Introduction
Type 2 diabetes mellitus (T2DM) presents a global
epidemic with an estimated global prevalence of 8.3%, and with the
World Health Organization predicting that the current number
(170,000,000) of patients with diabetes diagnosed with T2DM are
likely to more than double by 2030 (1). It is well-established that T2DM is a
metabolic disorder, characterized by impaired pancreatic β-cell
function and insulin resistance in target tissues (2). Abnormalities of glucose and insulin
result in hyperglycemia, together with dysregulation of
carbohydrate, fat and protein metabolism. Severe complications,
including retinopathy (3),
nephropathy (4,5) and cardiovascular disease (6,7),
contribute to the deleterious consequences of T2DM. Although the
progression of clinical complications of T2DM can be prevented or
delayed by effective glycemic control (8), the current medical management for
T2DM may yield unsatisfactory results due to the lack of
comprehensive awareness with regards to the physiological basis of
T2DM. Currently, an unhealthy lifestyle and high-fat diet (HFD), as
well as genetic background, are considered to be important
indicators of T2DM and T2DM-associated obesity (9). In animal models and in humans, T2DM
and T2DM-associated obesity have been found to be correlated with a
decrease in whole body insulin sensitivity, which is also referred
to as insulin resistance (10).
Improvements in current understanding of the development of insulin
resistance and pathogenesis in T2DM may lead to the development of
novel approaches for the prevention and control of this chronic
disease.
Nuclear receptors are ligand-activated transcription
factors, which govern aspects of major metabolic pathways by
regulating gene expression via binding to specific response
elements in the promoters of target genes. Farnesoid X receptor
(FXR) is a member of the nuclear receptor family, and is expressed
at high levels in the liver (11).
FXR was found to serve as a receptor for physiological
concentrations of several bile acids (BA), among which
chenodeoxycholic acid (CDCA) is the most potent (12). FXR is important in maintaining BA,
cholesterol, glucose and lipid levels by promoting BA efflux from
the liver, inhibiting hepatic BA synthesis and intestinal
absorption (11). Furthermore, FXR
regulates lipid metabolism, insulin sensitivity and energy
homeostasis, which are closely associated with T2DM. In diabetic
liver tissue samples, FXR expression levels are significantly
lower, compared with those of a normal control (13), and administration of FXR agonists
has been shown to lower blood glucose and lipid levels (14–16).
FXR activation suppresses hepatic gluconeogenic expression, and
increases hepatic glycogen synthesis and glycogen content via a
mechanism involving enhanced insulin sensitivity (14). Therefore, therapeutic strategies,
which target FXR may to hold promise for the treatment of T2DM.
However, the beneficial effects of an FXR agonist in metabolic
disorders has been challenged in several previous studies. A study
in obese FXR-knockout animals demonstrated that genetic ablation of
FXR, as opposed to FXR activation, improved glucose intolerance
(17). Another investigation
reported that FXR knockout in mice gained less body weight in
ob/ob mice (18).
Therefore, the potential therapeutic value of FXR activation on
metabolism and lipid profile remain in dispute.
The present study investigated the therapeutic
effects of FXR activation by CDCA in a rat T2DM model, which was
induced by feeding with a HFD for 8 weeks with a single
streptozotocin (STZ) injection. The expression levels of several
metabolism-associated proteins, including phosphoenolpyruvate
carboxykinase (PEPCK), glucose 6-phosphatase (G6Pase), short
heterodimer partner (SHP) and peroxisome proliferator-activated
receptor-γ coactivator-1 (PGC-1α) were evaluated in liver tissue
samples using reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) and western blotting. The study aimed to
determine whether, in the HFD and STZ-injection induced diabetic
model, FXR activation improved or impaired the metabolic profile in
diabetes.
Materials and methods
Animals
Male Wistar rats (8-week-old; n=40) were purchased
from the Animal Center of Tongji Medical College (Huazhong
University of Science and Technology, Wuhan, China). The animals
were housed in controlled conditions (temperature 23±2°C, humidity
60±10% and lighting 8 a.m–8 p.m) with access to water ad
libitum. All experiments were performed in accordance with the
National Institutes of Health Guidelines on the Use of Laboratory
Animals (19) and were approved by
the Animal Care Committee of Hubei Integrated Traditional Chinese
and Western Medicine Hospital, Hubei University of Chinese Medicine
(Wuhan, China).
T2DM model and treatment
The rats were fed a HFD consisting of 30% fat, 50%
carbohydrate, 18% protein and 2% fiber (Wuhan Feiyi Technology Co.,
Ltd., Wuhan, China) for 4 weeks. At the end of the fourth week, the
rats were administered with a single low-dose (30 mg/kg)
intraperioneal injection of STZ (Sigma-Aldrich, St. Louis, MO,
USA). Rats fed a regular diet were administered with an
intraperioneal injection of 0.1 mol/l sodium citrate buffer
(Sigma-Aldrich) as a vehicle. The provision of the HFD and regular
diet continued for a further 4 weeks, following which blood was
collected from the femoral vein following 12 h fasting, and rats
with plasma glucose >16 mmol/l were identified as having
diabetes. The control and the diabetic rats were randomly separated
into two subgroups (n=10) and treated for 10 days with either an
intraperitoneal injection of CDCA (10 mg/kg body weight/day;
Sigma-Aldrich) or saline. The 2 h postprandial blood glucose
concentration was then assayed. The following day, subsequent to
overnight fasting, the rats were anesthetized with intraperitoneal
phenobarbital sodium (40 mg/kg; Sigma-Aldrich) and blood was
collected from the tail for the determination of fasting plasma
glucose, lipid and insulin concentrations. Blood glucose
concentration was measured using the blood obtained from a tail
vein at the indicated time points with a OneTouch Ultra Glucometer
(Lifescan, Burnaby, Canada). Body weight was recorded following
anesthetization. The biochemical assays were performed using a
Hitachi-7060C Autoanalyzer (Hitachi, Ltd., Tokyo, Japan) using kits
supplied by Roche Diagnostics (Basel, Switzerland; cat. no.
11213070201). Fasting plasma insulin was assayed using a
commercially available radioimmunoassay kit obtained from the China
Institute of Atomic Energy (Beijing, China; cat. no. 20120125). In
addition, liver tissue samples were collected for subsequent
experiments. Left lateral liver tissue was dissected and washed in
ice-cold deionized water three times. Subsequently, the rats were
injected with phenobarbital sodium (200 mg/kg) for sacrifice.
RT-qPCR
RT-qPCR was performed, as previously described
(20). The mRNA expression levels
of FXR, PEPCK, G6Pase, SHP and PGC-1α were assayed using RT-qPCR on
a 7300 Real-Time PCR Detection system (ABI; Applied Biosystems;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and normalized to
β-actin. Tissues were homogenized in TRIzol® reagent.
RNA was isolated from the liver tissue samples using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), and 2 µg total RNA was reverse transcribed to cDNA
using reverse transcriptase (Takara Bio, Inc., Dalian, China). The
RT-qPCR reaction mixture contained 0.4 µM primers (5′-3′;
Invitrogen; Thermo Fisher Scientific, Inc.) and 2 µl cDNA in
SYBR® Green Supermix (Toyobo, Osaka, Japan) (21). The experimental mRNA expression
levels were expressed as the percentage change relative to the
control (β-actin). The thermocycling steps were as follows: 95°C
for 60 sec, 95°C for 15 sec and 60°C for 60 sec for 40 cycles. The
primers used are listed in Table
I. Data were normalized using β-actin as an internal control
and calculated using the comparative Cq method (2−ΔΔCq)
as described previously (22).
 | Table ISequences of the primers used for
reverse transcription-quantitative polymerase chain reaction. |
Table I
Sequences of the primers used for
reverse transcription-quantitative polymerase chain reaction.
| Gene | Accession
number | Primer | Fragment length
(bp) |
|---|
| FXR | NM_021745 |
5′-GCGAAAGTGCTGGGCTTTG-3′ | 118 |
| |
5′-TGTGCTTCTGGGATGGTGGT-3′ | |
| PEPCK | NM_001108377.2 |
5′-ACCAGTGATGGCGGTGTGTA-3′ | 132 |
| |
5′-AAAGCGAGAGTTTGGATGCG-3′ | |
| G6-Pase | NM_013098.2 |
5′-CAGCTCCGTGCCTCTGATAAA-3′ | 281 |
| |
5′-CAATGCCTGACAAGACTCCAG-3′ | |
| SHP | NM_057133.1 |
5′-TCTCTTCCTGCTTGGGTTGG-3′ | 146 |
| |
5′-GTGAGGGTTGTGGTGGGTCT-3′ | |
| PGC-1α | NM_031347.1 |
5′-ACAGGTCGTGTTCCCGATCA-3′ | 259 |
| |
5′-CTTTCAGACTCCCGCTTCTCA-3′ | |
| β-actin | NM_031144 |
5′-CGTTGACATCCGTAAAGACCTC-3′ | 110 |
| |
5′-TAGGAGCCAGGGCAGTAATCT-3′ | |
Western blot analysis
Western blot analysis was performed, as previously
described (23). The protein
expression levels of FXR, PEPCK, G6Pase, SHP, and PGC-1α were
assayed using western blot analysis. Liver tissues were homogenized
in radioimmunoprecipitation assay lysis solution and centrifuged at
12,000 × g. The supernatant was collected and the protein
concentration was determined using a BCA assay (Beyotime Institute
of Biotechnology, Haimen, China). Liver proteins (30–50 µg)
were separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (Sigma-Aldrich) and blotted onto nitrocellulose
membranes (Millipore, Bedford, MA, USA). The membrane was blocked
using 4% evaporated milk for 4 h. The membranes were then incubated
with the following antibodies: Goat anti-FXR (cat. no. sc-1204;
1:200; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), mouse
anti-PEPCK (cat. no. sc-271204; 1:200; Santa Cruz Biotechnology,
Inc.), goat anti-G6Pase (cat. no. sc-33839; 1:200; Santa Cruz
Biotechnology, Inc.), mouse anti-SHP (cat. no. sc-271470; 1:200;
Santa Cruz Biotechnology, Inc.), rabbit anti-PGC-1α (cat. no.
ab-54481; 1:1,500; Abcam, Cambridge, UK) or rabbit actin (cat. no.
sc-1616R; 1:2,000; Santa Cruz Biotechnology, Inc.) polyclonal
antibodies. Following being washed with phosphate buffered-saline
with Tween 20 (PBST; 5 min, three times), the membranes were then
incubated with corresponding HRP-conjugated goat anti-mouse IgG
(cat. no. 074-1806) goat anti-rabbit IgG (cat. no. 074-1506) and
biotinylated goat anti-rat IgG (cat. no. 71-00-31) secondary
antibodies (1:3,000 dilution; KPL, Inc., Gaithersburg, MD, USA).
Subsequently, the membranes were washed with PBST again (5 min,
three times). The protein bands were visualized using enhanced
chemiluminescence reagents (Millipore) and analyzed with Quantity
One Software (version 4.62; Bio-Rad Laboratories, Inc., Hercules,
CA, USA) (24). The membranes used
in the western blot assays were re-probed with rabbit anti-β-actin
polyclonal antibody (Santa Cruz Biotechnology, Inc.) to confirm
equal loading of proteins for each sample.
Statistical analysis
All results are expressed as the mean ± standard
error of the mean. The results were analyzed using either a
two-tailed Student's t-test or one-way analysis of variance,
followed by a Newman-Keuls post-hoc test. Statistical analyses were
performed using Prism 4 (GraphPad software, Inc., La Jolla, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of FXR activation on blood
biomedical parameters in T2DM rats
Body weight, blood TG levels, fasting insulin
levels, fasting glucose levels and 2 h postprandial plasma glucose
levels in the T2DM rats were significantly higher, compared with
those in the rats fed regular diets (Fig. 1A–E). CDCA treatment had no effect
on body weight gain (Fig. 1A).
However, CDCA treatment decreased the levels of blood TG (Fig. 1B), fasting insulin (Fig. 1C), fasting glucose (Fig. 1D) and 2 h postprandial plasma
glucose (Fig. 1E). These results
indicated that FXR activation improves glucose and lipid metabolism
in the T2DM rats.
Effects of FXR activation on the
expression levels of FXR in liver tissue samples
The mRNA and protein expression levels of FXR were
significantly lower in the liver tissues of the T2DM rats, compared
with those of the control rats (Fig.
2). FXR activation by CDCA treatment significantly increased
the mRNA and protein expression levels of FXR in the liver tissue
samples of rats with T2DM (Fig.
2).
Effects of FXR activation on hepatic
gluconeogenesis in the liver
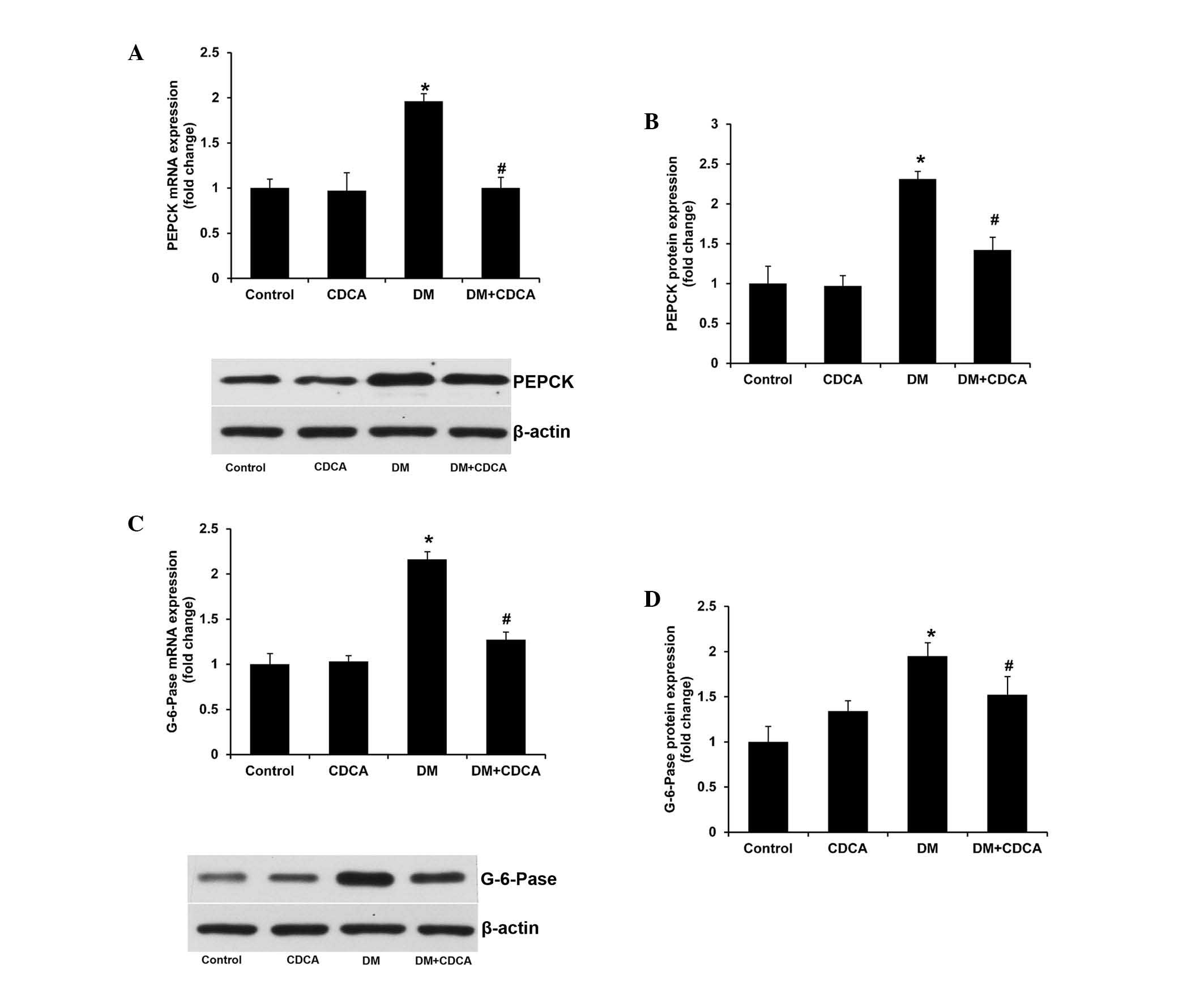
PEPCK and G6Pase are two important enzymes
catalyzing the rate-limiting steps of hepatic gluconeogenesis
(25). The mRNA and protein
expression levels of PEPCK and G6Pase were significantly increased
in the liver tissue samples of the T2DM rats (Fig. 3). FXR activation lowered the
upregulated expression levels of PEPCK and G6Pase in the T2DM rats
(Fig. 3). These results indicated
that CDCA treatment repressed hepatic gluconeogensis in
diabetes.
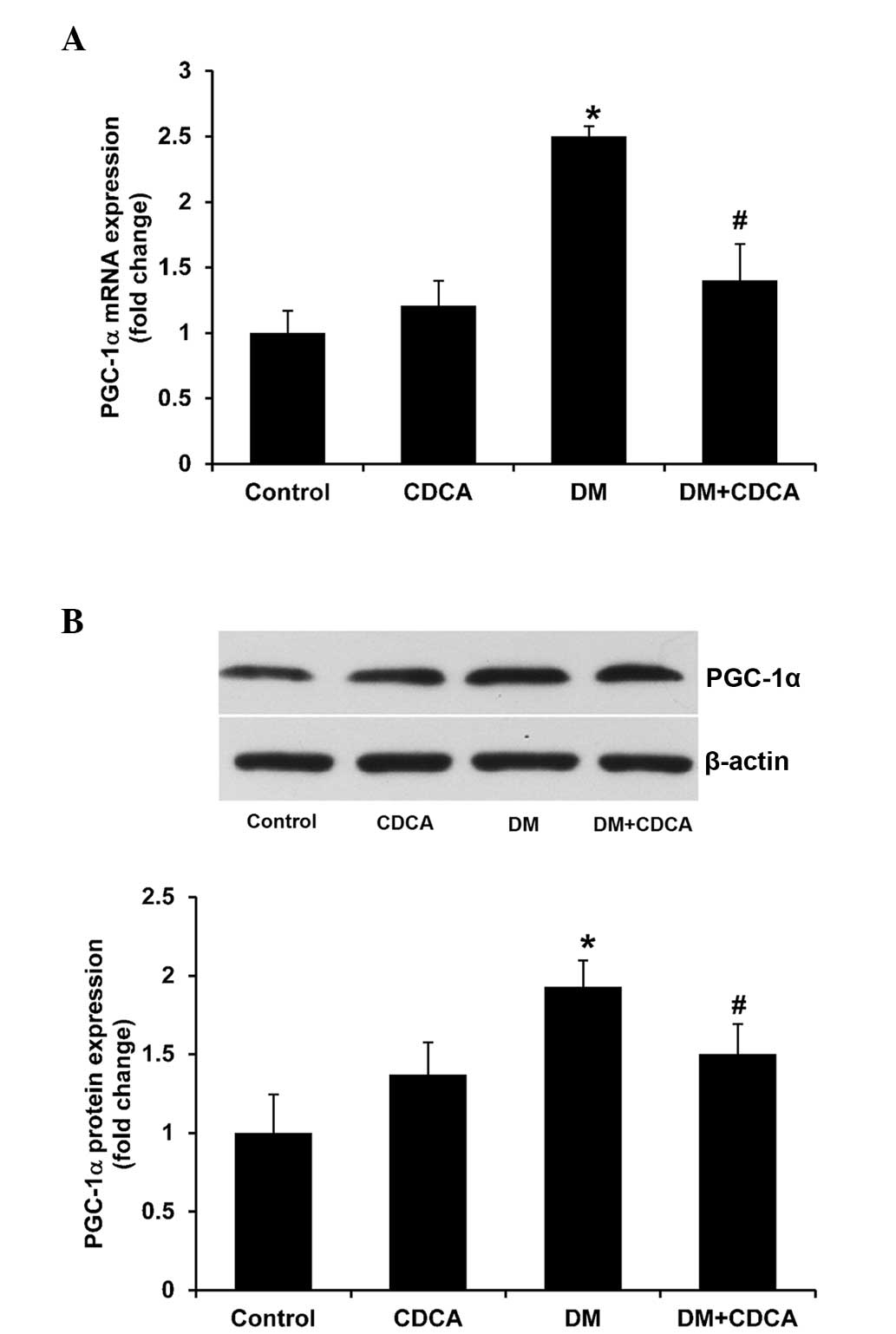
Effects of FXR activation on
FXR-associated gene expression levels in the liver
PGC-1α and SHP are two FXR-associated genes, and
PGC-1α activates FXR-mediated transcription in a ligand-dependent
manner (26), whereas the nuclear
receptor, SHP, is a target of FXR (27). In the present study, the mRNA and
protein expression levels of PGC-1α (Fig. 4) were significantly higher in the
liver tissues of the T2DM rats, compared with those in the control
rats, and these expression levels were significantly decreased
following treatment with CDCA (Fig.
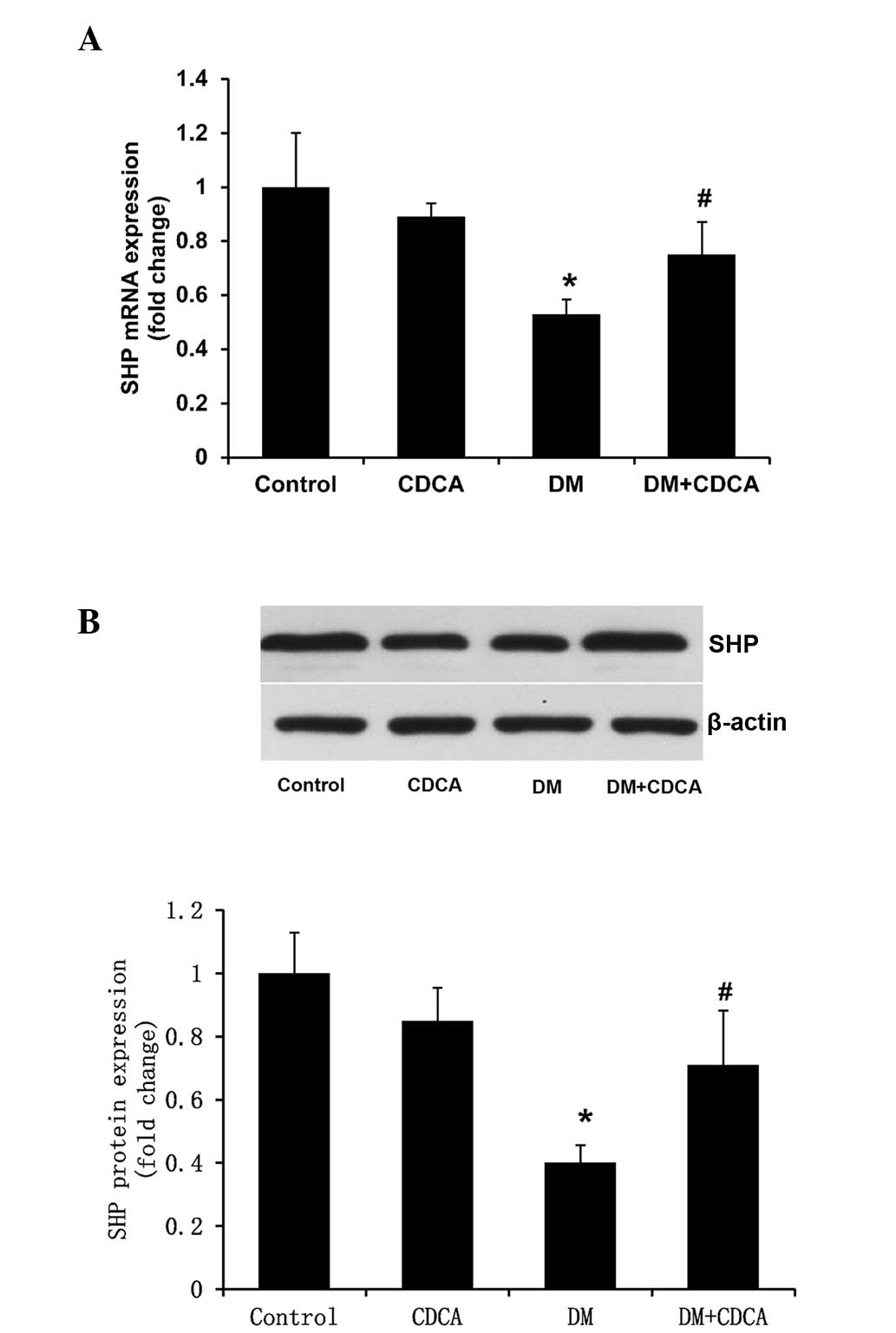
4). Notably, the mRNA and protein expression levels of SHP in
the liver tissues of the T2DM rats were significantly lower,
compared with those in the control rats (Fig. 5), and treatment with CDCA partly
reversed this decreased expression (Fig. 5).
Discussion
The present study examined the effects of FXR
activation by CDCA in a rat diabetic model. The results confirmed
that FXR activation lowered body weight, and levels of blood
glucose and insulin in the rats. In addition, FXR activation
partially inhibited the downregulation in the mRNA and protein
expression of FXR in T2DM rats. Furthermore, the upregulated
expression levels of PEPCK, G6Pase and PGC-1α, as well as the
downregulated expression of SHP in the liver tissues of the T2DM
rats were partly reversed by FXR activation. These results
supported the hypothesis that the activation of FXR is beneficial
in diabetes treatment.
T2DM is one of the most prevalent endocrine
disorders in developing and developed countries. Despite advances
in understanding of the pathophysiology of T2DM, and substantial
progress in efforts to control T2DM, the global burden of this
disease remains high (28).
Several novel therapeutic strategies have been developed. Novel
therapeutic targets include glucagon-like peptide-1, dipeptidyl
peptidase-4 and sodium-glucose co-transporter 2 inhibitors
(29). However, there remains a
requirement to identify novel molecules, which are more specific
and effective against T2DM. The primary aim of the present study
was to determine whether CDCA, a synthetic FXR-specific agonist, is
able to reduce hyperglycemia and hyperinsulinemia in rats with
T2DM, which was induced by a HFD for 8 weeks and a low-dose STZ
injection. Administration of CDCA significantly decreased levels of
fasting and post-prandial plasma glucose, TG concentrations, and
insulin levels in the rats with T2DM. RT-qPCR and western blotting
demonstrated that CDCA treatment partly reversed the abnormal mRNA
and protein expression levels of FXR, G6Pase, SHP and PGC-1α in the
T2DM rats.
The beneficial effects of FXR have been reported in
numerous studies. Activation of FXR by GW4064 or hepatic
overexpression of constitutively active FXR significantly lowered
blood glucose levels in obese db/db and wild-type
mice (14). Treatment with GW4064
also improves whole body insulin resistance in obese
ob/ob mice in vivo (16). The activation of FXR also promotes
insulin sensitivity in the liver and skeletal muscles (30,31).
However, other studies did not report concordant results. Prawitt
et al (17) demonstrated
that the deletion of FXR improved adipose tissue, but not hepatic
insulin sensitivity, in ob/ob mice, suggesting that
FXR deficiency, not activation, beneficially affects body weight
and glucose homeostasis in obesity. Furthermore, the previous study
demonstrated that total, but not liver-specific, FXR deficiency
protects from diet-induced obesity and insulin resistance (17), indicating that liver FXR may not be
important for glucose and lipid metabolism in obesity. Another
investigation also reported that FXR knockout mice gained less body
weight in an ob/ob mice background (18), indicating that loss of FXR prevents
diet-induced or genetic obesity, and accelerates liver
carcinogenesis under diabetic conditions. Furthermore, in a
vertical sleeve gastrectomy model, Ryan et al (32) reported that the preoperative weight
of wild-type mice was already markedly higher, compared with that
of FXR-deficient mice, and that sham-operated wild-type mice gained
an additional 10 g during the experiment, whereas sham-operated
FXR-deficient mice remained at preoperative weight. This suggested
that FXR exerts a detrimental effect on the control of body weight
in diabetes. In the present study, the results demonstrated that
FXR activation led to reduced body weight gain, lower blood
glucose/insulin levels and improved metabolism in a T2DM rat model
established by a HFD and a single injection of STZ. These data
support the beneficial effect of FXR activation in diabetes.
Previous studies have indicated that PGC-1α, G6Pase,
SHP and PEPCK may be involved in the beneficial effects of FXR
agonist in different animal models (33,34).
PGC-1α may be one of the most critical metabolic switches in
diabetes (35), obesity (36) and exercise-inducing effects
(37,38). Wang et al (39) reported that the orphan nuclear
receptor, SHP, represses the promoter activity of PGC-1α. and
PGC-1α has also been demonstrated to be closely associated with the
gluconeogenic gene, PEPCK, and G6Pase (34,40).
Therefore, a reduction in the levels of FXR in T2DM may inhibit the
expression of SHP and PGC-1a, resulting in an increase in PEPCK and
G6Pase activities. However, the direct effect of FXR activation on
expression levels of PGC-1α, G6Pase, SHP and PEPCK in the liver has
not been reported. The results of the RT-qPCR and western blotting
in the present study demonstrated that FXR activation by CDCA
downregulated the expression levels of PGC-1α, G6Pase and PEPCK,
and upregulated the expression of SHP in liver tissues. Deficits of
PGC-1α, G6Pase and PEPCK may be an important underlying cause of
insulin resistance (41). In
addition, the overexpression of SHP recovered impaired
glucose-stimulated insulin secretion (42). Therefore, the reversal of changes
to the expression levels of PGC-1α, G6Pase, PEPCK and SHP by FXR
activation suggested the presence of improved insulin sensitivity
in the rat T2DM model. These results were concordant with a
putative role for these proteins in the pathogenesis and treatment
of T2DM (43), suggesting that the
FXR agonist is able to attenuate the development of insulin
resistance and T2DM in the rat model.
In conclusion, the results of the present study
demonstrated that the FXR agonist reduced blood glucose/insulin
levels and partly inhibited the changes in the expression of
metabolism-associated genes in the liver tissue s of T2DM rats.
These results may provide additional evidence to support the
current hypothesis that FXR agonists may be useful in the treatment
of T2DM and hypertriglyceridemia.
Acknowledgments
The present study was supported by a grant from the
Hubei Province Young Scientists Fund (grant no. QJX2008-17).
References
|
1
|
Shaw JE, Sicree RA and Zimmet PZ: Global
estimates of the prevalence of diabetes for 2010 and 2030. Diabetes
Res Clin Pract. 87:4–14. 2010. View Article : Google Scholar
|
|
2
|
Tripathy D and Chavez AO: Defects in
insulin secretion and action in the pathogenesis of type 2 diabetes
mellitus. Curr Diab Rep. 10:184–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pugliese G, Solini A, Zoppini G, Fondelli
C, Zerbini G, Vedovato M, Cavalot F, Lamacchia O, Buzzetti R,
Morano S, et al: High prevalence of advanced retinopathy in
patients with type 2 diabetes from the renal insufficiency and
cardiovascular events (RIACE) italian multicenter study. Diabetes
Res Clin Pract. 98:329–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roscioni SS, de Zeeuw D, Hellemons ME,
Mischak H, Zürbig P, Bakker SJ, Gansevoort RT, Reinhard H, Persson
F, Lajer M, et al: A urinary peptide biomarker set predicts
worsening of albuminuria in type 2 diabetes mellitus. Diabetologia.
56:259–267. 2013. View Article : Google Scholar
|
|
5
|
Moosavi SM and Karimi Z: Cooperative
mechanisms involved in chronic antidiuretic response to
bendroflumethiazide in rats with lithium-induced nephrogenic
diabetes insipidus. Acta Physiol Hung. 101:88–102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Look AHEAD Research Group; Wing RR, Bolin
P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM,
Egan CM, Espeland MA, et al: Cardiovascular effects of intensive
lifestyle intervention in type 2 diabetes. N Engl J Med.
369:145–154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Torres-Jacome J, Gallego M,
Rodriguez-Robledo JM, Sanchez-Chapula JA and Casis O: Improvement
of the metabolic status recovers cardiac potassium channel
synthesis in experimental diabetes. Acta Physiol (Oxf).
207:447–459. 2013. View Article : Google Scholar
|
|
8
|
Duckworth W, Abraira C, Moritz T, Reda D,
Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, et
al: Glucose control and vascular complications in veterans with
type 2 diabetes. N Engl J Med. 360:129–139. 2009. View Article : Google Scholar
|
|
9
|
Winzell MS and Ahren B: The high-fat
diet-fed mouse: A model for studying mechanisms and treatment of
impaired glucose tolerance and type 2 diabetes. Diabetes. 53(Suppl
3): S215–S219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haslam DW and James WP: Obesity. Lancet.
366:1197–1209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baptissart M, Vega A, Martinot E, Baron S,
Lobaccaro JM and Volle DH: Farnesoid X receptor alpha: A molecular
link between bile acids and steroid signaling? Cell Mol Life Sci.
70:4511–4526. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lefebvre P, Cariou B, Lien F, Kuipers F
and Staels B: Role of bile acids and bile acid receptors in
metabolic regulation. Physiol Rev. 89:147–191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Duran-Sandoval D, Mautino G, Martin G,
Percevault F, Barbier O, Fruchart JC, Kuipers F and Staels B:
Glucose regulates the expression of the farnesoid X receptor in
liver. Diabetes. 53:890–898. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Lee FY, Barrera G, Lee H, Vales
C, Gonzalez FJ, Willson TM and Edwards PA: Activation of the
nuclear receptor FXR improves hyperglycemia and hyperlipidemia in
diabetic mice. Proc Natl Acad Sci USA. 103:1006–1011. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma K, Saha PK, Chan L and Moore DD:
Farnesoid X receptor is essential for normal glucose homeostasis. J
Clin Invest. 116:1102–1109. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cariou B, van Harmelen K, Duran-Sandoval
D, van Dijk TH, Grefhorst A, Abdelkarim M, Caron S, Torpier G,
Fruchart JC, Gonzalez FJ, et al: The farnesoid X receptor modulates
adiposity and peripheral insulin sensitivity in mice. J Biol Chem.
281:11039–11049. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Prawitt J, Abdelkarim M, Stroeve JH,
Popescu I, Duez H, Velagapudi VR, Dumont J, Bouchaert E, van Dijk
TH, Lucas A, et al: Farnesoid X receptor deficiency improves
glucose homeostasis in mouse models of obesity. Diabetes.
60:1861–1871. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Ge X, Heemstra LA, Chen WD, Xu J,
Smith JL, Ma H, Kasim N, Edwards PA and Novak CM: Loss of FXR
protects against diet-induced obesity and accelerates liver
carcinogenesis in ob/ob mice. Mol Endocrinol. 26:272–280. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th.
National Academies Press; Washington, DC: 2011
|
|
20
|
Wang P, Xu TY, Guan YF, Su DF, Fan GR and
Miao CY: Perivascular adipose tissue-derived visfatin is a vascular
smooth muscle cell growth factor: Role of nicotinamide
mononucleotide. Cardiovasc Res. 81:370–380. 2009. View Article : Google Scholar
|
|
21
|
Wang P, Du H, Zhou CC, Song J, Liu X, Cao
X, Mehta JL, Shi Y, Su DF and Miao CY: Intracellular
NAMPT-NAD+-SIRT1 cascade improves post-ischaemic vascular repair by
modulating Notch signalling in endothelial progenitors. Cardiovasc
Res. 104:477–488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee J, Hong SW, Park SE, Rhee EJ, Park CY,
Oh KW, Park SW and Lee WY: Exendin-4 regulates lipid metabolism and
fibroblast growth factor 21 in hepatic steatosis. Metabolism.
63:1041–1048. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang P, Xu TY, Guan YF, Tian WW, Viollet
B, Rui YC, Zhai QW, Su DF and Miao CY: Nicotinamide
phosphoribosyltransferase protects against ischemic stroke through
SIRT1-dependent adenosine monophosphate-activated kinase pathway.
Ann Neurol. 69:360–374. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang P, Xu TY, Wei K, Guan YF, Wang X, Xu
H, Su DF, Pei G and Miao CY: ARRB1/β-arrestin-1 mediates
neuroprotection through coordination of BECN1-dependent autophagy
in cerebral ischemia. Autophagy. 10:1535–1548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bechmann LP, Hannivoort RA, Gerken G,
Hotamisligil GS, Trauner M and Canbay A: The interaction of hepatic
lipid and glucose metabolism in liver diseases. J Hepatol.
56:952–964. 2012. View Article : Google Scholar
|
|
26
|
Kanaya E, Shiraki T and Jingami H: The
nuclear bile acid receptor FXR is activated by PGC-1alpha in a
ligand-dependent manner. Biochem J. 382:913–921. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goodwin B, Jones SA, Price RR, Watson MA,
McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, et al:
A regulatory cascade of the nuclear receptors FXR, SHP-1 and LRH-1
represses bile acid biosynthesis. Mol Cell. 6:517–526. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim Y, Keogh J and Clifton P: A review of
potential metabolic etiologies of the observed association between
red meat consumption and development of type 2 diabetes mellitus.
Metabolism. 64:768–779. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Inzucchi SE, Bergenstal RM, Buse JB,
Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R and
Matthews DR: Management of hyperglycemia in type 2 diabetes, 2015:
A patient-centered approach: Update to a position statement of the
American diabetes association and the european association for the
study of diabetes. Diabetes Care. 38:140–149. 2015. View Article : Google Scholar
|
|
30
|
Cipriani S, Mencarelli A, Palladino G and
Fiorucci S: FXR activation reverses insulin resistance and lipid
abnormalities and protects against liver steatosis in Zucker
(fa/fa) obese rats. J Lipid Res. 51:771–784. 2010. View Article : Google Scholar :
|
|
31
|
Mencarelli A, Cipriani S, Renga B, D'Amore
C, Palladino G, Distrutti E, Baldelli F and Fiorucci S: FXR
activation improves myocardial fatty acid metabolism in a rodent
model of obesity-driven cardiotoxicity. Nutr Metab Cardiovasc Dis.
23:94–101. 2013. View Article : Google Scholar
|
|
32
|
Ryan KK, Tremaroli V, Clemmensen C,
Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE,
Sandoval DA, Kohli R, Bäckhed F and Seeley RJ: FXR is a molecular
target for the effects of vertical sleeve gastrectomy. Nature.
509:183–188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barthel A and Schmoll D: Novel concepts in
insulin regulation of hepatic gluconeogenesis. Am J Physiol
Endocrinol Metab. 285:E685–E692. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee J, Padhye A, Sharma A, Song G, Miao J,
Mo YY, Wang L and Kemper JK: A pathway involving farnesoid X
receptor and small heterodimer partner positively regulates hepatic
sirtuin 1 levels via microRNA-34a inhibition. J Biol Chem.
285:12604–12611. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Haase TN, Ringholm S, Leick L, Biensø RS,
Kiilerich K, Johansen S, Nielsen MM, Wojtaszewski JF, Hidalgo J,
Pedersen PA and Pilegaard H: Role of PGC-1α in exercise and
fasting-induced adaptations in mouse liver. Am J Physiol Regul
Integr Comp Physiol. 301:R1501–R1509. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang LN, Zhou HY, Fu YY, Li YY, Wu F, Gu
M, Wu LY, Xia CM, Dong TC, Li JY, et al: Novel small-molecule
PGC-1α transcriptional regulator with beneficial effects on
diabetic db/db mice. Diabetes. 62:1297–1307. 2013. View Article : Google Scholar :
|
|
37
|
Perry CG: Is muscle hypertrophy following
resistance exercise regulated by truncated splice variants of
PGC-1alpha? Acta Physiol (Oxf). 212:122–124. 2014. View Article : Google Scholar
|
|
38
|
Lundberg TR, Fernandez-Gonzalo R, Norrbom
J, Fischer H, Tesch PA and Gustafsson T: Truncated splice variant
PGC-1α4 is not associated with exercise-induced human muscle
hypertrophy. Acta Physiol (Oxf). 212:142–151. 2014. View Article : Google Scholar
|
|
39
|
Wang L, Liu J, Saha P, Huang J, Chan L,
Spiegelman B and Moore DD: The orphan nuclear receptor SHP
regulates PGC-1alpha expression and energy production in brown
adipocytes. Cell Metab. 2:227–238. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Park MJ, Kong HJ, Kim HY, Kim HH, Kim JH
and Cheong JH: Transcriptional repression of the gluconeogenic gene
PEPCK by the orphan nuclear receptor SHP through inhibitory
interaction with C/EBPalpha. Biochem J. 402:567–574. 2007.
View Article : Google Scholar :
|
|
41
|
Schinner S, Scherbaum WA, Bornstein SR and
Barthel A: Molecular mechanisms of insulin resistance. Diabet Med.
22:674–682. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Suh YH, Kim SY, Lee HY, Jang BC, Bae JH,
Sohn JN, Bae JH, Suh SI, Park JW, Lee KU and Song DK:
Overexpression of short heterodimer partner recovers impaired
glucose-stimulated insulin secretion of pancreatic beta-cells
overexpressing UCP2. J Endocrinol. 183:133–144. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Teodoro JS, Rolo AP and Palmeira CM:
Hepatic FXR: Key regulator of whole-body energy metabolism. Trends
Endocrinol Metab. 22:458–466. 2011. View Article : Google Scholar : PubMed/NCBI
|