Introduction
Bladder cancer is one of the most common tumor types
of the urinary system. However, its etiopathogenisis has remained
to be fully elucidated. Study of the multifocal and polyclonal
origins of bladder cancer as well as various experiments and
epidemiological studies have shown that the persistence of
carcinogenic substances in urine is an important cause of bladder
cancer and also a major reason for bladder tumor recurrence after
treatment (1–3).
The activation of numerous small guanine
triphosphatases (GTPases) of the Ras superfamily is a crucial step
in the regulation of a variety of cellular processes via complex
cellular signaling networks. Phosphatidylinositol-specific
phospholipase C (PLC) has emerged as one of the important signaling
nodes in these complex networks, acting as a target as well as a
regulator of small GTPases. Six major families of PLC (PLCβ, PLCγ,
PLCδ, PLCε, PLCζ and PLCη) are known (4), which are characterized by regulatory
regions unique to each family. PLCε is a recent addition to the
growing list of Ras-associated effectors (5). Most suggested Ras effectors show
either kinase or guanine nucleotide exchange factor activity.
However, Ras has been implicated in regulating another class of
enzyme, namely phosphoinositide-specific PLC, which is involved in
the generation of well-characterized secondary messengers.
Studies have demonstrated that PLCε is an effector
of the Ras family of small GTPases, which bind directly to the
Ras-associated domain of PLCε (6–9).
Further studies have revealed that PLCε is also activated by small
GTPase Ras homolog gene family, member A as well as heterotrimeric
G proteins Gα12 and Gβ1γ2 (9,10).
Via these multiple regulatory mechanisms, PLCε mediates signals
originating from a large variety of cell surface receptors
(11,12). In addition, PLCε exerts a function
as a guanine nucleotide exchange factor for Ras-related protein 1
(Rap1) via its CDC25 homology domain (13). Mice homozygous regarding the
functionally inactivated PLCε allele (PLCε−/− mice)
exhibit semilunar valvulogenetic defects, which lead to cardiac
dilation (14–16). Bai et al (17) demonstrated that PLCε−/−
mice were resistant to 7,12-dimethylbenz(a)anthracene-induced skin
tumor formation with 12-O-tetradecanoylphorbol-13-acetate (TPA) as
a tumor promoter. In addition, PLCε−/− mice were void of
basal layer cell proliferation and epidermal hyperplasia,
suggesting the role of PLCε in tumor promotion.
The Ras gene is one of the most common genes
associated with bladder cancer. It has been confirmed that PLCε is
an effector of Ras and is in turn regulated by Ras in a
GTP-dependent manner. The association between bladder transitional
cell carcinoma and PLCε has not been fully elucidated. The present
study hypothesized that PLCε has a significant role in the
development of bladder transitional cell carcinoma. In order to
verify this hypothesis, the effects of PLCε knockdown on
N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN)-mediated
induction of bladder cancer were investigated in mice. BBN is able
to induce bladder cancer by targeting PLCε and activating Ras to
drive the clonal expansion of initiated cells (18). The present study suggested that
PLCε has a crucial role in the development of cancer downstream of
Ras signaling.
Materials and methods
Animals
PLCε−/− mice were obtained from the
Laboratory of Experimental Molecular Biology at Kobe University
(Kobe, Japan) and maintained at the 302 Hospital of the Chinese
People's Liberation Army (Beijing, China). PLCε−/−
homozygous mice and wild-type (PLCε+/+) littermates were
obtained by crossing C57BL/6J PLCε+/− and 129S4
PLCε+/− mice. Mice carrying inactivated PLC allele
(PLCε−) were generated using in-frame deletion of an
exon encoding the catalytic X domain, as described previously
(14). All animals were housed
under standard conditions (22±1°C; 12-h light/dark cycle; 50–55%
humidity) with free access to food pellets and tap water.
Experiments were performed on 6–8 week-old male mice, and a total
of 72 PLCε+/− and 72 PLCε+/− mice were used
in the present study. The mean weight of the animals was 20.3±0.1 g
for the PLCe+/+ mice and 20.3±0.15 g for the
PLCe−/− mice. Disparate groups of mice were
used in each experiment. Animal experiments were approved by the
Animal Care and Use Committee of The 463 hospital of Chinese
People's Liberation Army (Shenyang, China).
Materials
Antibodies used included anti-mouse PLCε antibody
(Dako Cytomation, Copenhagen, Denmark), rabbit anti-mouse
polyclonal anti-vascular endothelial growth factor-A (VEGF-A)
antibody (cat. no. ab51745; Abcam, Cambridge, MA, USA), rabbit
anti-mouse poly-clonal anti-GAPDH antibody (cat. no. sc-25778;
Santa Cruz Biotechnologies, Dallas, TX, USA), rabbit anti-mouse
anti-cyclooxygenase-2 (COX-2) antibody (cat. no. 160126; Cayman,
Ann Arbor, MI, USA) and goat anti-rabbit immunoglobulin
G-horseradish peroxidase conjugated secondary antibodies (Santa
Cruz Biotechnologies).
BBN-induced mouse model of bladder
cancer
A BBN-induced mouse model of bladder cancer was
prepared according to the method described by Vecchione et
al (19) with certain
modifications. Briefly, six-week-old male
PLCε−/− mice (n=72) and
PLCε+/+ mice (n=72) were sub-divided into BBN
treatment groups (n=48) and control groups (n=24) without treatment
of BBN (Table I). BBN-treated mice
were given tap water containing 0.1% BBN for 12 weeks. Thereafter,
they had access to tap water without BBN. Control mice were given
water without BBN throughout the experiment. Mice were sacrificed
at 8, 12 and 18 weeks after the cessation of BBN treatment. Bladder
specimens were harvested and analyzed for pathology
[hematoxylin-eosin (HE; Beyotime Institute of Biotechnology,
Shanghai, China) staining and ultrastructural assessment] and
protein (western blot and immunofluorescence).
 | Table IStatistics of control mice and mouse
models of bladder cancer. |
Table I
Statistics of control mice and mouse
models of bladder cancer.
| Group | Type | Treatment | Normal n (%) | Atypical
hyperplasia, n (%) | Transitional cell
carcinoma, n (%) | Dead animals
(n) | Surviving animals
(n) |
|---|
| A |
PLCε+/+ | Control, none | 24 (100) | 0 (0) | 0 (0) | 1 | 23 |
| B |
PLCε+/+ | BBN (0.1 %)a | 8 (18.18) | 8 (18.18) | 28 (63.64) | 4 | 44 |
| C |
PLCε−/− | Control, none | 24 (100) | 0 (0) | 0 (0) | 0 | 24 |
| D |
PLCε−/− | BBN (0.1 %)a | 12 (26.09) | 14 (30.43) | 20 (43.48) | 2 | 46 |
Pathological analysis
The mice were anesthetized with sodium
pentobarbitone (40 mg/kg, i.p.; Sigma-Aldrich, St. Louis, MO, USA)
and then transcardially perfused with 10 ml 0.9% saline, followed
by 30 ml 0.1 M phosphate buffer (PB; pH 7.4) containing 4%
paraformaldehyde for 5 min. At necropsy, urinary bladders were
removed and placed in 4% paraformaldehyde for HE staining
immunofluorescence or in 2.5% glutaraldehyde (Sigma-Aldrich) for
ultrastructural study. For HE staining, each bladder was dissected,
processed for routine paraffin embedding, cut into 5 µm
sections and mounted onto polylysine-coated slides. Sections were
de-waxed in xylene (Sinopharm, Shanghai, China), re-hydrated in a
descending series of ethanols and processed for routine HE. For
ultrastructural study, sections were washed with 0.01 M
phosphate-buffered saline (PBS), post-fixed with 10 g/l
OsO4 in PB for 45 min, de-hydrated through a graded
ethanol series and propylene oxide, and flat-embedded with Epon-812
(Nanjing Tansi Technology Co., Ltd., Nanjing, China). The sections
were examined under a light microscope (AE31; Motic, Xiamen, China)
and regions containing all layers of the bladder were investigated
under an electron microscope. Tissue samples from the selected
regions were cut into sections on an ultramicrotome (EM UC7; Leica,
Wetzlar, Germany) and prepared for electron microscopic analysis
(H-7100; Hitachi, Tokyo, Japan).
Western blot analysis
Protein was extracted from each sample using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology). The concentrations of proteins were detected using
the bicinchoninic acid assay (Beyotime Institute of Biotechnology).
Protein samples were separated by 10% SDS-PAGE and then transferred
onto nitrocellulose membranes. Then membranes were blocked with 5%
non-fat milk followed by incubation with antibodies against COX2
(1:500), VEGF-A (1:5,000) and GAPDH (1:1,000), respectively, at
37°C for another 1.5 h. Membranes were subsequently washed with
Tris-buffered saline containing Tween 20 (TBST) and incubated with
the corresponding secondary antibodies conjugated with horseradish
peroxidase (1:5,000) for 45 min at 37°C. Following washing with
TBST, a RapidStep™ ECL reagent (Millipore, Bedford, MA, USA) and
x-ray film were used to capture images of the blots.
Immunofluorescence
Detection of PLCε, COX2 and VEGF-A was performed on
consecutive sections obtained from paraffin-embedded tissues using
an immunofluorescence double labeling method. Sections were dried
at room temperature, de-paraffinized, re-hydrated and then treated
with 2% hydrogen peroxide at 37°C for 5 min. Antigen retrieval was
performed by pepsin (Biosharp, Hefei, China) treatment at 40°C for
10 min. After blocking with 5% bovine serum albumin, the sections
were incubated with anti-PLCε and COX2 (1:200) or VEGF-A antibody
(1:500) overnight at 4°C. Sections were washed with 0.01 M PBS and
incubated with fluorescein isothiocyanate-labeled anti-goat
immunoglobulin (Ig)G antibody (1:500; Sigma-Aldrich) for PLCε and
Texas Red-labeled anti-rabbit IgG antibody (1:1,000; Molecular
Probes, Breda, Netherlands) for COX2 and VEGF-A at 37°C for 2 h.
The sections were thoroughly rinsed in 0.01 M PBS between and after
the incubation steps. The sections were examined using confocal
laser-scanning microscopy (FV1000; Olympus, Tokyo, Japan). Digital
images were captured using EZ-C1 3.50 software (Nikon).
Statistical analysis
All statistical analyses were performed using SPSS
10.0 software (SPSS, Inc., Chicago, IL, USA). The Kaplan-Meier test
was used to analyze survival data. The incidence and multiplicity
of urinary bladder cancer were analyzed by the Fisher's exact and
Wilcoxon's rank-sum tests, respectively. Each experiment was
repeated three times and representative results are presented.
Results
General observations
In the present study, one hundred and forty-four
mice were used, out of which seven died during the experiment (5%;
Group A, 1; group B, 4; group D, 2). These mice showed no
significant difference from others in weight and activity levels
prior to their death. A higher susceptibility of PLCε+/+
mice to BBN-induced tumor development was observed. The tumors of
PLCε−/− mice were smaller than those of
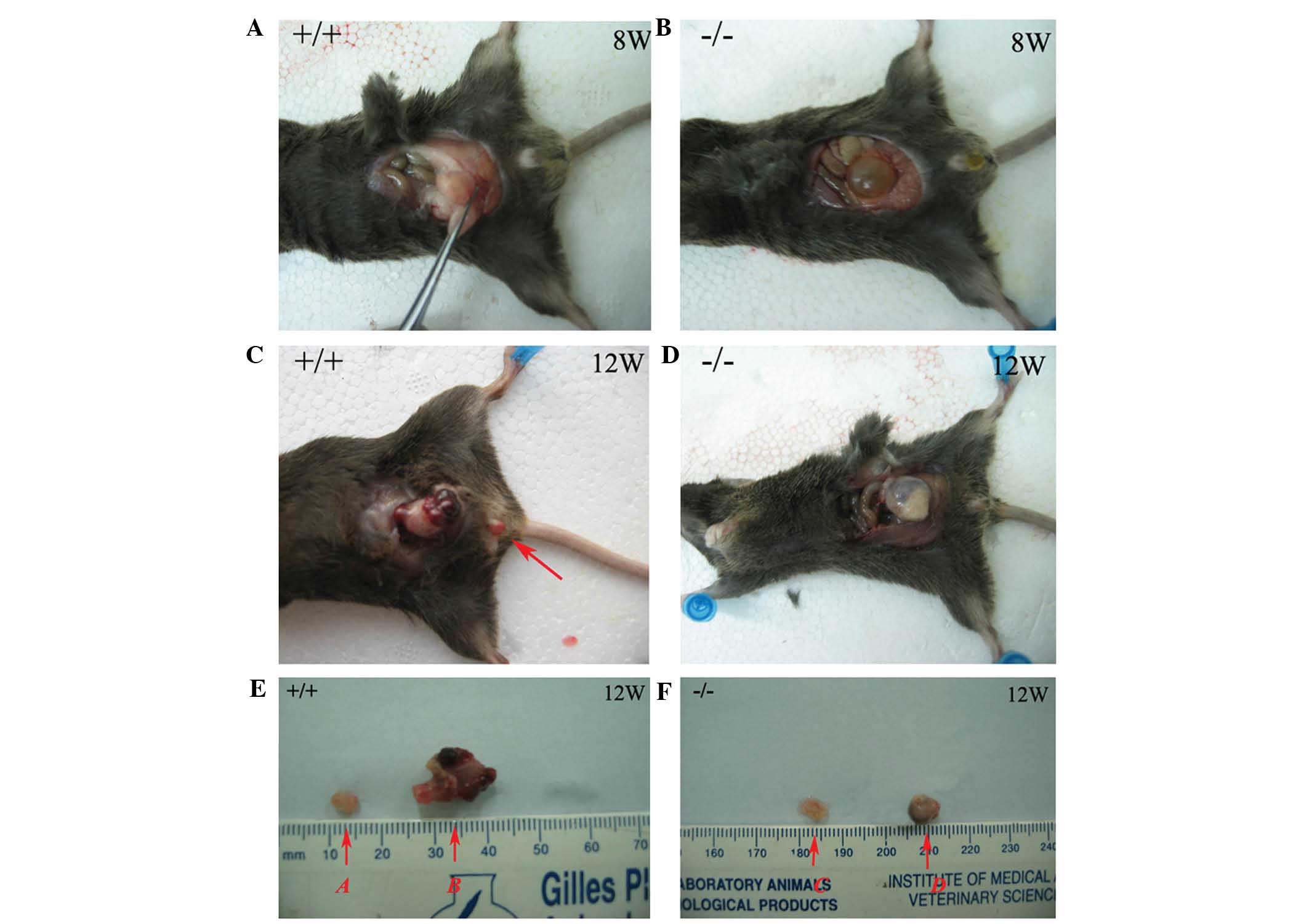
PLCε+/+ mice at all time-points examined (Fig. 1A–D). In Group B, eight mice
(18.18%) had normal urinary bladder mucous membranes, eight mice
(18.18%) showed atypical hyperplasia and 63.6% (28/44) presented
with neoplastic lesions (with in situ or invasive
carcinoma), and in group D, these numbers were 26.09% (12/46),
30.43% (14/46) and 43.5% (20/46), respectively (Table I). Pre-neoplastic lesions in the
urothelium adjacent to advanced tumors were frequently observed. By
contrast, in the control groups A and C, atypical hyperplasia and
neoplastic lesions were not observed. The size of the tumors in
group B was significantly larger than that in group D (Fig. 1E and F). No significant differences
were observed between groups A and C.
Pathological changes
In the present study, pathological changes in the
development of BBN-induced bladder carcinoma in PLCε−/−
and PLCε+/+ mice were monitored. The following
pathological changes were observed by light microscopy: Smooth
mucosa of bladder walls were present in groups A and C without the
presence of ulcers, congestion or neoplasms (Fig. 2A and B). The morphology of the
mucosa of the bladder walls in groups B and D changed gradually
with increasing time of BBN intake. In general, tumor formation in
PLCε−/− mice was delayed and its incidence was reduced
compared with that in PLCε+/+ mice. Electron microscopy
further confirmed the morphological findings in the experimental
groups: While no differences in morphology of the bladder
transitional epithelium were observed between groups A and C
(Fig. 2E and F), the following
pathological changes were visible in group B at week 12: Chromatin
was distributed across the nuclear membrane, nucleolar hypertrophy
was present and fiber structure around the nuclei was disordered
(Fig. 2G). However, heterogenous
transitional cells were observed in the mucosa of mice in group D
at week 12 (Fig. 2H). All of the
above results demonstrated that knockout of PLCε attenuated
BBN-induced bladder tumorigenesis.
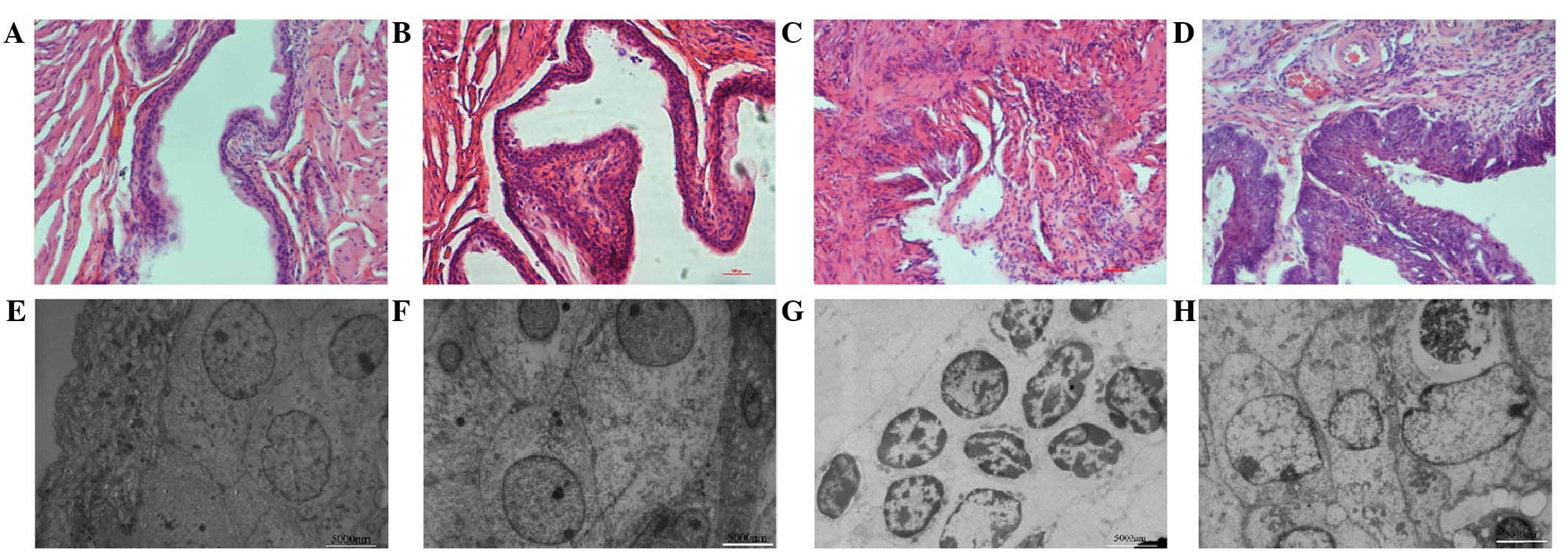 | Figure 2Representative histological HE
staining images and electron micrographs of bladder mucosa of the
mice in the experimental groups at 12 weeks. HE staining of normal
bladder mucosa in (A) group A and (B) group C. (C) In group B, HE
staining revealed significantly heterogenous bladder mucosa. (D) HE
staining of bladder mucosa in group D, revealing thickened,
carcinomatous bladder mucosa (magnification, ×200). Ultrastructures
of bladder mucosa of mice in (E) group A and (F) group C. (G)
Ultrastructure of transitional epithelial membrane cells of mice of
group B. The chromatin was distributed across the nuclear membrane,
nucleolar hypertrophy was observed and fiber structure around the
nuclei could not be distinguished. (H) Ultrastructure of bladder
mucosa of mice in group D, revealing significantly heterogenous
transitional cells in the mucosa. Scale bar, 5000 nm. Groups: A,
untreated PLCε+/+ control mice; B, PLCε+/+
mice induced with 0.1% BBN; C, untreated PLCε−/− mice;
D, PLCε−/− mice induced with 0.1% BBN. BBN,
N-butyl-N-(4-hydroxybutyl) nitrosamine; PLC,
phospholipase C; HE, hematoxylin and eosin. |
Role of PLCε in BBN-induced expression of
inflammatory and angiogenesis-associated molecules
In order to investigate the underlying mechanisms of
the involvement of PLCε in tumor development, the present study
examined the expression of representative inflammatory and
angiogenesis-associated proteins in tumors from the bladders of
mice in groups B and D. Previous studies have revealed that PLCε is
important in TPA-induced skin inflammation and tumor promotion
(17,20). Inflammation is known to exert its
highest effects on tumorigenesis in the later stage, in which
tumors progress to high-grade adenomas (21–23).
Therefore, the present study investigated the role of PLCε in
inflammation associated with late-stage bladder tumorigenesis. In
grade-matched tumors from PLCε+/+ and PLCε−/−
mice at week 18, the expression of COX-2 and VEGF-A was assessed;
these proteins were selected to be analyzed as representative
signaling molecules due to their intrinsic functions in
inflammation and angiogenesis, respectively, and because they have
been implicated in bladder tumorigenesis (24). Western blot analysis revealed that
the expression levels of COX-2 and VEGF-A were influenced by the
PLCε background of the mice, with upregulation in the BBN-treated
PLCε+/+ mice, but not in BBN-treated PLCε−/−
mice (Fig. 3). In addition, the
expression of COX-2 and VEGF-A was detected by immunofluorescence
analysis. Groups A and B showed stronger green fluorescence (PLCε)
than groups C and D, which was consistent with their genetic
backgrounds. Consistent with the western blot results, marked
increased in the VEGF-A signals in tumor epithelial cells of
high-grade adenomas in PLCε+/+ mice were observed, which
were less apparent in PLCε−/− mice (Fig. 4A). The expression patterns of COX-2
were similar to those of VEGF-A, with increased expression in
groups B and D (Fig. 4B). However,
a locally elevated level of COX-2 was also identified. These
results demonstrated that the protective role of PLCε knockout
against bladder carcinogenesis may be associated with inflammatory
responses and angiogenesis.
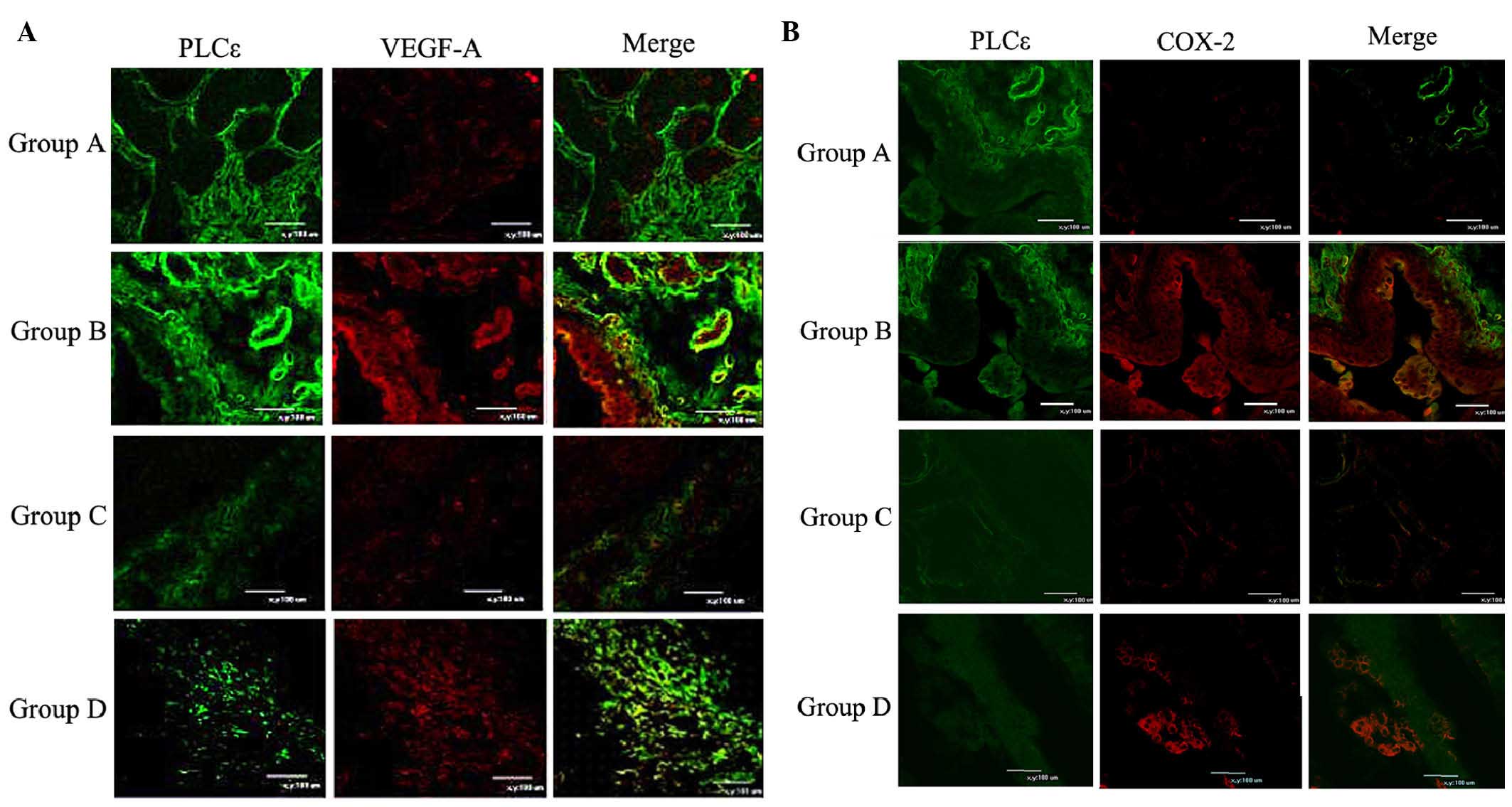 | Figure 4Representative immunofluorescence
images. (A) Expression of VEGF-A in the bladder mucosa of mice in
Groups A, B, C and D at week 18. Green fluorescence, PLCε; red
fluorescence, VEGF-A. (B) Expression of COX-2 in the bladder mucosa
of mice in Groups A, B, C and D at week 18. Green fluorescence,
PLCε; red fluorescence, COX-2. Scale bar, 100 µm. Groups: A,
untreated PLCε+/+ control mice; B, PLCε+/+
mice induced with 0.1% BBN; C, untreated PLCε−/− mice;
D, PLCε−/− mice induced with 0.1% BBN. BBN,
N-butyl-N-(4-hydroxybutyl) nitrosamine; PLC,
phospholipase; C COX, cyclooxygenase; VEGF, vascular endothelial
growth factor. |
Discussion
Numerous chemicals are known to evoke bladder
carcinoma, among which nitroso compounds, including BBN and
N-methyl-N-nitrosourea (MNU), and nitrofuran
compounds, such as
N-[4-(5-nitro-2-furyl)-2-thiazolyl]-formamide (FANFT) are
most potent (25,26). BBN and FANFT are indirect
carcinogens through oral intake, while MNU is a carcinogen
requiring direct bladder instillation. Due to its high potency to
induce bladder cancer, BBN is the most suitable reagent to generate
in vivo models of bladder cancer and to study bladder
carcinogenesis. The carcinogenicity of BBN is limited to the
bladders of rats, mice and dogs (27). No marked difference between the
bladder cancer was observed between humans and mice, and rats and
dogs. Therefore, BBN-induced bladder cancer is similar to
transitional cell carcinoma in patients in both kinetic and
histological features (28). PLCε
is important in the development and progression of human cancer
types (29). The present study
used BBN to induce bladder cancer, and knockout of PLCε attenuated
BBN-induced tumorigenesis of bladder cancer. This indicated that
PLCε is an oncogene and may be a therapeutic target for the
treatment and prevention of bladder cancer. The downstream
metabolite of BBN, N-butyl-N-(3-carboxy-propyl)
nitrosamine (BCPN), is subjected to urinary excretion and comes in
direct contact with the urinary tract, resulting in epithelial cell
DNA damage and carcinogenesis (30). Compared with a high, single dose of
BBN, smaller and multiple doses of BBN induce a higher rate of
bladder cancer with larger tumor size, poorer differentiation and a
higher rate of infiltration.
Bladder carcinoma is the most common tumor type of
the urinary system. Its occurrence and development involve numerous
genes and processes, as well as congenital and acquired factors
(31–33). Ras was the first oncogene
identified in human bladder carcinoma and has been proved to be
highly relevant to its development (34–36).
PLCε, as a downstream effector protein of Ras, may have an
important role in the occurrence and development of bladder
carcinoma.
PLCε was first identified in Caenorhabditis
elegans by Shibatohge et al (37) in 1998 as a novel sub-type of the
PLC family. PLCε has been reported to act as an effector protein
for the products of the oncogene Ras and the tumor suppressor gene
Rap (5,7,8). In
recent years, the role of PLCε in tumors has received increasing
attention. PLCε−/− mice were successfully established in
Kataoka's laboratory at Kobe University (Kobe, Japan) by Bai et
al (17). In these
PLCε−/− mice, the tumor incidence was significantly
decreased and the progression of chemically induced skin tumors was
inhibited (17), suggesting that
PLCε has an important role in tumor development. Consistent with
the hypothesis of the present study, Bourguignon et al
(38) found that PLCε is involved
in human head and neck squamous cell carcinoma (38); furthermore, Cheng et al
(39) and Ling et al
(40) reported that small hairpin
RNA-mediated knockdown of PLCε inhibited bladder cancer cell
proliferation and cell cycle progression in vitro. PLCε was
also demonstrated to be association with invasion and migration of
bladder cancer (41).
The present study observed almost complete squamous
commitment of the neoplastic lesions. After 18 weeks of BBN
treatment, 43.48% of PLCε-knockout mice developed invasive bladder
cancer, which was significantly lower than the incidence observed
in wild-type mice. This result is consistent with the notion that
wild-type PLCε mice develop bladder tumors more rapidly and
frequently after chronic intake of BBN than PLCε−/−
mice, further suggesting that the presence of PLCε sensitizes
tissues to carcinogenesis. The present study also observed that the
incidence of atypical hyperplasia in PLCε−/− mice was
significantly higher than that observed in wild-type mice. This
indicates that wild-type mice are pre-disposed to developing
bladder cancer. Differences in the incidence of bladder tumors
between these groups of mice indicated that urothelial cells
require two intact PLCε alleles in order to respond efficiently to
the damaging action of chemical carcinogens.
PLCε has been demonstrated to be associated with
inflammatory responses (42).
Ikuta et al (20) have
shown that two TPA targets, Ras guanyl-releasing protein 3 and
protein kinase C, are involved in TPA-induced inflammation through
the activation of PLCε, leading to tumor promotion. Li et al
(43) suggested that PLCε has
crucial roles in intestinal tumorigenesis through two distinct
mechanisms - augmentation of angiogenesis and inflammation. In
order to determine whether PLCε has a role in bladder tumorigenesis
through augmentation of angiogenesis and inflammation in
BBN-induced bladder cancer, the present study also examined
inflammatory and angiogenesis-associated factors COX-2 and VEGF-A,
respectively. The results showed that VEGF-A was upregu-lated by
BBN treatment in the tumors of PLCε+/+ mice, while no
marked changes in PLCε−/− mice were observed. COX-2
showed a similar pattern to that of VEGF-A, however, with local
elevation. These results suggested that bladder cancer is induced
by BBN through two distinct mechanisms, augmentation of
angiogenesis and inflammation, and that PLCε has a pivotal role in
tumorigenesis of bladder cancer induced by BBN.
In conclusion, the present study revealed that PLCε
has a crucial role in BBN-induced carcinogenesis of bladder
epithelial cells, as knockout of PLCε attenuated bladder
carcinogenesis induced by BBN. The present study also provided
solid in vivo evidence for the importance of PLCε signaling
in carcinogenesis. These results indicated that specific inhibitors
of PLCε may be useful for the treatment and prevention of certain
types of cancer.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81101938 and
81271316) and the Science and Technology Development Project of
Liaoning Province (no. 2012225020).
References
|
1
|
Beland FA, Beranek DT, Dooley KL, Heflich
RH and Kadlubar FF: Arylamine-DNA adducts in vitro and in vivo:
Their role in bacterial mutagenesis and urinary bladder
carcinogenesis. Environ Health Perspect. 49:125–134. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Akaza H, Murphy WM and Soloway MS: Bladder
cancer induced by noncarcinogenic substances. J Urol. 131:152–155.
1984.PubMed/NCBI
|
|
3
|
Cohen SM: Role of urinary physiology and
chemistry in bladder carcinogenesis. Food Chem Toxicol. 33:715–730.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katan M: New insights into the families of
PLC enzymes: Looking back and going forward. Biochem J. 391:e7–e9.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kelley GG, Reks SE, Ondrako JM and Smrcka
AV: Phospholipase C(epsilon): A novel Ras effector. EMBO J.
20:743–754. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mitin N, Rossman KL and Der CJ: Signaling
interplay in Ras superfamily function. Curr Biol. 15:R563–R574.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lopez I, Mak EC, Ding J, Hamm HE and
Lomasney JW: A novel bifunctional phospholipase c that is regulated
by Galpha 12 and stimulates the Ras/mitogen-activated protein
kinase pathway. J Biol Chem. 276:2758–2765. 2001. View Article : Google Scholar
|
|
8
|
Song C, Hu CD, Masago M, Kariyai K,
Yamawaki-Kataoka Y, Shibatohge M, Wu D, Satoh T and Kataoka T:
Regulation of a novel human phospholipase C, PLCepsilon, through
membrane targeting by Ras. J Biol Chem. 276:2752–2757. 2001.
View Article : Google Scholar
|
|
9
|
Bunney TD and Katan M: Phospholipase C
epsilon: Linking second messengers and small GTPases. Trends Cell
Biol. 16:640–648. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smrcka AV, Brown JH and Holz GG: Role of
phospholipase Cε in physiological phosphoinositide signaling
networks. Cell Signal. 24:1333–1343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Edamatsu H, Satoh T and Kataoka T: Ras and
Rap1 activation of PLCepsilon lipase activity. Methods Enzymol.
407:99–107. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kelley GG, Kaproth-Joslin KA, Reks SE,
Smrcka AV and Wojcikiewicz RJ: G-protein-coupled receptor agonists
activate endogenous phospholipase Cepsilon and phospholipase Cbeta3
in a temporally distinct manner. J Biol Chem. 281:2639–2648. 2006.
View Article : Google Scholar :
|
|
13
|
Satoh T, Edamatsu H and Kataoka T:
Phospholipase Cepsilon guanine nucleotide exchange factor activity
and activation of Rap1. Methods Enzymol. 407:281–290. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tadano M, Edamatsu H, Minamisawa S,
Yokoyama U, Ishikawa Y, Suzuki N, Saito H, Wu D, Masago-Toda M,
Yamawaki-Kataoka Y, et al: Congenital semilunar valvulogenesis
defect in mice deficient in phospholipase C epsilon. Mol Cell Biol.
25:2191–2199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang H, Oestreich EA, Maekawa N, Bullard
TA, Vikstrom KL, Dirksen RT, Kelley GG, Blaxall BC and Smrcka AV:
Phospholipase C epsilon modulates beta-adrenergic
receptor-dependent cardiac contraction and inhibits cardiac
hypertrophy. Circ Res. 97:1305–1313. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kariya K, Bui YK, Gao X, Sternberg PW and
Kataoka T: Phospholipase Cepsilon regulates ovulation in
Caenorhabditis elegans. Dev Biol. 274:201–210. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bai Y, Edamatsu H, Maeda S, Saito H,
Suzuki N, Satoh T and Kataoka T: Crucial role of phospholipase
Cepsilon in chemical carcinogen-induced skin tumor development.
Cancer Res. 64:8808–8810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fujita J, Ohuchi N, Ito N, Reynolds SH,
Yoshida O, Nakayama H and Kitamura Y: Activation of H-ras oncogene
in rat bladder tumors induced by
N-butyl-N-(4-hydroxybutyl)nitro-samine. J Natl Cancer Inst.
80:37–43. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vecchione A, Sevignani C, Giarnieri E,
Zanesi N, Ishii H, Cesari R, Fong LY, Gomella LG, Croce CM and
Baffa R: Inactivation of the FHIT gene favors bladder cancer
development. Clin Cancer Res. 10:7607–7612. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ikuta S, Edamatsu H, Li M, Hu L and
Kataoka T: Crucial role of phospholipase C epsilon in skin
inflammation induced by tumor-promoting phorbol ester. Cancer Res.
68:64–72. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Greten FR, Eckmann L, Greten TF, Park JM,
Li ZW, Egan LJ, Kagnoff MF and Karin M: IKKbeta links inflammation
and tumorigenesis in a mouse model of colitis-associated cancer.
Cell. 118:285–296. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karin M and Greten FR: NF-kappaB: Linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bartoletti R, Cai T, Nesi G, Sardi I and
Rizzo M: Qualitative and quantitative analysis of angiogenetic
factors in transitional cell bladder carcinoma: Relationship with
clinical course at 10 years follow-up. Oncol Rep. 14:251–255.
2005.PubMed/NCBI
|
|
25
|
Cui L, Shi Y, Dai G, Pan H, Chen J, Song
L, Wang S, Chang HC, Sheng H and Wang X: Modification of
N-Methyl-N-Nitrosourea initiated bladder carcinogenesis in Wistar
rats by terephthalic acid. Toxicol Appl Pharmacol. 210:24–31. 2006.
View Article : Google Scholar
|
|
26
|
Ariel I, Ayesh S, Gofrit O, Ayesh B,
Abdul-Ghani R, Pizov G, Smith Y, Sidi AA, Birman T, Schneider T, et
al: Gene expression in the bladder carcinoma rat model. Mol
Carcinog. 41:69–76. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ding J, Xu D, Pan C, Ye M, Kang J, Bai Q
and Qi J: Current animal models of bladder cancer: Awareness of
translatability (Review). Exp Ther Med. 8:691–699. 2014.PubMed/NCBI
|
|
28
|
Gofrit ON, Birman T, Dinaburg A, Ayesh S,
Ohana P and Hochberg A: Chemically induced bladder cancer-a
sonographic and morphologic description. Urology. 68:231–235. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang RY, Du WQ, Zhang YC, Zheng JN and
Pei DS: PLCε signaling in cancer. J Cancer Res Clin Oncol. June
25–2015.Epub ahead of print. View Article : Google Scholar
|
|
30
|
Gofrit ON, Birman T, Dinaburg A, Ayesh S,
Ohana P and Hochberg A: Chemically induced bladder cancer-a
sonographic and morphologic description. Urology. 68:231–235. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chu H, Wang M and Zhang Z: Bladder cancer
epidemiology and genetic susceptibility. J Biomed Res. 27:170–178.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Murta-Nascimento C, Schmitz-Drager BJ,
Zeegers MP, Steineck G, Kogevinas M, Real FX and Malats N:
Epidemiology of urinary bladder cancer: from tumor development to
patient's death. World J Uro. 25:285–295. 2007. View Article : Google Scholar
|
|
33
|
Malats N and Real FX: Epidemiology of
bladder cancer. Hematol Oncol Clin North Am. 29:177–189. vii2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Burchill SA, Neal DE and Lunec J:
Frequency of H-ras mutations in human bladder cancer detected by
direct sequencing. Br J Urol. 73:516–521. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Traczyk M, Borkowska E, Rozniecki M,
Purpurowicz R, Jędrzejczyk A, Marks P, Pietrusiński M, Jabłonowski
Z, Sosnowski M and Kałużewski B: Polymorphic variants of H-RAS
protooncogene and their possible role in bladder cancer etiology.
Cent European J Urol. 65:84–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ahmad I, Patel R, Liu Y, Singh LB, Taketo
MM, Wu XR, Leung HY and Sansom OJ: Ras mutation cooperates with
β-catenin activation to drive bladder tumourigenesis. Cell Death
Dis. 2:e1242011. View Article : Google Scholar
|
|
37
|
Shibatohge M, Kariya K, Liao Y, Hu CD,
Watari Y, Goshima M, Shima F and Kataoka T: Identification of
PLC210, a Caenorhabditis elegans phospholipase C, as a putative
effector of Ras. J Biol Chem. 273:6218–6222. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bourguignon LY, Gilad E, Brightman A,
Diedrich F and Singleton P: Hyaluronan-CD44 interaction with
leukemia-associated RhoGEF and epidermal growth factor receptor
promotes Rho/Ras co-activation, phospholipase C
epsilon-Ca2+ signaling and cytoskeleton modification in
head and neck squamous cell carcinoma cells. J Biol Chem.
281:14026–14040. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cheng H, Luo C, Wu X, Zhang Y, He Y, Wu Q,
Xia Y and Zhang J: shRNA targeting PLCε inhibits bladder cancer
cell growth in vitro and in vivo. Urology. 78:474.e477–411. 2011.
View Article : Google Scholar
|
|
40
|
Ling Y, Chunli L, Xiaohou W and Qiaoling
Z: Involvement of the PLCε/PKCα pathway in human BIU-87 bladder
cancer cell proliferation. Cell Biol Int. 35:1031–1036. 2011.
View Article : Google Scholar
|
|
41
|
Du HF, Ou LP, Yang X, Song XD, Fan YR, Tan
B, Luo CL and Wu X: A new PKCα/β/TBX3/E-cadherin pathway is
involved in PLCε-regulated invasion and migration in human bladder
cancer cells. Cell Signal. 26:580–593. 2014. View Article : Google Scholar
|
|
42
|
Nagano T, Edamatsu H, Kobayashi K,
Takenaka N, Yamamoto M, Sasaki N, Nishimura Y and Kataoka T:
Phospholipase cε, an effector of ras and rap small GTPases, is
required for airway inflammatory response in a mouse model of
bronchial asthma. PLoS One. 9:e1083732014. View Article : Google Scholar
|
|
43
|
Li M, Edamatsu H, Kitazawa R, Kitazawa S
and Kataoka T: Phospholipase Cepsilon promotes intestinal
tumorigenesis of Apc (Min/+) mice through augmentation of
inflammation and angiogenesis. Carcinogenesis. 30:1424–1432. 2009.
View Article : Google Scholar : PubMed/NCBI
|