Introduction
Hepatitis B virus (HBV) infection, an infection that
can cause chronic liver disease, and which raises the risks of
mortality from liver cirrhosis and cancer, is a major public health
problem worldwide (1). It is
estimated that ~2 billion individuals worldwide exhibited
serological evidence of current or past HBV infection, and
~350,000,000 individuals are suffering from chronic HBV infection
(2). As a non-cytopathic virus,
HBV fails to directly destroy the host cells, however, it could
influence disease progression and prognosis by activating the
innate or adaptive immune response (3). Dendritic cells (DCs), which are
powerful antigen-presenting cells in vivo, have been shown
to form a critical interface between the innate and adaptive immune
response (4), and serve as
important components of the regulation of immune responses
(5). DC dysfunction in patients
with chronic HBV (CHB), which can cause inefficient antigen
presenting and inadequate special antiviral immune response, was an
important reason for persistent infection of HBV (6). In addition, previous studies have
suggested that the significantly lower frequency of myeloid DCs
(mDCs) and plasmacytoid DCs (pDCs) in the umbilical cord blood,
compared with that in the peripheral blood of healthy adults, can
comprise the mechanism of easily-acquired chronicity among newborns
following HBV infection (7).
Large quantities of HBV particles and viral
proteins, including the hepatitis B virus surface antigen (HBsAg),
were identified in the peripheral blood of HBV-infected patients.
The HBsAg can accumulate up to concentrations of 100 µg/ml
(8). In acute HBV infection, the
cellular and humoral immune response induced by HBsAg may be
important in the control of HBV replication and life cycle
(9). In addition, it has
previously been suggested that HBsAg can alter the immune response,
which may contribute to the establishment of chronic infection;
Wang et al (10)
demonstrated an association between HBsAg and decreased cytokine
production, in particular that of interleukin (IL)-12, which may
have been induced via the toll-like receptor 2 ligand in peripheral
blood mononuclear cells (PBMCs) from patients with CHB. These
findings can lead to the understanding of the mechanism through
which HBV evades host immunity. It has also been demonstrated that
HBsAg may directly interfere with pDC function through the
HBsAg-mediated upregulated expression of the suppressor of cytokine
signaling 1 and blood DC antigen-2 ligation (11,12).
T cell immunoglobulin and mucin domain-containing
molecule-3 (Tim-3), a member of the Tim family, was found to be
involved in the pathogenesis of numerous diseases, including virus
infections, cancer, autoimmune diseases, and also allergic
reactions and transplantation rejection (13). Khademi et al (14) first reported the high mRNA
expression levels of Tim-3 in human T helper 1 cells (Th1) in
vitro, therefore Tim-3 was initially considered as a special
membrane protein expressed on the Th1 cells (15); however, it was revealed that Tim-3
was also expressed on other cell types, including cluster of
differentiation (CD)8+T, Th17, nature killer (NK), mast,
endothelial and regulatory cells, as well as monocytes,
macrophages, DCs and neurogliocytes (13). A previous study showed that Tim-3
was also expressed in tumor cells and could serve as an independent
prognostic factor for cancer (16).
In the present study, human monocyte-derived DCs
(MD-DCs) from normal adult peripheral blood were selected in order
to investigate the effects of HBsAg on the immune function of
MD-DCs in vitro, and the possible role of the Tim-3
signaling molecule in the regulation of MD-DCs.
Materials and methods
Generation of MD-DCs
DCs were cultured, as previously described with
slight modifications (17). The
PBMCs were obtained from healthy adult donors by centrifugation
with Ficoll-Paque (Sigma-Aldrich, St. Louis, MO, USA). Written
informed consent was obtained from all donors prior to blood
donation. The present study was approved by the ethics committee at
the Affiliated Taizhou Hospital of Wenzhou Medical University
(Linhai, China). The cells were cultured in RPMI-1640 medium in
6-well plates for 2 h at 37°C. Non-adherent cells were removed by
gently swirling the plate and adherent cells (monocytes) were
subsequently cultured in RPMI-1640 medium, supplemented with 20%
heat-inactivated fetal bovine serum (HyClone™; GE Healthcare Life
Sciences, Logan, UT, USA) in 6-well plates with recombinant human
granulocyte-macrophage colony-stimulating factor (rhGM-CSF; 750
U/ml; R&D Systems, Inc., Minneapolis, MN, USA) and rhIL-4 (800
U/ml; R&D Systems, Inc.). Fresh medium containing GM-CSF and
IL-4 was added every 3 days. Phosphate-buffered saline (PBS) was
used to replace GM-CSF and IL-4 in the control group.
Flow cytometry
MD-DCs were collected in test tubes, incubated in
PBS and stained with fluorescein isothiocyanate
(FITC)-phycoerythrin (PE)-allophycocyanin (APC)- or
peridinin-chlorophyll-protein (PerCP)-labeled monoclonal antibodies
(anti-human CD80, CD86, CD11c and human leukocyte antigen (HLA)-DR,
or the relevant isotype control; eBioscience Inc., San Diego, CA,
USA), according to the manufacturer's protocol. Following
incubation at room temperature in the dark for 15 min, the cells
were washed twice with PBS buffer and analyzed using a FACSort cell
analyzer (FACSCalibur; Becton-Dickinson, San Jose, CA, USA). The
flow cytometry data was analyzed using the FlowJo software, version
7.6.5 (FLOWJO, LLC, Ashland, Oregon, USA).
Treatment of MD-DCs with HBsAg
HBsAg (5 µg/ml; ProSpec-Tany TechnoGene Ltd.,
Ness Ziona, Israel) was added to the medium of MD-DCs on day 7
following culture, while the control group was treated with 0.2%
bovine serum albumin (BSA). The cells were treated for 48 h.
Inhibition of the Tim-3 signaling
pathway
Initially, the Ultra-Leaf purified anti-human Tim-3
(10 µg/ml; BioLegend, San Diego, CA, USA) was added to the
medium of MD-DCs on day 7 following culture to inhibit the Tim-3
signaling pathway, while the control group was treated with BSA
(0.2%). After 1 h, HBsAg (5 µg/ml) was added to the medium
for 48 h.
Mixed leukocyte reaction
MD-DCs treated with mitomycin C (25 µg/ml)
for 30 min, were used as the stimulating cells. Allogenic
lymphocytes, which were obtained from other healthy adult donors,
as previously described, were distributed at in 96-well plates a
density of 1×105 cells/well and were incubated for 96 h
in certain ratio of stimulating cells (DC/lymphocytes = 1/5 or
1/10). MTS-phenazine methosulfate (20 µl/well; Promega
Corp., Madison, USA) was added and incubated for 4 h at 37°C, after
which the absorbance (A) at 450 nm was determined using a
Microplate Reader (CLARIOstar®; BMG LABTECH GmbH,
Ortenberg, Germany). The stimulation index (SI) was calculated as
follows: SI = (Aexperimental − Abackground) /
(Acontrol − Abackground) (18).
Western blotting
The proteins from the MD-DCs were collected through
progressions of pyrolysis and extraction, and the total protein
concentration was determined using a bicinchoninic acid protein
detection kit (Sigma-Aldrich). The total protein (40 µg) was
separated using 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (Sigma-Aldrich), and the proteins were transferred
onto polyvinylidene difluo-ride membranes (Sigma-Aldrich). The
membranes were first incubated for 2 h at room temperature with 1%
BSA in PBS, and then overnight at 4°C with rabbit anti-human Tim-3
polyclonal antibody (dilution, 1:1,000; cat. no. 3808-100;
BioVision, Inc., Milpitas CA, USA), and anti-NF-κB (dilution,
1:1,000; cat. no. 1559-1; Epitomics, Inc., Burlingame, CA, USA),
and anti-GAPDH monoclonal (dilution, 1:1,000; cat. no. 5632-1;
Epitomics, Inc.) antibodies. For signal detection, horseradish
peroxidase-conjugated mouse anti-rabbit immunoglobulin G (dilution,
1:10,000; cat. no. 5618-1; Epitomics, Inc.) and the enhanced
chemiluminescence detection system (Cell Signaling Technology,
Inc., Danvers, MA, USA) were used. According to the gray value
ratio of target protein against GAPDH, the relative expression of
the target protein was calculated using the Quantity One software,
version 4.6.2 (Bio-Rad Laboratories, Inc., Hercules, CA, USA), as
demonstrated in a previous study (19).
Enzyme-linked immunosorbent assay
(ELISA)
The concentrations of inflammatory cytokines in the
supernatant were determined using the following ELISA kits: Human
IL-6 Quantikine ELISA kit, Human IL-10 Quantikine ELISA kit and
Human IFN-γ Quantikine ELISA kit (R&D Systems, Inc.). The
experiments were performed according to the manufacturer's
protocol.
Statistical analysis
Continuous variables are expressed as the mean ±
standard deviation. Student's t-test was used to determine the
statistical differences between two groups, one-way analysis of
variance for multiple groups and the least significant difference
t-test was used for multiple comparisons. Statistical analyses were
conducted using the GraphPad Prism software, version 5.01 (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Identification of MD-DCs
Following 7 days of incubation with RPMI-1640 medium
containing rhGM-CSF and rhIL-4, the majority of the cells
aggregated to form suspended colonies, which were subsequently
observed using an optical microscope. Electron microscopy revealed
that the morphology of the MD-DCs was irregular, with burr cells
observed on the surface. These findings were consistent with the
typical DC morphology (20). The
expression of DC-associated cell phenotypes was revealed to be
significantly increased with CD11c 70.09±0.57%, HLA-DR 79.83±2.12%,
CD80 48.33±7.34% and CD86 44.21±5.35% (P<0.01; Fig. 1).
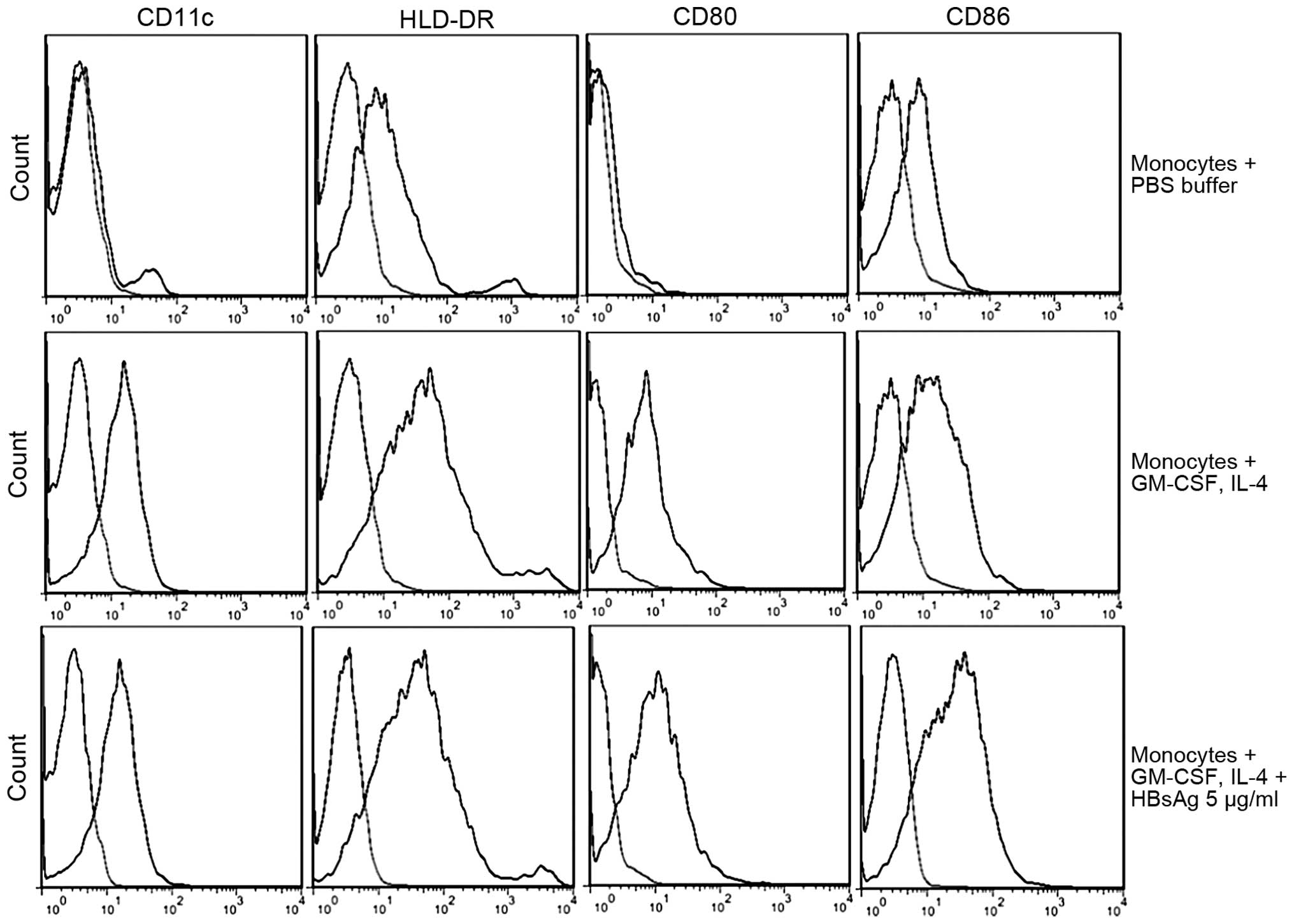 | Figure 1Phenotypic analysis of human
monocyte-derived dendritic cells. The phenotypic expression values
of monocytes cultured with PBS buffer were as follows: CD11c
(9.41±6.38%), HLA-DR (26.13±6.61%), CD80 (3.46±2.00%) and CD86
(6.70±3.21%). The expression of monocytes cultured with GM-CSF and
IL-4 was significantly increased (P<0.01): CD11c (70.09±0.57%),
HLA-DR (79.83±2.12%), CD80 (48.33±7.34%) and CD86 (44.21±5.35%). In
addition, HBsAg treatment with GM-CSF and IL-4, increased the
expression of CD80 (t=3.75; P<0.05) and CD86 (t=7.61;
P<0.01). The cell phenotypic expression values were as follows:
CD11c (69.71±7.42%), HLA-DR (81.60±8.79%), CD80 (68.87±6.01%), CD86
(75.48±4.70%). The statistical differences between the groups were
determined using Student's t-test. The graphs were generated using
FlowJo software, version 7.6.5. The light regions on the graphs
represent the control samples. GM-CSF, granulocyte-macrophage
colony-stimulating factor, HLA, human leukocyte antigen; CD,
cluster of differentiation; HBsAg, hepatitis B virus surface
antigen; IL, interleukin; PBS, phosphate-buffered saline. |
Effects of HBsAg on MD-DCs
Compared with the control group, HBsAg treatment
resulted in an enhanced expression of co-stimulatory molecules
(P<0.05) with CD80 (68.87±6.01%) and CD86 (75.48±4.70%). The
changes in the cell phenotype expression of CD11c and HLA-DR
revealed no statistically significant difference (P>0.05;
Fig. 2). A higher stimulation
index (SI) represented a more marked lymphocyte stimulatory
capacity. The SI of HBsAg-treated MD-DCs was higher compared with
the control BSA treated group, with a 1/5 or 1/10 ratio of
DC/lymphocytes (P<0.01; Fig.
2). Treatment of the MD-DCs with HBsAg resulted in the
upregulation of NF-κB (t=7.55, P<0.01; Fig. 3B), as well as an enhancement in the
cytokine secretion levels of IL-6 (t=6.05, P<0.01), IL-10
(t=7.02, P<0.01) and interferon (IFN)-γ (t=11.69, P<0.01;
Fig. 4).
 | Figure 4Cytokine secretion levels. Following
HBsAg stimulation, in vitro, levels of the downstream
cytokines, IL-6, IL-10 and IFN-γ, were significantly increased,
which indicated an enhanced immune response of MD-DCs to HBsAg
stimulation. This immune response, was reduced following inhibition
of the Tim-3 signaling pathway, using anti-Tim-3 prior to HBsAg
stimulation. The secretion of IL-6, IL-10 and IFN-γ cytokines was
significantly downregulated. (*P<0.01). Statistical
differences between the groups were determined using one-way
analysis of variance and the least significant difference t-test.
Data are presented as the mean ± standard deviation. HBsAg,
hepatitis B virus surface antigen; IL, interleukin; IFN-γ,
interferon-γ; MD-DCs, human monocyte-derived dendritic cells;
Tim-3, T cell immunoglobulin and mucin domain-containing
molecules-3; BSA, bovine serum albumin. |
Role of Tim-3 in the regulation of
MD-DCs
HBsAg stimulation in vitro increased the
expression of Tim-3 (t=5.83, P<0.01) in MD-DCs (Fig. 3A) and enhanced the immune function
of MD-DCs; however, reduced the immune response of MD-DCs to HBsAg
stimulation was observed when the Tim-3 signaling pathway was
inhibited prior to treatment. The NF-κB expression was revealed to
be significantly decreased (t=2.61, P<0.05) and the cytokine
secretion level of IL-6 (t=3.99, P<0.01), IL-10 (t=5.03,
P<0.01) and IFN-γ (t=6.30, P<0.01) were significantly
downregulated (Fig. 4).
Discussion
The present study demonstrated that the HBsAg
enhanced the immune response of MD-DCs in vitro. Previous
studies have revealed large quantities of HBV particles and viral
proteins in the peripheral blood of HBV-infected patients, which
enabled multiple interactions among the virus, its viral proteins
and the immunocytes (21). HBsAg
enhanced the innate or adaptive immune response found in the mice
bone marrow-derived DCs treated with HBsAg in vitro, as well
as affect the differentiation of T helper cells (22). Different forms of HBsAg elicited
various types of B and T cell immunity (23), and both HBV DNA vaccine and HBsAg
induced the humoral immunity (24). In the present study, MD-DCs were
treated with HBsAg in vitro, which resulted in the enhanced
expression of co-stimulatory molecules and the lymphocyte
stimulatory capacity of MD-DCs, as well as the upregulated
secretion of inflammatory cytokines. Consistent with the present
study, Jan et al (19) also
used the MD-DCs from healthy adult donors and reported that HBsAg
increases the expression of CD80, CD83, CD86 and major
histocompatibility complex class II of MD-DCs. This treatment also
increases the production of interleukin IL-12 and IL-10, as well as
enhances T cell-stimulatory capacity. In addition, treated with
different concentrations of HBsAg, the immature DCs, which were
cultured from PBMCs of patients with CHB, differentiate into mature
DCs (25); and DCs stimulated with
HBsAg more efficiently presented antigen and induced a specific T
cell immune response (26).
However, Op den Brouw et al (21) reported that both HBV particles and
purified HBsAg had an immune modulatory capacity and directly
contributed to the dysfunction of mDCs in patients with chronic
HBV; since the mDCs were directly isolated from PBMCs, the
differences between circulating mDCs and MD-DCs in immune function,
as well as the exact molecular association between the HBsAg and
DCs, remained unclear.
In the present study, in vitro HBsAg
stimulation increased the expression of the Tim-3 signal molecule
and enhanced the immune function of MD-DCs. Tim-3 was expressed in
a variety of immune cells, exerts an important role in regulating
acquired or innate immune responses, and was revealed to be closely
associated with the pathogenesis of infection, cancer and other
diseases (27). The interaction
between Tim-3 and its natural ligand, galectin-9, induces
intracellular calcium efflux, resulting in Th1 cell aggregation and
apoptosis, followed by immune tolerance and inhibition of acquired
immune response (15). In the
innate immune response, Tim-3 acts as a promoter or inhibitor
through its interaction with galectin-9 or other ligands. Anderson
et al (28) reported that
Tim-3, expressed on antigen presenting cells, cooperates with
Toll-like receptors to promote the secretion of proin-flammatory
cytokines. Kanzaki et al (29) reported that the galectin-9-Tim-3
signaling pathway induces TNF-α production in cultured DCs in a
dose-dependent manner; however, Chiba et al (30) revealed that the high expression of
Tim-3 on tumor-associated DCs inhibited the immune response
mediated by nucleic acid. Therefore, different immune environments
may lead to different roles of Tim-3-mediated immune response.
The Tim-3 signal molecule may positively regulate
the immune response of MD-DCs. As described in the present study, a
reduced immune response of MD-DCs to HBsAg stimulation was observed
when the Tim-3 signaling pathway was inhibited in advance; however,
this conclusion was not consistent with previously published
results. Ju et al (31)
found that the inhibition of the Tim-3 signaling pathway with
anti-Tim-3 antibodies or Tim-3-Fc fusion proteins resulted in an
increased cytotoxicity for NK92 cells and an elevated IFN-γ
production. The increased expression of Tim-3 on PBMCs, circulating
NK and CD8+T cells in patients with CHB led to immune response
dysfunction. In addition, partial restoration of immune function
was observed when the Tim-3 signaling pathway was inhibited prior
to treatment (31,32). The opposite regulatory effects of
Tim-3 may be attributed to the relatively different immune
environment between MD-DCs with simple HBsAg stimulation and
patients with chronic HBV infection; however, the exact mechanism
remains to be elucidated.
In conclusion, in vitro HBsAg treatment
resulted in an enhanced immune response of MD-DCs, which may be
positively regulated by Tim-3. However, further study is required
in order to determine the regulatory role of Tim-3 on the immune
function of DCs in the intracorporeal immune environment of
patients with CHB and the exact mechanism of Tim-3-mediated
regulation.
Abbreviations:
|
BSA
|
bovine serum albumin
|
|
CHB
|
chronic hepatitis B
|
|
DCs
|
dendritic cells
|
|
MD-DCs
|
monocyte derived DCs
|
|
HBV
|
hepatitis B virus
|
|
HBsAg
|
HBV surface antigen
|
|
IFN
|
interferon
|
|
NF-κB
|
nuclear factor κB
|
|
NK
|
nature killer cell
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
Th1
|
T helper 1 cells
|
|
TH17
|
T helper 17 cells
|
|
SI
|
stimulation index
|
|
Tim-3
|
T cell immunoglobulin and mucin
domain-containing molecules-3
|
Acknowledgments
The present study received financial support from
the Zhejiang Bureau of Public Health (no. 2012KYB237) and the
Zhejiang Province Major Science and Technology Programs (no.
2012C13018-3). The authors would also like to thank the staff of
the Medical Research Center for their helpful advice and
guidance.
References
|
1
|
Trépo C, Chan HL and Lok A: Hepatitis B
virus infection. Lancet. 384:2053–2063. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hatzakis A, Van Damme P, Alcorn K, Gore C,
Benazzouz M, Berkane S, Buti M, Carballo M, Cortes Martins H,
Deuffic-Burban S, et al: The state of hepatitis B and C in the
Mediterranean and Balkan countries: Report from a summit
conference. J Viral Hepat. 20(Suppl 2): 1–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hösel M, Quasdorff M, Wiegmann K, Webb D,
Zedler U, Broxtermann M, Tedjokusumo R, Esser K, Arzberger S,
Kirschning CJ, et al: Not interferon, but interleukin-6 controls
early gene expression in hepatitis B virus infection. Hepatology.
50:1773–1782. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mildner A and Jung S: Development and
function of dendritic cell subsets. Immunity. 40:642–656. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hänninen A: New dimensions for dendritic
cells. Duodecim. 130:883–891. 2014.In Finnish.
|
|
6
|
Wang FS, Xing LH, Liu MX, Zhu CL, Liu HG,
Wang HF and Lei ZY: Dysfunction of peripheral blood dendritic cells
from patients with chronic hepatitis B virus infection. World J
Gastroenterol. 7:537–541. 2001. View Article : Google Scholar
|
|
7
|
Hao HX, Zhang YL, Li MH, Zhang LX, Yi W,
Hu YH, Yi N, Cheng J, Liu SA and Xie Y: Dendritic cell subsets and
function in newborns from mothers of different HBV infection
status. Zhong Hua Shi Yan He Lin Chuang Bing Du Xue Za Zhi.
27:112–114. 2013.In Chinese.
|
|
8
|
Ganem D and Prince AM: Hepatitis B virus
infection-natural history and clinical consequences. N Engl J Med.
350:1118–1129. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Z, Cheng Y, Xu Y, Liao J, Zhang X, Hu
Y, Zhang Q, Wang J, Zhang Z, Shen F and Yuan Z: Expression profiles
and function of Toll-like receptors 2 and 4 in peripheral blood
mononuclear cells of chronic hepatitis B patients. Clin Immunol.
128:400–408. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang S, Chen Z, Hu C, Qian F, Cheng Y, Wu
M, Shi B, Chen J, Hu Y and Yuan Z: Hepatitis B virus surface
antigen selectively inhibits TLR2 ligand-induced IL-12 production
in monocytes/macrophages by interfering with JNK activation. J
Immunol. 190:5142–5151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu Y, Hu Y, Shi B, Zhang X, Wang J, Zhang
Z, Shen F, Zhang Q, Sun S and Yuan Z: HBsAg inhibits TLR9-mediated
activation and IFN-alpha production in plasmacytoid dendritic
cells. Mol Immunol. 46:2640–2646. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kondo Y, Ninomiya M, Kakazu E, Kimura O
and Shimosegawa T: Hepatitis B surface antigen could contribute to
the immunopatho-genesis of hepatitis B virus infection. ISRN
Gastroenterol. 2013:9352952013. View Article : Google Scholar
|
|
13
|
Zhang XM and Shan NN: The role of T cell
immunoglobulin and mucin domain-3 in immune thrombocytopenia. Scand
J Immunol. 79:231–236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khademi M, Illés Z, Gielen AW, Marta M,
Takazawa N, Baecher-Allan C, Brundin L, Hannerz J, Martin C, Harris
RA, et al: T Cell Ig- and mucin-domain-containing molecule-3
(TIM-3) and TIM-1 molecules are differentially expressed on human
Th1 and Th2 cells and in cerebrospinal fluid-derived mononuclear
cells in multiple sclerosis. J Immunol. 172:7169–7176. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu C, Anderson AC, Schubart A, Xiong H,
Imitola J, Khoury SJ, Zheng XX, Strom TB and Kuchroo VK: The Tim-3
ligand galectin-9 negatively regulates T helper type 1 immunity.
Nat Immunol. 6:1245–1252. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang J, Jin MS, Kong F, Cao D, Ma HX, Jia
Z, Wang YP, Suo J and Cao X: Decreased galectin-9 and increased
Tim-3 expression are related to poor prognosis in gastric cancer.
PLoS One. 8:e817992013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chapuis F, Rosenzwajg M, Yagello M, Ekman
M, Biberfeld P and Gluckman JC: Differentiation of human dendritic
cells from monocytes in vitro. Eur J Immunol. 27:431–441. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van der Molen RG, Sprengers D, Binda RS,
de Jong EC, Niesters HG, Kusters JG, Kwekkeboom J and Janssen HL:
Functional impairment of myeloid and plasmacytoid dendritic cells
of patients with chronic hepatitis B. Hepatology. 40:738–746. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jan RH, Lin YL, Chen CJ, Lin TY, Hsu YC,
Chen LK and Chiang BL: Hepatitis B virus surface antigen can
activate human monocyte-derived dendritic cells by nuclear factor
kappa B and p38 mitogen-activated protein kinase mediated
signaling. Microbiol Immunol. 56:719–727. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Romani N, Reider D, Heuer M, Ebner S,
Kämpgen E, Eibl B, Niederwieser D and Schuler G: Generation of
mature dendritic cells from human blood. An improved method with
special regard to clinical applicability. J Immunol Methods.
196:137–151. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Op den Brouw ML, Binda RS, van Roosmalen
MH, Protzer U, Janssen HL, van der Molen RG and Woltman AM:
Hepatitis B virus surface antigen impairs myeloid dendritic cell
function: A possible immune escape mechanism of hepatitis B virus.
Immunology. 126:280–289. 2009. View Article : Google Scholar :
|
|
22
|
Jan RH, Lin YL, Chen LK, Huang MT, Wang LC
and Chiang BL: Hepatitis B virus surface antigen can activate
dendritic cells and modulate T helper type immune response.
Microbiol Immunol. 55:51–59. 2011. View Article : Google Scholar
|
|
23
|
Shen M, Wang S, Ge G, Xing Y, Ma X, Huang
Z and Lu S: Profiles of B and T cell immune responses elicited by
different forms of the hepatitis B virus surface antigen. Vaccine.
28:7288–7296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Du X, Wang J, Kang Y, Xiao W, Zhao G and
Wang B: Suppression of the antigen-specific T cell immune response
by co-immunization with the HBV DNA vaccine and recombinant HBsAg.
Wei Sheng Wu Xue Bao. 49:938–942. 2009.In Chinese. PubMed/NCBI
|
|
25
|
Tong HS, Zhang Y, Yuan K and Fu XW: HBsAg
loading on dendritic cells in patients with chronic hepatitis B:
Expressions of phenotypic molecules. Hepatobiliary Pancreat Dis
Int. 5:56–59. 2006.PubMed/NCBI
|
|
26
|
Kang P, Luo SL and Li SC: Comparative
study on dendritic cells stimulated with HBsAg or HBcAg in patients
with chronic hepatitis B. Zhong Hua Shi Yan He Lin Chuang Bing Du
Xue Za Zhi. 21:250–252. 2007.In Chinese.
|
|
27
|
Han G, Chen G, Shen B and Li Y: Tim-3: An
activation marker and activation limiter of innate immune cells.
Front Immunol. 4:4492013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anderson AC, Anderson DE, Bregoli L,
Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW,
Hirashima M, et al: Promotion of tissue inflammation by the immune
receptor Tim-3 expressed on innate immune cells. Science.
318:1141–1143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kanzaki M, Wada J, Sugiyama K, Nakatsuka
A, Teshigawara S, Murakami K, Inoue K, Terami T, Katayama A, Eguchi
J, et al: Galectin-9 and T cell immunoglobulin mucin-3 pathway is a
therapeutic target for type 1 diabetes. Endocrinology. 153:612–620.
2012. View Article : Google Scholar
|
|
30
|
Chiba S, Baghdadi M, Akiba H, Yoshiyama H,
Kinoshita I, Dosaka-Akita H, Fujioka Y, Ohba Y, Gorman JV, Colgan
JD, et al: Tumor-infiltrating DCs suppress nucleic acid-mediated
innate immune responses through interactions between the receptor
TIM-3 and the alarmin HMGB1. Nat Immunol. 13:832–842. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ju Y, Hou N, Meng J, Wang X, Zhang X, Zhao
D, Liu Y, Zhu F, Zhang L, Sun W, et al: T cell immunoglobulin- and
mucin-domain-containing molecule-3 (Tim-3) mediates natural killer
cell suppression in chronic hepatitis B. J Hepatol. 52:322–329.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu W, Shi Y, Li S, Zhang Y, Liu Y, Wu Y
and Chen Z: Blockade of Tim-3 signaling restores the virus-specific
CD8+ T-cell response in patients with chronic hepatitis
B. Eur J Immunol. 42:1180–1191. 2012. View Article : Google Scholar : PubMed/NCBI
|


















