therapy vectors consist of two major categories, viral vectors
(adenoviruses, retroviruses, adeno-associated viruses, lentiviruses
and the herpes simplex virus) and non-viral vectors (bacterial
vectors, artificial carriers and liposome vectors). Non-viral
vectors have no limitations in the size of inserted elements,
possess low immunogenicity and are easy to produce; however, their
low transfection efficiency in vivo has reduced their
popularity compared with viral vectors (14). Among the widely used viral vectors,
the adenovirus system is considered to be an effective vector for
introducing exogenous genes and small interfering RNA into all
types of cells (dividing and non-dividing) (15). Adenoviruses possess a high
transfection efficiency (~100%), low genotoxicity, a marginal
possibility of carcinogenic mutation (its genome is not integrated
into the host cell genome), and is easy to use with recombinant DNA
techniques. In addition, the adenoviral genome is large (36 kb),
therefore, the insertion of larger fragments is straightforward.
Furthermore, by using adenoviral vectors, a transfection efficiency
of 50~90% is more likely without inhibiting DC activity and immune
function. However, the adenovirus system is not flawless; the
reduced duration of action in transient transfection and the
immunogenicity of the adenovirus vector remain to be resolved
(16,17).
Thus, the present study aims to construct a
pAdxsi-GFP-PSMA adenovirus and develop a novel type of DC vaccine
to target PCa, as well as to investigate its therapeutic effect
in vitro. This may enable its further application in
vivo and provide a novel approach for DC-based immunotherapy
for the treatment of PCa.
Materials and methods
Reagents
Restriction endonuclease was purchased from New
England Biolabs (UK) Ltd., Co., (Hitchin, UK); T4 DNA ligase was
purchased from JingMei Biological Engineering Co., Ltd. (Shenzhen,
China); Alkaline Phosphatase, Calf Intestinal (CIP) enzyme was
obtained from Promega Corporation (Madison, WI, USA); Invitrogen
Pfx DNA polymerase was purchased from Thermo Fisher Scientific,
Inc. (Waltham, MA, USA); dNTP was purchased from Sangon Biotec Co.,
Ltd. (Shangai, China) and primers were synthesized by Sangon Biotec
Co., Ltd. A DNA purification kit (column-type centrifugal), and a
DNA gel extraction and purification kit (column-type centrifugal)
were purchased from Zweig Las Biotechnology Co., Ltd. (Beijing,
China); a 1-kb DNA ladder was purchased from DingGuo Biotech., Co.
(Shanghai, China); DNA Marker DL2000 was obtained from Takara
Biotechnology Co., Ltd. (Dalian, China); the total protein cleaving
enzyme and protease inhibitors were purchased from Beyotime
Institute of Biotechnology (Haimen, China); a three-color
pre-stained protein marker was purchased from Beyotian
Biotechnology Co. (Shanghai, China); rabbit anti-human PSMA
monoclonal antibody and rabbit anti-human β-actin polyclonal
antibody were purchased from Jiangsu Kangwei Biological Co., Ltd.
(Dongtai, China). RPMI-1640 and fetal bovine serum (FBS) were
purchased from Thermo Fisher Scientific Inc. (Gibco); recombinant
human granulocyte-macrophage colony-stimulating factor (GM-CSF),
recombinant human interleukin (IL)-4, IL-2 and tumor necrosis
factor (TNF) were purchased from Tebao Biological engineering Co.,
Ltd. (Xiamen, China). Lymphocyte separation medium was purchased
from Haoyang Biotechnology Co., Ltd. (Tianjin, China);
phosphate-buffered saline (PBS) was purchased from Boster
Biological Technology, Ltd. (Wuhan, China); phycoerythrin
(PE)-labeled mouse anti-human CD80 (cat no. 557227) and CD86 (cat.
no. 560957)antibodies, peridinium chlorophyll protein
(PerCP)-labeled mouse anti-human HLA-DR antibody (Cat. no. 551375)
and allo-phycocyanin (APC)-labeled mouse anti-human CD83 antibody
(cat. no. 551073) were purchased from BD Pharmingen (San Diego, CA,
USA). The Cell Counting Kit-8 (CCK-8) assay kit was purchased from
Skylight Biotech Co., Ltd. (Akita, Japan); interferon (IFN)-γ and
IL-10 ELISA kits were purchased from Dakewe Biotech Co., Ltd.
(Shenzhen, China) and a PE/7AAD apoptosis kit was purchased from
KeyGen Biotech. Co., Ltd. (Nanjing, China).
Target gene, vector plasmid and cell
strains
A cDNA plasmid for pCMV-SPORT6/PSMA construction was
purchased from Mitaka Biotechnology Co., Ltd (Wuhan, China). The
shuttle plasmid for pShuttle-CMV-EGFP (Shanghai Jikai Gene Chemical
Technology Co. Ltd., Shanghai, China), pAdxsi adenovirus backbone
plasmid (Shanghai Jikai Gene Chemical Technology Co. Ltd.), super
chemically competent Escherichia coli DH5α and HEK293 cells
(Shanghai Jikai Gene Chemical Technology Co. Ltd.) were maintained
in the laboratory at China Medical University (Shenyang, China).
The PCa cell strains, LNCap, Du145 and 22RV were also preserved in
this lab.
Construction and identification of PSMA
adenovirus plasmid
Using PSMA as the reference sequence, two primers,
containing SfiI restriction sites at the 5′-end, were
designed as follows: 5′-AAAAGGCCGCTGCGGCCACCATGTGGAATCTCCTTCA-3′
for P1 and 5′-AAAAGGCCTGTTTGGCCTTAGGCTACTTCACTCA-3′ for P2.
Construction and identification of
shuttle plasmid, pShuttle-GFP-PSMA
pCMV-SPORT6/PSMA served as a template, and P1, P2,
and the Pfu enzyme (pfx DNA polymerase) were used for
polymerase chain reaction (PCR) amplification. The amplified
product was processed by SfiI digestion to obtain 2.2-Kb
PSMA gene fragments. The adenovirus shuttle plasmid,
pShuttle-CMV-EGFP was also digested with SfiI, and agarose
gel electrophoresis was performed to retrieve a 5.1-Kb vector DNA.
The amplified PSMA gene and retrieved vector DNA fragment were
joined using T4 DNA ligase at 22°C for 4 h (molecular concentration
ratio, 3:1). The products were transformed into chemically
competent E. coli DH5α. The positive clones were selected
using kanamycin (Shenyang Boermei Reagent Co., Shenyang, China),
and underwent DNA sequencing following identification by
restriction enzyme digestion. The recombinant shuttle plasmid,
pShuttle-GFP-PSMA and the empty vector, pShuttle-CMV-EGFP were
digested by SfiI, and underwent 1% agarose gel
electrophoresis to identify any PSMA gene fragments. Additionally,
the recombinant vector, pShuttle-GFP-PSMA was sent to Shanghai
Shenggong Co., Ltd. (Shangai, China) for sequencing to ensure the
insertion sequence and reading frame were correct.
Construction and identification of
recombinant adenovirus plasmid, pAdxsi-GFP-PSMA
The shuttle plasmid, pShuttle-GFP-PSMA and the
pAdxsi vector were processed by I-CeuI and I-SceI
double digestion. The target gene of the shuttle plasmid was
retrieved on gel, while the digested vector was collected using an
ethanol precipitation method following CIP dephosphorylation. The
treated vector fragment and the insertion element were joined by T4
DNA ligase, and the 3-µl ligation product was transformed
into a chemically competent DH5α cell strain and smeared onto an
ampicillin-resistant solid-medium plate. Positive clones were
seeded onto the ampicillin-resistant liquid medium and incubated
overnight at 4°C. The plasmid was purified using a small/medium
quantity plasmid extraction purification kit, and the recombinant
adenovirus plasmid, pAdxsi-GFP-PSMA was obtained. XhoI
enzyme digestion was performed, followed by 1% agarose gel
electrophoresis, to identify whether the recombinant adenovirus
plasmid had been constructed correctly.
Packaging, amplification and purification
of recombinant human PSMA adenovirus
HEK293 cells were cultured in Dulbecco's modified
Eagle's medium (Shenyang Boermei Reagent Co.) containing 10% FBS
and incubated at 37°C in an atmosphere of 5% CO2. The
cells were transfected with the recombinant adenovirus at a density
of 80–90%. For trans-fection, an adenovirus vector plasmid carrying
the PSMA gene was linearized with the restriction enzyme
PacI and Lipofectamine 2000 (Shenyang Boermei Reagent Co.)
was used to mediate the transfection and packaging of the HEK293
cells. The packaged cells were incubated at 37°C in an atmosphere
of 5% CO2 for 7–12 days before cytopathic effects (CPEs)
and green fluorescent protein (GFP) could be observed under an
ordinary microscope and a fluorescence microscope, respectively.
The cells were collected, centrifuged at 800 × g for 5 min,
resuspended with 1 ml PBS, then underwent three cycles of repeated
freezing and thawing (−80°C to 37°C). The supernatant containing
the recombinant viruses was collected following centrifugation. The
above-mentioned supernatant (40–50%) was used for re-transfection
of the HEK293 cells, and the same freeze/thaw method was repeated
four times before the supernatant was collected and the virus was
purified by CsCl density gradient centrifugation. The virus was
divided into small portions and preserved at −80°C for subsequent
experiments.
Extraction, identification and titer
determination of recombinant adenovirus plasmid
The recombinant virus supernatant (10 µl) was
added to proteinase K (10 mg/ml), incubated at 50°C for 1 h and
boiled at 100°C for 5 min. Following centrifugation, 2 µl
supernatant served as a template for PCR identification. The HEK293
cells were plated in 96-well plates and the concentrated virus
solution was added into the cultured cells at different dilutions.
The infected cells were incubated at 37°C for 10 days and the
number of wells per line that were exhibiting CPE was recorded
under a fluorescence microscope. The ratio of positive wells was
calculated. According to the 50% Tissue Culture Infectious Dose
(TCID50) formula method, for a 100-µl sample, the titer (T)
= 101+d(s−0.5) (where d is log10 dilution and s is the
sum of the ratio of positive wells at each dilution). In order to
convert from TCID50/ml to pfu/ml the following formula was used: T
= a × 10b TCID50/ml = a × 10b−0.7 pfu/ml.
Induction of peripheral blood mononuclear
cell (PBMC)-derived dendritic cells and determination of the
efficiency of adenovirus infection in vitro
Blood cell separator was applied for separation and
enrichment of PBMCs obtained from ten healthy volunteers (mean age,
39±5 years; 3 male, 7 female). Blood collection performed at The
First Affiliated Hospital, China Medical University (Shenyang,
China). Ficoll lymphocyte separation medium was used with density
gradient centrifugation for preliminary purification of the
mononuclear cells. The cells were rinsed twice with PBS, counted
and stained with trypan blue (Shenyang Boermei Reagent Co.) to
detect cell viability. The cells were resuspended with RPMI-1640
medium containing 10% FBS at a density of 2×106/ml and
seeded in 25-cm2 flasks. The cells were incubated at
37°C in an atmosphere of 5% CO2 for 2 h, and the
non-adherent cells were extracted into another culture flask for
CTL induction in vitro. The adherent cells were cultured in
RPMI-1640 medium containing 10% FCS, with 100 ng/ml GM-CSF and 50
ng/ml IL-4. Half of the medium was changed every three days and
fresh cytokines were added. After six days of induction, 1,000 U/ml
TNF-α was added to stimulate DC maturation and the mature DCs were
collected on day seven or eight.
Transfection of the empty viral plasmid,
Ad-GFP into immature DCs and detection of transfection efficiency
at different MOI
On day five, immature DCs that were half-adherent
were collected and seeded in 24-well plates in 0.2 ml medium
containing 10% FBS (5×105 cells/well). The concentration
of Ad-GFP was adjusted to 2×107 pfu/µl. The
immature DCs were divided into five groups (three wells per group),
and the Ad-GFP virus was added to the DCs in each group at MOI
values of 50, 100, 200, 300 and 400. DCs containing the virus were
incubated at 37°C in 5% CO2 for 2 h, during which the
culture plates were agitated every 15 min to ensure uniform
infection of the virus. Then RPMI-1640 medium containing 10% FCS,
100 ng/ml GM-CSF and 50 ng/ml IL-4 was added to each well and the
cells were observed under a fluorescence microscope at 12, 24 and
48 h to detect the expression of GFP at one MOI value. At 48 h,
flow cytometry was used to detect the GFP-positive rate at
different MOI values, and a PE-7AAD apoptosis and necrosis kit was
used to detect the apoptosis rate of the DCs, in order to determine
the optimal MOI.
Changes in DC immune function following
infection with the PSMA gene recombinant adenovirus DC and CTL
culture in vitro
The method of DC culture is described above. The
non-adherent cells were seeded in an additional flask and
differentiated into CTLs in the presence of IL-2.
Expression of PSMA in recombinant
adenovirus-mediated DCs
On the fifth day, half-adherent immature DCs were
collected and made into a single cell suspension. The appropriate
quantities of Ad-PSMA and Ad-GFP were added into the
above-mentioned DC suspension according to the optimal MOI value,
creating two groups of DCs: The Ad-PSMA-DC and Ad-GFP-DC groups.
After 48 h of incubation at 37°C in a 5% CO2 atmosphere,
cells were processed with radioimmunoprecipitation assay lysis
buffer and protease inhibitors. The cell lysate was centrifuged at
4°C for 5 min at 800 × g, and the supernatant was transferred to a
clean Eppendorf (EP) tube and preserved at −20°C. Separating gel
(10%) and 5% stacking gel were prepared for electrophoresis. The
protein was transferred onto a polyvinylidene fluoride membrane
(Shenyang Boermei Reagent Co.) and the membrane was blocked with 5%
skimmed milk at room temperature for 2 h on a thermostatic shaker.
The primary antibody (rabbit anti-human PSMA monoclonal antibody)
was added at a concentration of 1:2,000 in Tris-buffered saline
with Tween-20 (TBST) and incubated at 4°C overnight. The membrane
was washed with TBST, three times for 10 min each time, and the
horseradish peroxidase (HRP)-labeled secondary antibody (HRP-goat
anti-rabbit IgG) was added and the membrane was incubated at room
temperature for 1 h. The membrane was washed with TBST a further
three times (10 min each time), and visualized in a dark room by
enhanced chemiluminescence.
Detection of DC phenotype before and
after virus infection by direct immunofluorescence
Fluorescent antibodies were PE-labeled CD80 and
CD86, peridinin chlorophyll-labeled HLA-DR and
allophycocyanin-labeled CD83. Immature DCs on day 5, mature DCs on
day 8 and the cells infected with adenovirus for 48 h (Ad-PSMA-DC
and Ad-GFP-DC) were collected. The four types of cell were rinsed
twice with PBS, centrifuged at 800 × g for 5 min and the cell
density was adjusted to ~1×106/ml. Each type of cell
suspension (1 ml) was transferred to four EP tubes and the
fluorescent antibodies were added. After 30 min of incubation at
4°C, cells in each tube were rinsed twice with PBS, resuspended
with 0.5 ml PBS and transferred into FACS tubes (BD Pharmingen) for
phenotype analysis.
Detection of the IL-12 level in the
supernatant of the DC vaccine in each group by ELISA
On the fifth day of DC culture, the recombinant
virus was added to each group according to the optimal MOI value
and the DCs were divided into three groups: Ad-PSMA-DC, Ad-GFP-DC
and normal DCs. On the sixth day, 1,000 U/ml TNF-α was added to
each group for DC maturation stimulation. The supernatant of each
group was collected after 48 h of transfection, and the quantity of
IL-12 in each group was detected according to the ELISA kit
instructions.
Detection of CTL cytotoxicity to the
three PCa cell lines (LNCap, Du145 and 22RV) in the different
groups by CCK-8 assay
On the fifth day of DC culture, a recombinant virus
was added to each group according to the optimal MOI value and the
DCs were divided into three groups: Ad-PSMA-DC, Ad-GFP-DC and
normal DCs. The DCs were incubated at 37°C with 5% CO2
for 48 h to form stimulation cells. Prior to use, 10 µg/ml
mitomycin was added and the cells were incubated at 37°C for 30
min, rinsed with RPMI-1640 incomplete medium (serum free) three
times and adjusted to a density of 1×106/ml. The
non-adherent mononuclear cells were cultured in RPMI-1640 medium
containing 10% FBS and 1,000 U/ml IL-2. Every three days, fresh
medium and IL-2 were added to induce CTLs, the cell density was
adjusted to 1×106/ml and prepared as reaction cells. The
above-mentioned stimulation cells (each group of DCs) and reaction
cells (CTLs) were co-cultured at a ratio of 1:10 in complete medium
at a density of 2×106/ml for 96 h (37°C, 5%
CO2). In addition, the PCa cells (LNCap, Du145 and 22RV)
at the logarithmic growth phase were plated in 96-well plates and
cultured for 24 h for cell attachment. The DC-CTL of the three
groups and the CTL control were added to the plates containing
cancer cells at different effector to target (E:T) ratios (10:1,
20:1 or 40:1) and the mixtures were incubated for another 24 h
before the CCK-8 reagent was added (concentration, 20
µl/well). After 4 h of incubation at 37°C, the optical
density (OD) value of each well was detected with ELISA and the
cytotoxicity of CTLs in each group was calculated according to the
following formula: Killing rate (%) = [1-(ODexperimental
group-ODeffector cells alone)/ODtarget cell
alone] × 100.
Statistical analysis
Data were processed with SPSS 16.0 software (SPSS,
Inc., Chicago, IL, USA) and expressed as means ±standard deviation.
Multiple comparisons of sample means were analyzed by one-way
analysis of variance and P<0.05 was considered to indicate a
statistically significant difference.
Results
Identification of shuttle plasmid,
pShuttle-GFP-PSMA
The recombinant shuttle plasmid, pShuttle-GFP-PSMA
and empty vector, pShuttle-CMV-EGFP were digested by SfiI
and, by PCR amplification, two fragments of ~2.2 kb (consistent
with the size of the inserted fragment) and ~5.1 kb were obtained
from the pShuttle-GFP-PSMA plasmid, while only the 5.1-kb fragment
(recombinant shuttle vector fragment) was obtained from the empty
vector (Fig. 1). The results of
DNA sequencing were consistent with the reports obtained from
GenBank: PSMA (NC_004463.1; http://www.ncbi.nlm.nih.gov/gene/1054986) and FOLH1
(NC_000011.10; http://www.ncbi.nlm.nih.gov/gene/2346).
Identification of recombinant adenovirus
vector, pAdxsi-GFP-PSMA
The recombinant shuttle plasmid, pShuttle-GFP-PSMA
and empty vector, pShuttle-CMV-EGFP were digested by SfiI,
and loaded onto 1% agarose gel for electrophoresis. The results are
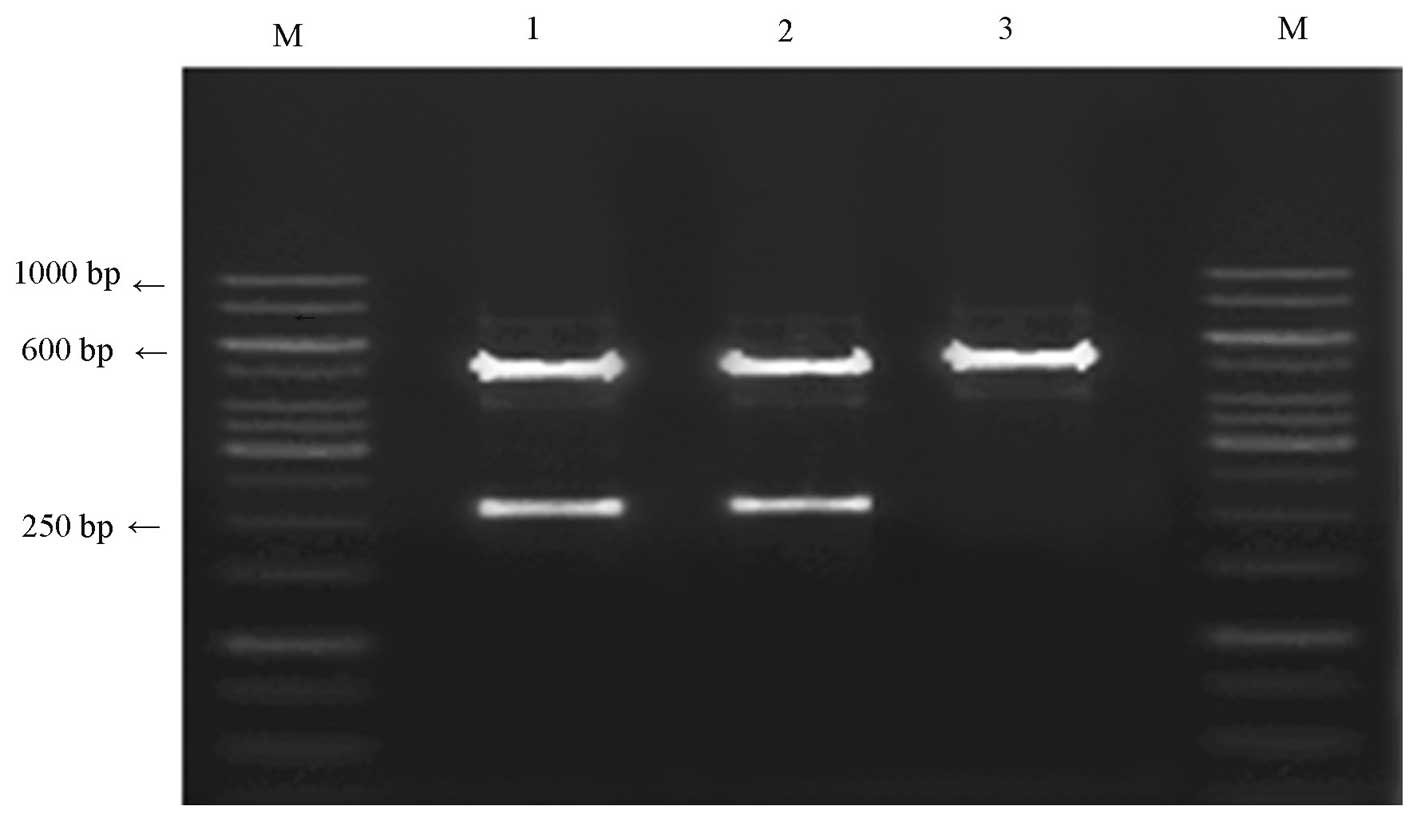
presented in Fig. 2.
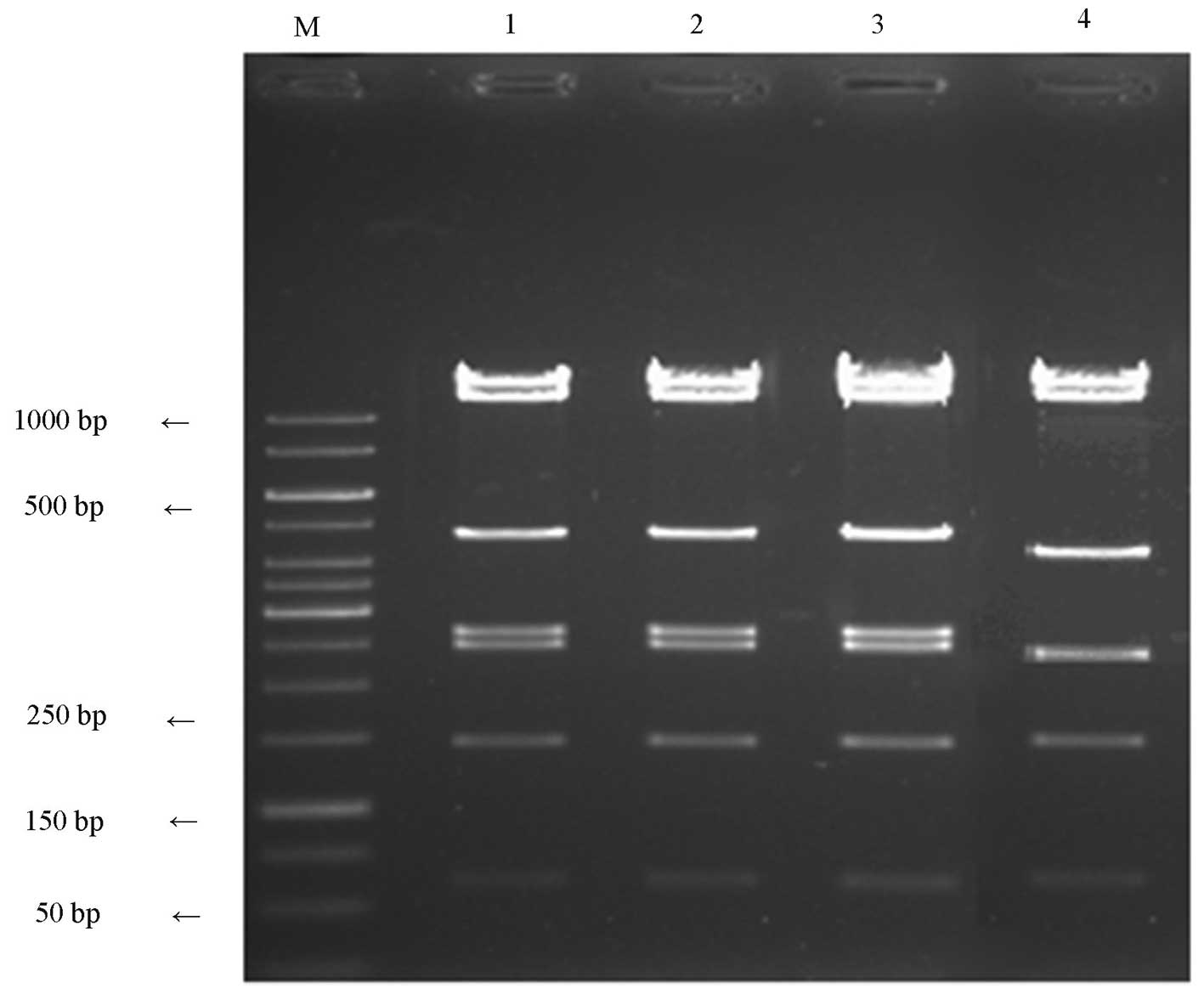 | Figure 2Identification results of
pAdxsi-GFP-FOLH1. Lane M, marker (1 kb DNA ladder); lanes 1-3,
positive clones 14, 11.8, 4.8, 2.66, 2.47, 1.45 and 0.6 κ; lane 4,
negative clones 14, 11.8, 4.0, 2.47, 1.45 and 0.6 kb. |
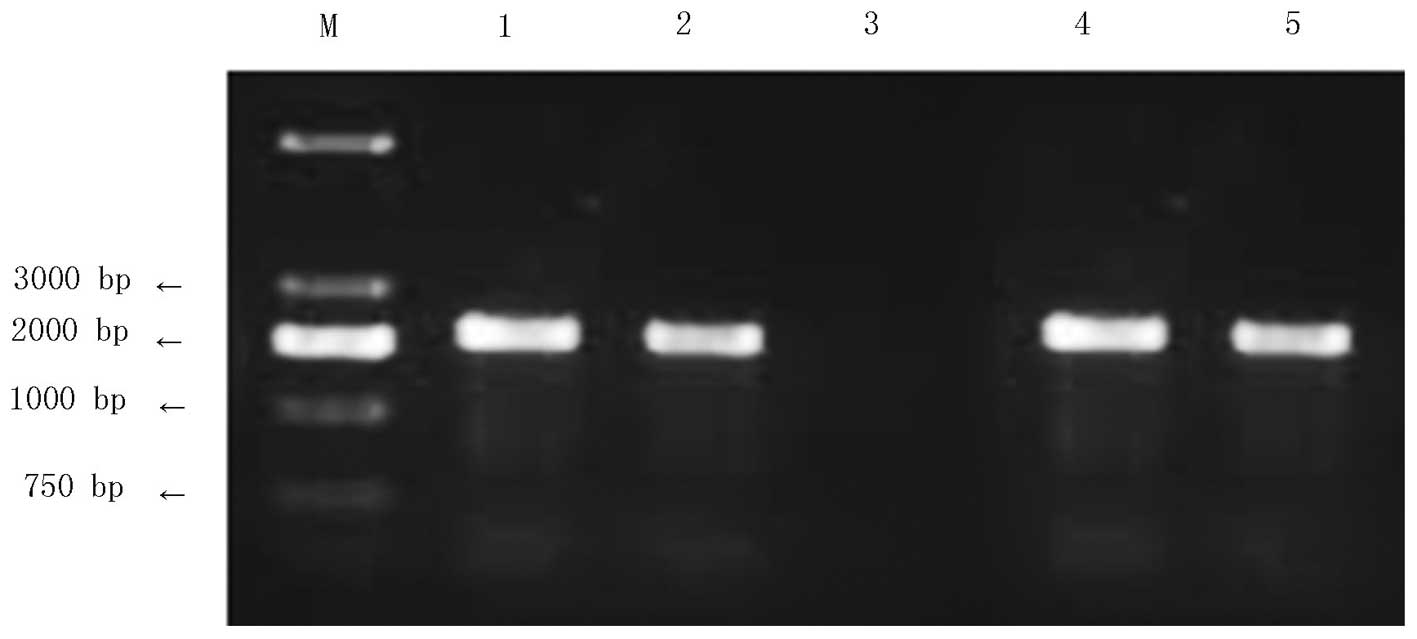
Construction and identification of the
human recombinant adenovirus carrying the PSMA gene
pAdxsi-GFP-PSMA was linearized with PacI for
transfection into HEK293 cells. After one week of culture, when the
transfected cell showed obvious vacuole-like changes under a
fluorescent microscope, the virus DNA was extracted and amplified
by PCR. The emergence of a 2,200-bp target band during
electrophoresis indicated successful recombination of the target
genes into the adenovirus genome (Fig.
3). Subsequent to identification, the concentrated virus
solution was used for re-infection of the HEK293 cells at various
concentrations and the virus titer was 2.0×1010
pfu/ml.
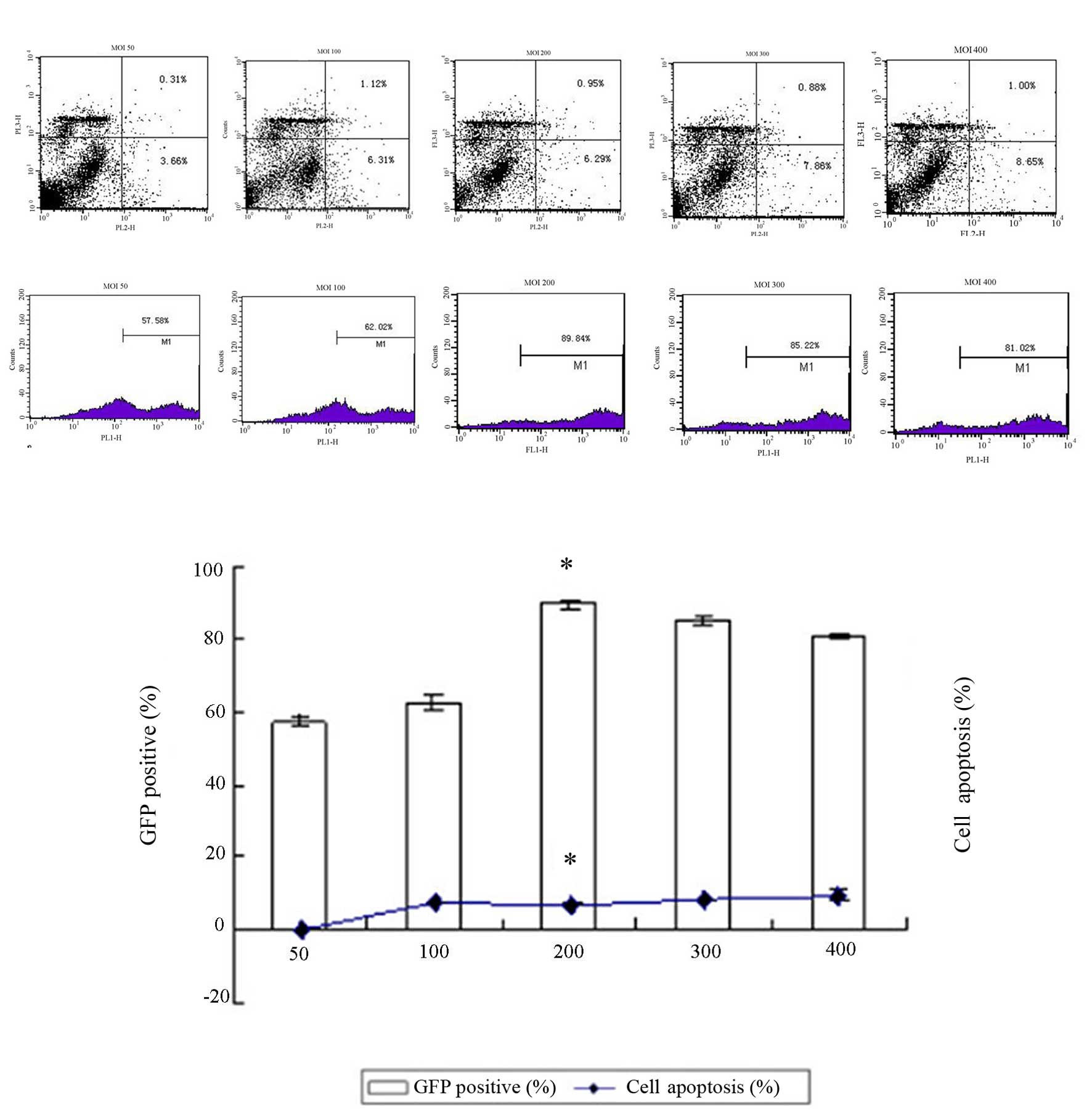
Efficiency of in vitro transfection of
the adenovirus into the DCs
Results of flow cytometry (Fig. 4) demonstrate that the transfection
efficiency reached a maximum value (89.84+1.13%) at an MOI value of
200, which was significantly different from that at MOI values of
100, 150, 300 and 400 (P<0.05). In addition, the apoptosis rate
of the DCs was significantly lower in the MOI 200 group
(6.29+0.14%) when compared with the other groups (P<0.05),
indicating a minimum impact on DC vitality at an MOI value of 200.
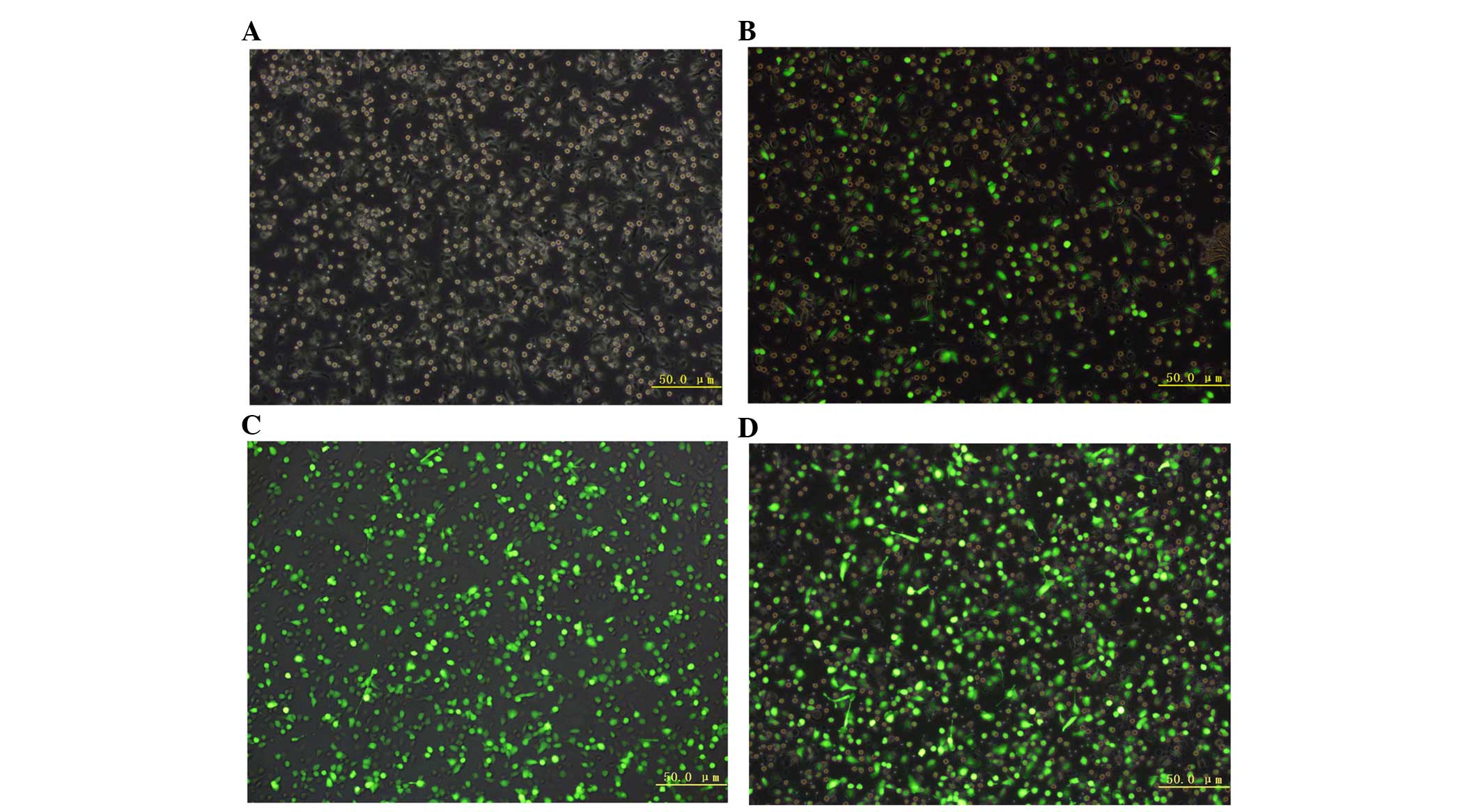
Therefore, the optimal MOI value was determined to be 200. When the
MOI value was fixed at 200, the GFP expression level was observed
under a fluorescent microscope 12, 24 and 48 h after transfection.
The results demonstrated that GFP expression was greatest (up to
85%) 48 h following transfection (Fig.
5A–D).
Changes in immune function of DC after
transfection with PSMA gene recombinant adenovirus
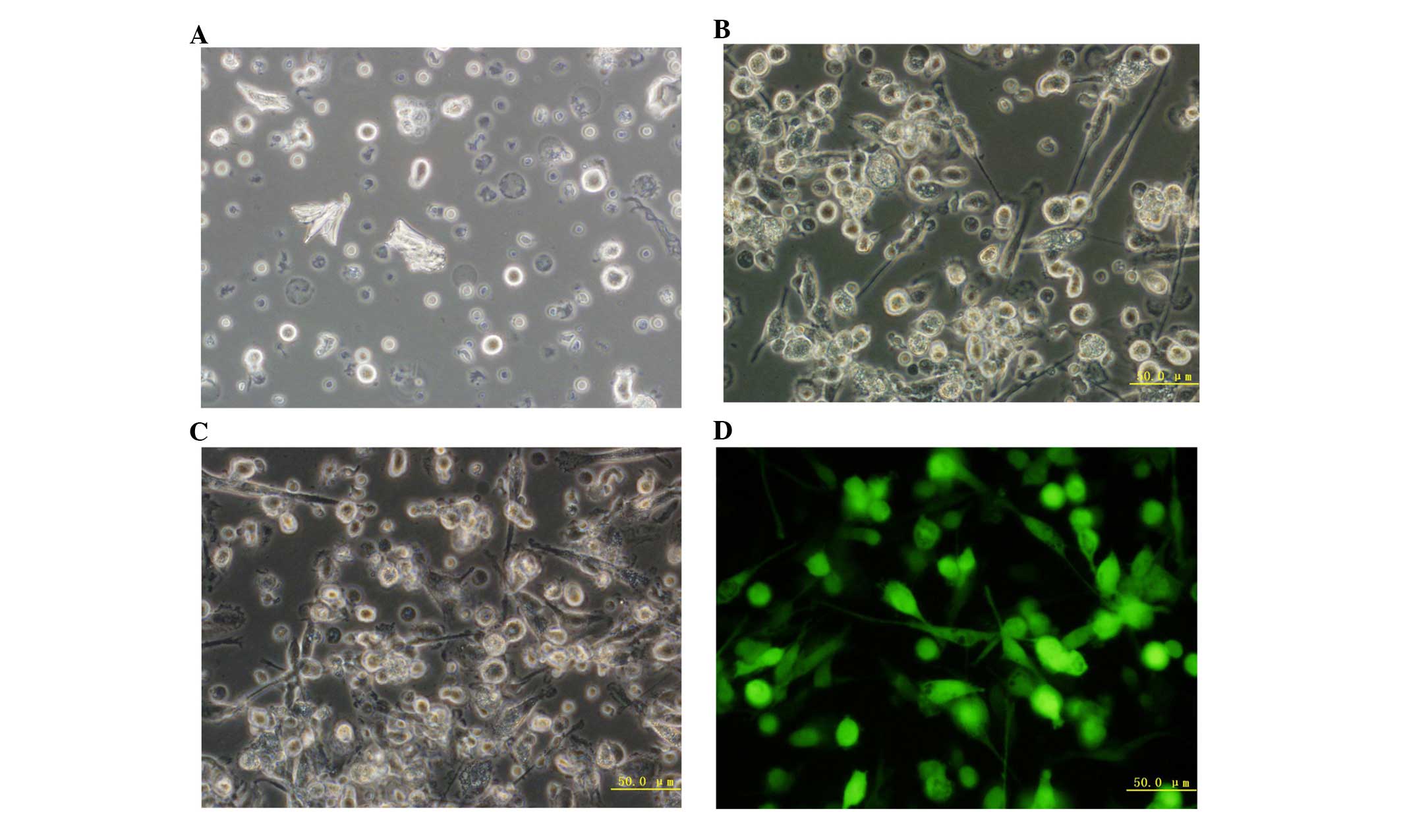
Morphology of DCs observed by
fluorescence microscopy
The DCs were observed on different days under a
fluorescence microscope. On day 1, cells were adherent, small,
irregularly shaped (with small pseudopodia and no branch-like
protrusions) and showed a uniform distribution of cell colonies. On
day 5, the majority of cells became larger, semi-adherent irregular
spindle cells that clustered and showed bacteria curtain-like
protrusions on the periphery. On the seventh day after Ad-GFP
transfection, the cell size increased, the spikes on the cell rim
became more apparent with obvious branches, and the cells were
star- or spindle-shaped, with obvious nuclei and grew in clusters.
All of these features were similar to those of DCs, which had been
cultured for seven days under normal conditions (Fig. 6A–D).
Expression levels of PSMA in DCs
observed by western blotting
Following transfection (48 h), the positive
expression rate of GFP was 85%. Western blot analysis revealed a
band at around 110 kd, which is consistent with the size of PSMA
proteins; however, no such band was observed in the control group
(Fig. 7).
Expression of co-stimulatory molecules
in DCs in each group following transfection
Flow cytometry revealed that immature DCs on day 5
expressed a reduced quantity of co-stimulatory and major
histocompatibility complex class II (MHC II) molecules (Table I). On day 8, the DCs in the
trans-fection and non-transfection groups all showed features of
mature DCs (high expression of the co-stimulatory and MHC II
molecules); the expression levels of CD80, CD83, CD86 and HLA-DR
were significantly higher in the transfected DCs than in the
non-transfected DCs (P<0.05). However, no difference was
observed between the Ad-PSMA-DC and Ad-GFP-DC groups (P>0.05).
The results demonstrate that transfection of the adenovirus
promoted DC maturation and significantly upregulated the expression
levels of co-stimulatory molecules.
 | Table IPhenotype analysis of DCs in each
group on days 5 and 8. |
Table I
Phenotype analysis of DCs in each
group on days 5 and 8.
| Group | DC marker
expression (%)
|
|---|
| CD80 | CD83 | CD86 | HLA-DR |
|---|
| 5d-DCs | 22.95±3.34 | 18.93±1.65 | 29.64±0.83 | 29.17±0.81 |
| Ad-PSMA-DC | 33.29±1.13b | 36.02±0.71b | 53.30±0.86b | 53.00±1.62b |
| Ad-GFP-DC | 29.68±0.61c | 32.62±1.07c | 50.70±0.83c | 49.29±0.53c |
| 8d-DCs | 28.27±1.04a | 28.08±1.16a | 41.05±1.33a | 46.87±1.12a |
Detection of IL-12 in the supernatant
of each group
Following transfection (48 h), the supernatant of
each group was collected, and the quantity of IL-12 in the
supernatant was measured by ELISA (Fig. 8). The IL-12 level in the Ad-PSMA-DC
(79.51±1.60 pg) and Ad-GFP-DC (69.67±1.43 pg) groups was
significantly higher than that in the non-transfection (normal-DC)
group (28.88±2.97 pg; P<0.05); however, no significant
difference was identified between the two trans-fected groups
(Ad-PSMA-DC and Ad-GFP-DC). These results indicate that adenovirus
transfection significantly promotes the ability of DCs to secrete
IL-12, regardless of whether the cell carries the PSMA gene.
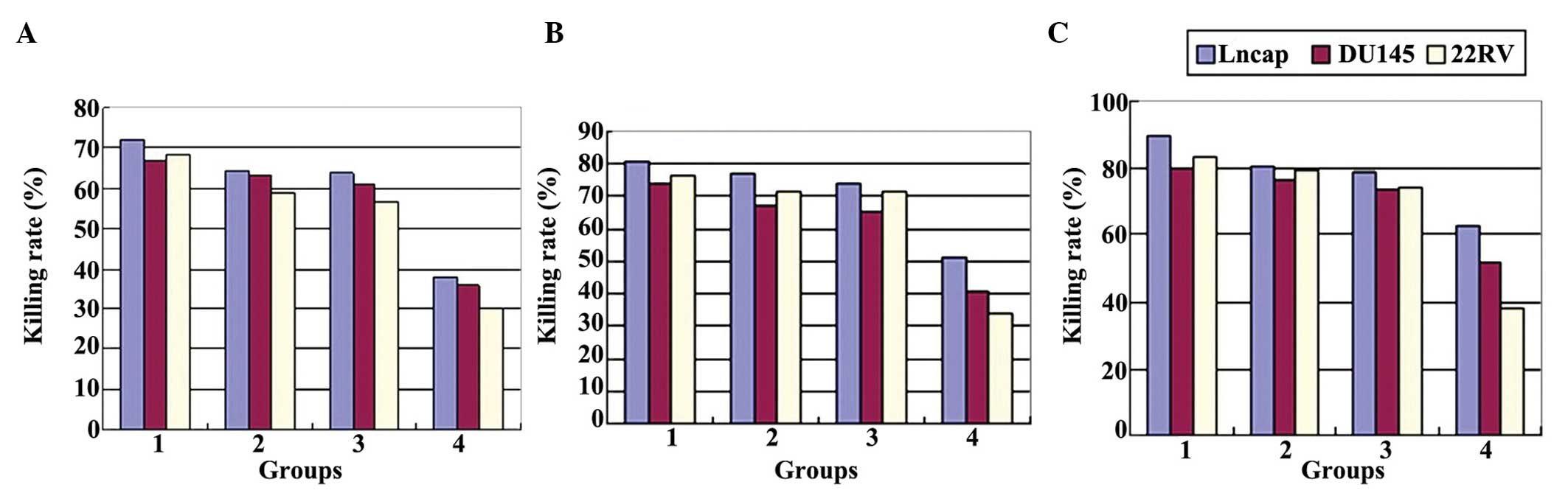
Cytotoxicity of effector cell (CTL) to
target cells (LNCap, Du145 and 22RV)
A CCK-8 assay was conducted to detect the OD value
of each well and the cytotoxicity of CTL in each group was
calculated based on the following formula: Killing rate
(%)=[1-(ODexperimental group-ODeffector cells
alone)/ODtarget cell alone]×100. Statistical
analysis revealed that CTLs that were stimulated by transfected DCs
were associated with a stronger killing rate against all three PCa
lines, when compared with CTLs that were stimulated by normal
cultured DCs or CTLs alone. Cytotoxicity reached a maximum value in
all of the groups when the E:T ratio was 40:1. Furthermore, at the
same E:T ratio, the killing rate of CTL in the Ad-PSMA-DC-CTL group
against the LNCap cells (exhibiting high PSMA expression levels)
was significantly higher than that against the Du145 and 22RV cells
(P<0.05). In addition, the killing rate in the Ad-PSMA-DC-CTL
group was significantly higher than that in the Ad-GFP-DC-CTL,
normal DC-CTL and CTL alone groups (Fig. 9A–C; P<0.05 and Table II). The results demonstrate that
DCs transfected with PSMA were more effective in eliciting the
differentiation of tumor-specific CTLs, which exert a particularly
potent killing effect on PCa cells with high PSMA expression
levels, such as LNCap cells.
 | Table IIKilling rate of effector cells (CTLs)
against three prostate cancer cell lines. |
Table II
Killing rate of effector cells (CTLs)
against three prostate cancer cell lines.
| Cell line
(E:T) | Killing rate (%)
|
|---|
| Ad-PSMA-DC-CTL | Ad-GFP-DC-CTL | Normal DC-CTL | CTL |
|---|
| LNCap | | | | |
| 10:1 | 72.18±0.43a,b | 64.28±1.63 | 63.96±1.47a | 37.90±1.39a |
| 20:1 | 80.87±1.50a,b | 76.92±0.92a | 74.51±0.85a | 51.08±0.86a |
| 40:1 | 89.76±0.44a | 80.97±1.12 | 78.75±0.99a | 62.35±0.97a |
| DU145 | | | | |
| 10:1 | 66.61±0.95a | 63.22±1.86 | 60.83±1.03 | 35.81±0.76 |
| 20:1 | 74.49±1.10a | 66.96±0.82 | 64.73±1.68 | 40.90±1.10 |
| 40:1 | 80.21±1.20a | 76.66±0.37 | 73.52±1.29 | 51.84±2.24 |
| 22RV | | | | |
| 10:1 | 68.16±0.82a | 58.81±0.48 | 56.34±1.23 | 29.85±0.38 |
| 20:1 | 76.15±0.71a | 71.52±0.74 | 71.87±1.75 | 33.46±1.38 |
| 40:1 | 83.50±0.85a | 79.66±0.64 | 74.50±1.45 | 37.98±0.49 |
Discussion
PSMA is a type II transmembrane glycoprotein, which
is secreted by prostate epithelial cells. It is composed of 750
amino acids, and has a relative molecular mass of ~100,000. There
are three PSMA domains, comprising the intracellular N-terminal,
and the transmembrane and extracellular regions. On the
intracellular and extracellular regions, there are a plurality of
epitopes that may be combined with a variety of monoclonal
antibodies (18). Compared with
PSA and PAP, PSMA is regarded as a more sensitive and a more
specific tumor maker. Its highly specific expression in PCa tissues
has established PSMA as one of the leading targets for use within
targeted therapies (19). Based on
the above-mentioned information, a replication-defective adenoviral
vector containing PSMA gene sequences was constructed for use in
the present study.
The Adxsi system, which comprised a shuttle plasmid
vector system, a virus backbone plasmid and the packaging cell
line, HEK293 was selected. The primary advantage of the Adxsi viral
packaging system is that the packaged virus is stable and of a high
titer, furthermore, the system may be used in the packaging of
cytotoxic genes, as well as genes that influence adenovirus
physiological processes, such as infection, duplication and
packaging (20). The PSMA gene was
amplified by PCR and the pShuttle-EGFP-PSMA was directly ligated
into the viral plasmid according to a dual restriction site
(I-Ceul and I-Scel) directional cloning method. There
are few restriction sites of I-Ceul and I-Scel in the
human genome, therefore, they can be used to identify longer gene
sequences. Additionally, the application of HEK293 cells for the
control of PSMA gene expression has improved the efficacy of virus
packaging and the virus titer (21). CsCl density gradient centrifugation
was performed for purification of the HEK293 cells, including
exemption of defective viral particles, cellular debris and a small
quantity of medium components, so as to maximize the reduction of
pollution-induced immunological responses (22,23)
and to obtain a high titer (2.0×1010 pfu/ml) PSMA
adenovirus with high productivity.
DCs are considered to be the most powerful
antigen-presenting cells currently known. They are derived from
hematopoietic stem cells, and are widely distributed in tissues and
organs, but not in the brain. However, the number of DCs in the
human body is limited (<1% in human PBMCs), with cancer patients
possessing even fewer DCs, which are functionally deficient, as
they are incapable of effective antigen presenting (24). In addition, the low immunogenicity
of tumor cells and their lack of specific antigens has consistently
presented a major issue during vaccine therapy in the activation of
human immune responses (25).
There are three stages involved in the in
vitro culture of DCs: i) Amplification, ii) differentiation
induction and iii) maturation promotion. In the present study,
PBMCs were enriched for the induction of DCs in the presence of
IL-4, GM-CSF and TNF-α. These cytokines inhibit the transformation
of PBMCs into precursor cells, and also induce the generation and
maturation of DCs (26,27). However, due to the immune
surveillance effect, and strong antigen uptaking and processing
capacity of immature DCs, the adenovirus-carrying PSMA gene was
added on the fifth day of culture to promote DC maturation and to
obtain abundant specific tumor antigen-loaded DCs (28). The present study has demonstrated
the validity of this method of constructing DC vaccines that are
specific to PCa.
As a result of the high expression levels of MHC I
and II molecules, co-stimulatory molecules (such as CD80 and CD86),
adhesion molecules (such as CD44 and CD54, integrin βl and integrin
β2) and characteristic markers (such as CD83), the predominant role
of mature DCs is the induction of immune activation. The mechanism
of the strong immune stimulatory capacity of DCs is via an
interaction between DCs and heat shock protein receptors (including
CD91 and Toll-like receptors) on the DC surface, which triggers a
series of reactions, including upregulation of surface factors,
increased secretion levels of IL-12 and IFN-γ, promotion of DC
maturation and improvement of antigen-presenting efficiency
(29,30).
In conclusion, in the current study, DCs induced by
the PSMA-adenovirus exhibited significantly higher expression
levels of CD80, CD86 and CD83, and a significantly higher secretion
level of IL-12, when compared with that of DCs that had not been
stimulated. However, no such difference was observed between the
DCs induced by the PSMA-adenovirus or the empty virus. Furthermore,
among all of the DC vaccines, the PSMA-DC vaccine demonstrated a
more obvious cytotoxic effect against all three cancer cell lines
(particularly against the LNCap cells) when compared with the other
vaccines, including the adenovirus-DC vaccine. This indicated that
the PSMA-targeted DC vaccine exerted a marked effect against the
PCa cells; however, its ability to stimulate DC differentiation and
maturation, and to improve their antigen-presenting efficiency was
dependent on the loaded adenovirus. The above result may have been
due to the expression of other co-stimulatory molecules that were
not identified in the present study; therefore further studies are
required to obtain a comprehensive perspective.
Acknowledgments
The present study was supported by a grant from the
Liaoning Province Science and Technology Plan Projects (grant no.
2010225034).
References
|
1
|
Zumwalt TH and Goel A: Immunotherapy of
metastatic colorectal cancer: prevailing Challenges and new
perspectives. Curr Colorectal Cancer Rep. 11:125–140. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang H and Huang B: Tumor cell-derived
microparticles: a new form of cancer vaccine. Oncoimmunology.
4:e10177042015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Onji M and Akbar SMF: On dendritic cell
based therapy for cancers. Zhejiang Univ Sci B. 6:1–3. 2005.
View Article : Google Scholar
|
|
4
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gu F: Epidemiological survey of benign
prostatic hyperplasia and prostate cancer in China. Chin Med J
(Engl). 113:299–302. 2000.In Chinese.
|
|
6
|
Basler M and Groettrup M: Advances in
prostate cancer immunotherapies. Drugs & Aging. 24:197–221.
2007. View Article : Google Scholar
|
|
7
|
Murphy GP, Tjoa BA, Simmons SJ, Jarisch J,
Bowes VA, Ragde H, Rogers M, Elgamal A, Kenny GM, Cobb OE, et al:
Infusion of dendritic cells pulsed with HLA-A2-specific
prostate-specific membrane antigen peptides: A phase II prostate
cancer vaccine trial involving patients with hormone-refractory
metastatic disease. Prostate. 38:73–78. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murphy GP, Tjoa BA, Simmons SJ, Rogers MK,
Kenny GM and Jarisch J: Higher-dose and less frequent dendritic
cellinfusions with PSMA peptides in hormone-refractory metastatic
prostate cancer patients. Prostate. 43:59–62. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou Y, Wang HP and Wang Q: Advances in
design strategy of dendritic cell vaccine. Medical Journal of
Chinese People's Liberation Army. 31:1205–1206. 2006.In
Chinese.
|
|
10
|
Ribas A, Butterfield LH, Glaspy JA and
Economou JS: Cancer immunotherapy using gene-modified dendritic
cells. Curr Gene Ther. 2:57–78. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu Q, Xia D, Carlsen S and Xiang J:
Adenovirus-mediated transgene-engineered dendritic cell vaccine of
cancer. Curr Gene Ther. 5:237–247. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Perner S, Hofer MD, Kim R, Shah RB, Li H,
Möller P, Hautmann RE, Gschwend JE, Kuefer R and Rubin MA: Prostate
specific membrane antigen expression as a predictor of prostate
cancer progression. Hum Pathol. 38:696–701. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang P, Chiu YC, Tostanoski LH and Jewell
CM: Polyelectrolyte multilayers assembled entirely from immune
signals on gold nanoparticle templates promote antigen-specific T
cell response. ACS Nano. 9:6465–6477. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiu K, Yu B, Huang H, Zhang P, Ji L and
Chao H: Tetranuclear ruthenium(II) complexes with oligo-oxyethylene
linkers as one-and two-photon luminescent tracking non-viral gene
vectors. Dalton Trans. 44:7058–7065. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xia D, Moyana T and Xiang J: Combinational
adenovirus-mediated gene therapy and dendritic cell vaccine in
combating well-established tumors. Cell Res. 16:241–259. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Yan and Shen Xi: The impact of
transfection with common viral vector on dendritic cells. Foreign
Medical Science (Molecular Biology Section). 25:188–190. 2003.In
Chinese.
|
|
17
|
Liu Qingxin and Song Jun: Viral
vector-mediated gene therapy for prostate cancer. International
Journal of Urology and Nephrology. 20:62–63. 2000.In Chinese.
|
|
18
|
Slovin SF, Kehoe M, Durso R, Fernandez C,
Olson W, Gao JP, Israel R, Scher HI and Morris S: A phase I dose
escalation trial of vaccine replicon particles (VRP) expressing
prostate-specific membrane antigen (PSMA) in subjects with prostate
cancer. Vaccine. 31:943–949. 2013. View Article : Google Scholar
|
|
19
|
Wang W and Mo ZN: Advances in
prostate-specific membrane antigen targeted therapies for prostate
cancer. Zhonghua Nan Ke Xue. 16:547–551. 2010.In Chinese.
PubMed/NCBI
|
|
20
|
Medin JA, Liang SB, Hou JW, Kelley LS,
Peace DJ and Fowler DH: Efficient transfer of PSA and PSMA cDNAs
into DCs generates antibody and T cell antitumor responses in vivo.
Cancer Gene Ther. 12:540–551. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeng H, Wu Q, Li H, Wei Q, Lu Y, Li X,
Wang F, Zhao F, Ding Z and Yang Y: Construction of
prostate-specific expressed recombinant plasmids with high
transcriptional activity of prostate-specific membrane antigen
(PSMA) promoter/enhancer. J Androl. 26:215–221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Q, Zaiss AK, Colarusso P, Patel K,
Haljan G, Wickham TJ and Muruve DA: The role of capsid-endothelial
interactions in the innate immune response to adenovirus vectors.
Hum Gene Ther. 14:627–643. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Philpott NJ, Nociari M, Elkon KB and
Falck-Pedersen E: Adenovirus-induced maturation of dendritic cells
through a PI3 kinase-mediated TNF-alpha induction pathway. Proc Nat
Acad Sci USA. 101:6200–6205. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Smyth MJ, Godfrey DI and Trapani JA: A
fresh look at tumor immunosurveillance and immunotherapy. Nat
Immunol. 2:293–299. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fan Y and Moon JJ: Nanoparticle drug
delivery systems designed to improve cancer vaccines and
immunotherapy. Vaccines. 3:662–685. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu A, Takahashi M, Narita M, Zheng Z,
Kanazawa N, Abe T, Nikkuni K, Furukawa T, Toba K, Fuse I and Aizawa
Y: Generation of functional and mature dendritic cells from cord
blood and bone marrow CD34+ cells by two-step culture combined with
calcium ionophore treatment. J Immunol Methods. 261:49–63. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hill JA, Ichim TE, Kusznieruk KP, Li M,
Huang X, Yan X, Zhong R, Cairns E, Bell DA and Min WP: Immune
modulation by silencing IL-12 production in dendritic cells using
small interfering RNA. J Immunol. 171:691–696. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weigel BJ, Panoskaltsis-Mortari A, Diers
M, Garcia M, Lees C, Krieg AM, Chen W and Blazar BR: Dendritic
cells Pulsed or fused with AML cellular antigen provide comparable
in vivo antitumor protective responses. Exp Hematol. 34:1403–1412.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rivoltini L, Castelli C, Carrabba M,
Mazzaferro V, Pilla L, Huber V, Coppa J, Gallino G, Scheibenbogen
C, Squarcina P, et al: Human tumor-derived heat shock protein 96
mediates in vitro activation and in vivo expansion of melanoma- and
colon carcinoma-specific T cells. J Immunol. 171:3467–3474. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mazzaferro V, Coppa J, Carrabba MG,
Rivoltini L, Schiavo M, Regalia E, Mariani L, Camerini T, Marchianò
A, Andreola S, et al: Vaccination with autologous tumor-derived
heat-shock protein gp96 after liver resection for metastatic
colorectal cancer. Clin Cancer Res. 9:3235–3245. 2003.PubMed/NCBI
|