Introduction
Morphological and functional changes in the aged
brain cause different types and degrees of neuropathological
changes and neurobiological alterations (1–4).
Aging of the brain leads to various changes, including oxidative
stress, protein repair ability, DNA damage, autophagic degradation
and neuronal activity in the aged brain (5–10).
Among various brain regions, the hippocampus, which is important in
the function of learning and memory, has been well established as a
most vulnerable and susceptible region to the aging process
(11,12).
Mammalian target of rapamycin (mTOR) regulates
cellular growth, proliferation and survival, and mediates a diverse
range of cellular functions, including translation and
transcription (13). It is
understood that the inhibition of mTOR extends lifespan, whereas
the overactivation of mTOR has been associated with the
pathophysiology of age-related neurodegenerative diseases (14–16).
In the hippocampus, the activation of mTOR is important for
synaptic protein synthesis, dendrite arborization and excitatory
synaptogenesis (17–19).
However, it has been widely accepted that Redd1 is a
negative regulator of mTOR (20,21).
Redd1, which is also known as RTP801/Dig2/DDIT4, is a
stress-induced protein expressed in neuronal and non-neuronal cells
(21,22). Redd1 is involved in the regulation
of reactive oxygen species, and is regulated in response to
hypoxia, oxidative stress, autophagy and cerebral ischemia
(21–23). The induction of Redd1 causes
diverse functional consequences, including protecting cells from
apoptosis induced by oxidative stress, as well as promoting
post-mitotic neuronal death (21,24).
In addition, Redd1 has been investigated as an essential regulator
of normal developmental neurogenesis and neuronal migration, as
well as a direct regulator of mitochondrial metabolism (25–27).
A number of studies have shown age-related changes
in the expression of mTOR in the hippocampus (9,28).
However, chronological changes in the expression of Redd1, as a
negative regulator of mTOR, in the hippocampus during the process
of normal aging remains to be fully elucidated. Therefore, in the
present study, age-related changes in the protein expression levels
of Redd1 were investigated in the hippocampus of the gerbil, which
is considered to be a unique and suitable animal model for
investigations on aging (29).
This was performed in groups of animals at various ages using
western blot and immunohistochemical analyses.
Materials and methods
Experimental animals
Male Mongolian gerbils (Meriones
unguiculatus) were obtained from the Experimental Animal
Center, Kangwon National University (Chuncheon, South Korea) at
postnatal month (PM) 3, representing young animals, PM 6 and PM 12,
representing adult animals, and PM 24, representing aged animals.
The animals (n=14 in each group) were housed in a
conventional state under adequate temperature (23°C) and humidity
(60%) control, with a 12-h light/12-h dark cycle, and were provided
with free access to food and water. The procedures for handling and
caring for animals adhered to the guidelines in compliance with the
Guide for the Care and Use of Laboratory Animals (30), and they were approved by the
Institutional Animal Care and Use Committee at Kangwon National
University (Chuncheon, South Korea; approval no. KW-130424-3). All
experiments were performed in a manner to minimize the number of
animals used and to minimize the suffering caused by the procedures
used in the present study.
Western blot analysis
To examine changes in the protein levels of Redd1,
mTOR and phosphorylated (p-)mTOR in the hippocampus between the
adult and aged groups, animals in each age group (n=7) were
used, and western blot analysis was performed, according to the
method used in our previous studies (6,23,31).
In brief, following sacrifice of the animals by cervical
dislocation, the hippocampus was removed. The hippocampal tissues
were homogenized in 50 mM phosphate-buffered saline PBS (pH 7.4)
containing 0.1 mM ethylene glycol bis (2 aminoethyl
ether)-N,N,N′,N′ tetraacetic acid (pH 8.0), 0.2% Nonidet P-40, 10
mM ethylendiamine tetraacetic acid (pH 8.0), 15 mM sodium
pyrophosphate, 100 mM β-glycerophosphate, 50 mM NaF, 150 mM NaCl, 2
mM sodium orthovanadate, 1 mM phenylmethylsulfonyl floride and 1 mM
dithiothreitol (DTT; all from Sigma-Aldrich). Following
centrifugation at 16,000 × g for 20 min at 4°C, the protein levels
were determined in the supernatants using a Micro BCA protein assay
kit (Sigma-Aldrich), with bovine serum albumin as the standard
(Pierce Biotechnology, Inc.; Thermo Fisher Scientific Inc.,
Waltham, MA, USA). Aliquots containing 20 µg total protein
were boiled in loading buffer containing 150 mM Tris (pH 6.8), 3 mM
DTT, 6% sodium dodecyl sulfate, 0.3% bromophenol blue and 30%
glycerol (all from Sigma-Aldrich). The aliquots were then loaded
onto a 10% polyacrylamide gel. Following electrophoresis, the gels
were transferred onto nitrocellulose transfer membranes (Pall Life
Sciences, East Hills, NY, USA). To reduce background staining, the
membranes were incubated with 5% non-fat dry milk (Sigma-Aldrich)
in PBS containing 0.1% Tween 20 (PBST; Sigma-Aldrich) for 45 min at
room temperature. Following washing with PBS (×3), the membranes
were incubated with polyclonal rabbit anti-human mTOR (cat. no.
2972; dilution, 1:1,000; Cell Signaling Technology, Inc., Danvers,
MA, USA), polyclonal rabbit anti-human phospho-mTOR (Ser2448; cat.
no. 2971; dilution, 1:1,000; Cell Signaling Technology, Inc.),
polyclonal rabbit anti-human Redd1 (cat. no. 10638-1-AP; dilution,
1:500; Proteintech, Chicago, IL, USA) or monoclonal mouse
anti-human β-actin (cat. no. A5316; dilution, 1:5,000;
Sigma-Aldrich) overnight at 4°C. Following washing with PBST (x3),
the membranes were incubated with peroxidase-conjugated donkey
anti-rabbit IgG (cat. no. sc-2305) or goat anti-mouse IgG (cat. no.
sc-2031; dilution, 1:1,000; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) for 1 hr at room temperature. An enhanced
chemiluminescence kit (Pierce; Thermo Fisher Scientific, Inc.) was
used to detect the protein expression. The results of the western
blot analyses were scanned, and densitometric analysis for the
quantification of the bands was performed using Image J 1.46
software (ImageJ, National Institutes of Health, Bethesda, MD,
USA), which was used to determine the relative optical density
(ROD). The levels of mTOR, p-mTOR and Redd1 were normalized with
the level of β-actin. A ratio of the ROD was calibrated as the
percentage, with the PM 3 group designated as 100%.
Immunohistochemical detection of the
expression of Redd1
For the immunohistochemical analyses, the animals
(n=7 per group) were anesthetized with zoletil 50 (30 mg/kg;
Virbac, Carros, France) and perfused transcardially with 0.1 M
phosphate-buffered saline (PBS; pH 7.4; Sigma-Aldrich), followed by
4% paraformaldehyde (Sigma-Aldrich) in 0.1 M phosphate-buffer (pH
7.4). The brain tissues were removed, cryoprotected and serially
sectioned on a cryostat (CM1520; Leica Microsystems; Wetzlar,
Germany) into 30 µm coronal sections, and these were
collected into six-well plates containing PBS.
According to the method used in our previous study
(6,23,31),
immunohistochemical staining for Redd1 was performed using the
polyclonal rabbit anti-human Redd1 primary antibody (1:200; cat.
no. 10638-1-AP; Proteintech) a overnight at 4°C. Following washing
with PBS (×3), the brain tissues were incubated with biotinylated
goat anti-rabbit IgG (BA-1000; 1:200; Vector Laboratories,
Burlingame, CA, USA) for 2 hr at room temperature and streptavidin
peroxidase complex (SA-5004; 1:200; Vector Laboratories) for 45 min
at room temperature. In order to establish the specificity of the
immunostaining, a negative control assessment was performed using
pre-immune serum (S-2012; 5%; Vector Laboratories, Inc.,
Burlingame, CA, USA), which was used in place of the primary
antibody. The negative control resulted in the absence of
immunoreactivity in any structures (data not shown).
A total of seven sections, with 90-µm
intervals, per animal were selected to quantitatively analyze Redd1
immunoreactivity. Digital images of the hippocampal subregions were
captured under an AxioM2 light microscope (Carl Zeiss AG, Jena,
Germany) equipped with a digital camera (Axiocam; Carl Zeiss AG)
connected to a PC monitor. Semi-quantification of the
immunostaining intensities in the pyramidal cells of the
hippocampus proper and in the granular cells of the dentate gyrus
were evaluated using ImageJ 1.46 software (National Institutes of
Health), according to the method of our previous study (6,31).
The mean intensity of the immunostaining in each of the
immunoreactive structures was measured using a 0–255 gray scale
system (32,33), in which white to dark signal
corresponded to values between 255 and 0, respectively). Based on
this approach, the level of immunoreactivity was scaled as −, ±, +,
++ or +++, representing no staining (gray scale value: ≥200),
weakly positive (gray scale value: 150–199), moderate (gray scale
value: 100–149), strong (gray scale value: 50–99) or very strong
(gray scale value: ≤49), respectively.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Differences between the mean ROD values among the groups
were statistically analyzed using one-way analysis of variance,
followed by Bonferroni's post-hoc multiple comparison test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Changes in the protein levels of mTOR,
p-mTOR and Redd1
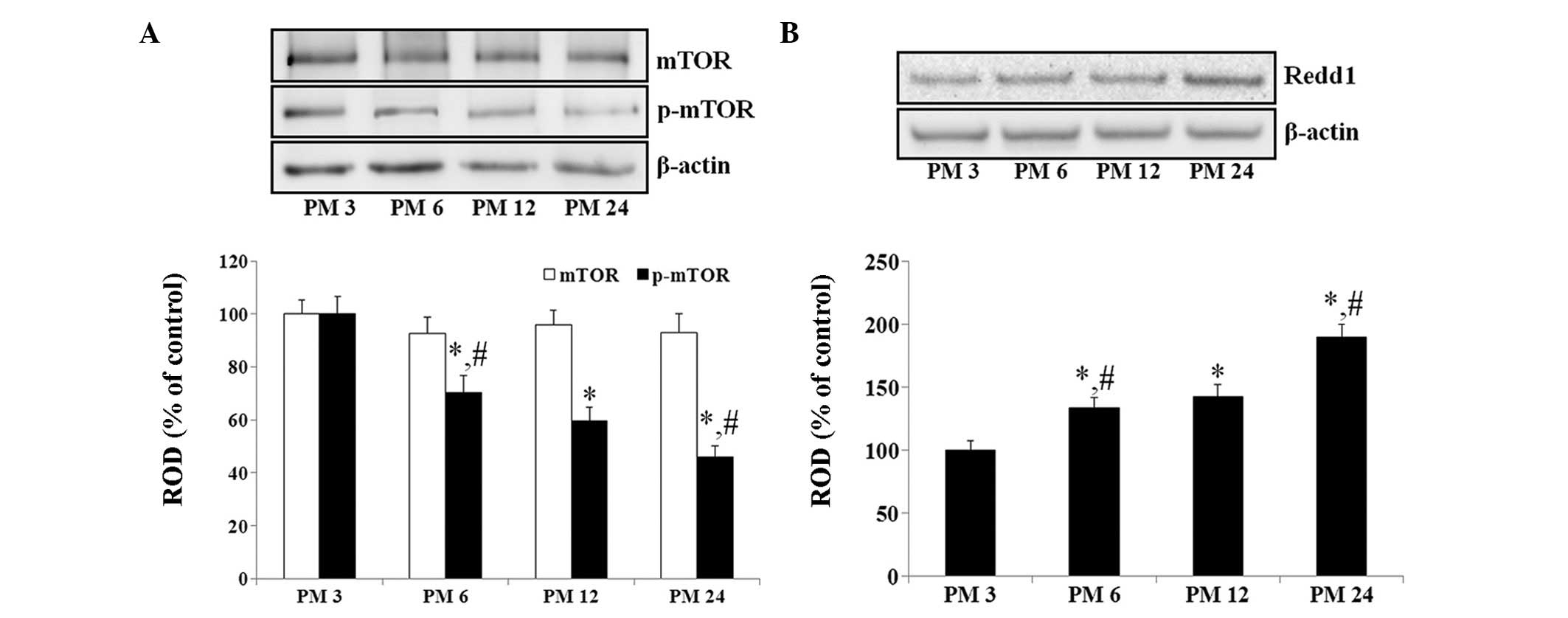
From the results of the western blot analysis, no
significant differences were observed in the levels of mTOR in the
hippo-campus among the experimental groups (Fig. 1A). However, the levels of p-mTOR in
the hippocampus decreased in an age-dependent manner (Fig. 1A).
By contrast, the protein level of Redd1 in the
hippocampus increased gradually during normal aging. The protein
levels of Redd1 were significantly increased in the PM 6 and PM 12
groups (133.2 and 142.5%, respectively), compared with the PM3
group. In addition, the protein level of Redd1 was markedly higher
at PM 24, compared with the PM 6 and PM 12 group (132.7% of the PM
12 group; Fig. 1B).
Changes in Redd1 immunoreactivity
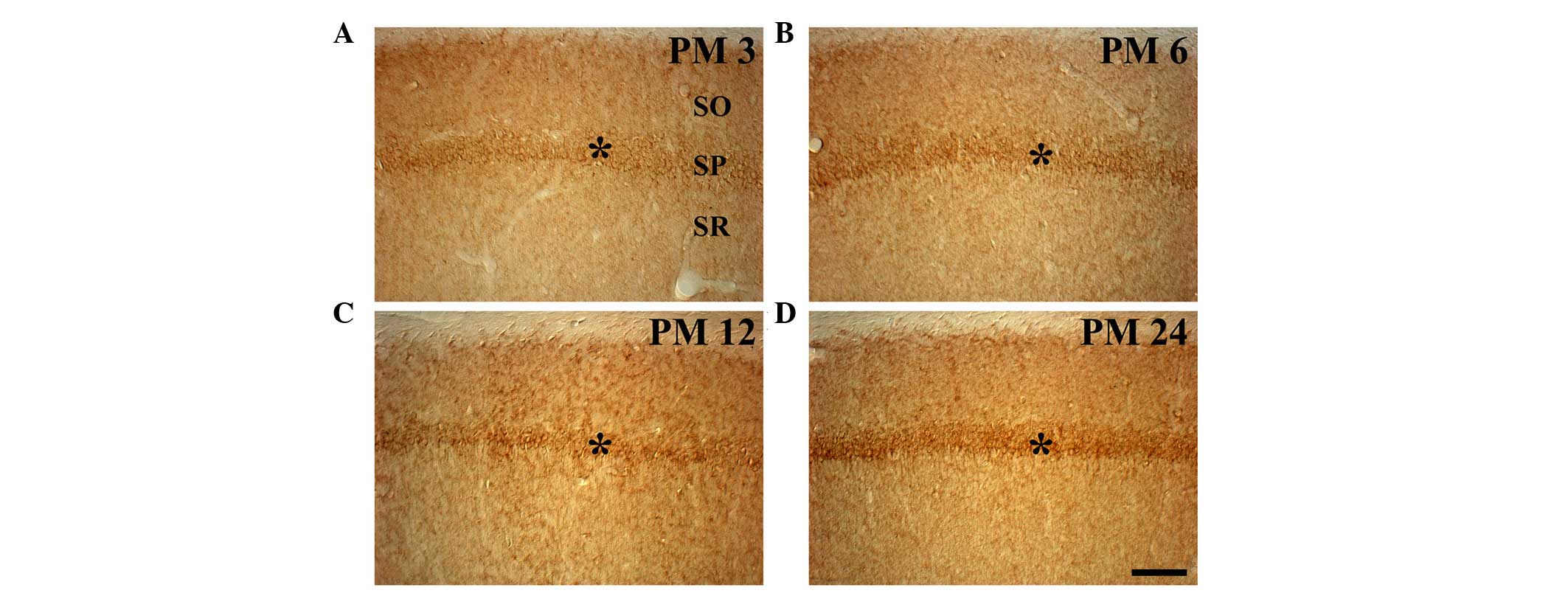
In the PM 3 group, weak Redd1 immunoreactivity was
observed in the pyramidal neurons of the stratum pyramidale (SP) of
the hippocampus proper (CA1-3 regions; Table I; Fig.
2A). In particular, Redd1 immunoreactivity in the pyramidal
neurons was detected in the cytoplasm, but not in the nucleus. In
the PM 6 group, Redd1 immunoreactivity was increased; and moderate
Redd1 immunoreactivity was observed in the SP of the hippocampus
proper, compared with that in the PM 3 group (Table I; Fig.
2B). The Redd1 immunoreactivity in the hippocampus proper of
the PM 12 group was similar to that observed in the PM 6 group
(Table I; Fig. 2C). In the PM 24 group, Redd1
immunoreactivity in the SP of the hippocampus proper was markedly
higher, compared with the PM 6 and PM 12 groups (Table I, Fig.
2D).
 | Table IMean intensity of Redd1
immunoreactivity in the principal cells of the gerbil hippocampus
during normal aging. |
Table I
Mean intensity of Redd1
immunoreactivity in the principal cells of the gerbil hippocampus
during normal aging.
| Cell type | PM 3 | PM 6 | PM 12 | PM 24 |
|---|
| Pyramidal cells of
hippocampus proper | + | ++ | ++ | +++ |
| Granule cells of
dentate gyrus | ± | + | + | ++ |
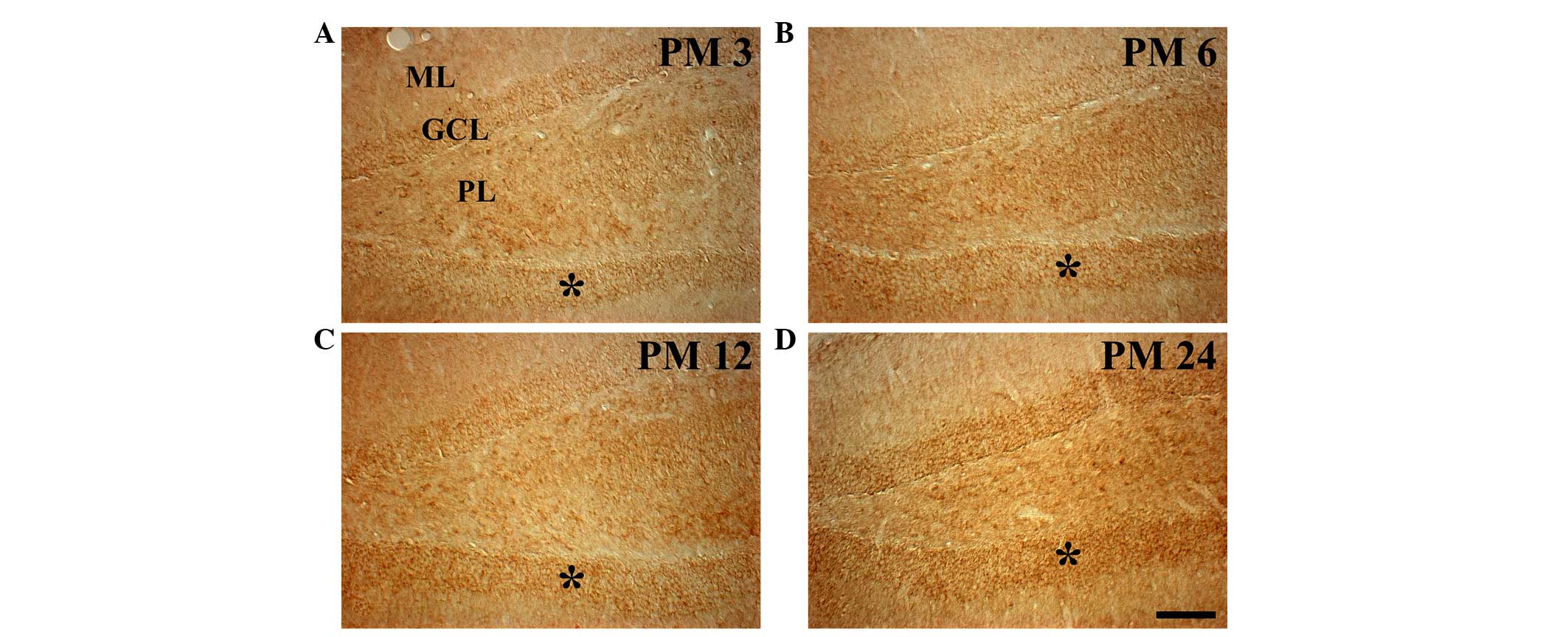
In the dentate gyrus of the PM 3 group, weak Redd1
immunoreactivity was observed in the granule cells of the granule
cell layer (GCL) and in the polymorphic cells of the polymorphic
layer (PL; Table I; Fig. 3A). The age-related change in Redd1
immunoreactivity in the GCL was similar to that observed in the
hippocampus proper. In the GCL, the levels of Redd1
immunoreactivity were moderate in the PM 6 and PM 12 groups, and
strong in the PM 24 group (Table
I; Fig. 3B-D). However, no
marked changes in Redd1 immunoreactivity were observed in the
polymorphic cells of the PL among the experimental groups (Fig. 3).
Discussion
It is well established that mTOR is necessary for
the normal development and viability of an organism, and that the
inhibition of mTOR prolongs lifespan (14,16,34).
The inhibition of mTOR through rapamycin administration ameliorates
age-related cognitive deficits, enhances learning and memory in
young mice, and maintains memory in aged mice (35,36).
In addition, rapamycin treatment partially prevents behavioral
decline in senescence-accelerated OXYS rats, which occurs by
normalizing certain brain structures (37). In the present study, age-related
changes were initially examined in the protein expression levels of
mTOR and p-mTOR in the hippocampus, and an age-dependent decrease
in the expression of p-mTOR Ser2448, but not mTOR, was found in the
gerbil hippocampus. This result is in accordance with the results
of previous studies, which demonstrated that the levels of p-mTOR
Ser2448 decreased significantly in aged mice, compared with young
mice (9,28). Yang et al (9) showed that a decline in mTOR signaling
activity was accompanied by a decline in brain-derived neurotrophic
factor/phosphoinositide 3-kinase/Akt activity, and suggested that
age-related dysfunction and diseases may be associated with the
failure, rather than the overactivation, of mTOR decline as aging
progresses. It was suggested that increased mTOR signaling in the
hippocampus of the young mice may be due to it being more necessary
for neuronal growth in young animals, than in adult and aged
animals. In addition, it has been suggested that age-related
cognitive decline might be related with a decrease of mTOR
activation in the hippocampus (28).
In the present study, the protein level of Redd1 was
also found to increase gradually during normal aging. In addition,
it was found that Redd1 immunoreactivity increased gradually with
age in the pyramidal and granule cells, which are known to be
principal neurons in the hippocampus (38). To the best of our knowledge, the
present study was the first study to demonstrate an age-dependent
increase in the expression of Redd1 in the hippocampus. Although it
is difficult to determine exactly why the expression of Redd1 was
increased in the aged hippo-campus, these finding may be supported
by certain reports that showed that Redd1 was closely associated
with oxidative stress and DNA damage, and that the accumulation of
DNA damage and increase in oxidative stress were associated with
the aging process in the brain (5,8,22).
In addition, Redd1 is a well-known negative regulator of mTOR
(20,21). Based on the previous and present
findings, it was hypothesized that the increase of Redd1 in the
aged hippocampus may be a compensatory mechanism against the
elevation of DNA damage and oxidative stress, and that the enhanced
expression of Redd1 may lead to a decline in mTOR activity.
In conclusion, the present study showed that the
protein expression of Redd1 in the hippocampus was markedly
increased with age during normal aging, and indicated that the
age-dependent increase in Redd1 may be closely associated with
age-related changes in the hippocampus, including an increase in
DNA damage and oxidative stress, as well as changes in mTOR
activity.
Acknowledgments
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea,
funded by the Ministry of Education (grant no. NRF-2014R1
A1A2058440), and by the Basic Science Research Program through the
National Research Foundation of Korea, funded by the Ministry of
Science, ICT and Future Planning (grant no.
NRF-2014R1A2A2A01005307)′.
References
|
1
|
Hou XQ, Wu DW, Zhang CX, Yan R, Yang C,
Rong CP, Zhang L, Chang X, Su RY, Zhang SJ, et al: Bushen-Yizhi
formula ameliorates cognition deficits and attenuates oxidative
stress-related neuronal apoptosis in scopolamine-induced senescence
in mice. Int J Mol Med. 34:429–439. 2014.PubMed/NCBI
|
|
2
|
Lister JP and Barnes CA: Neurobiological
changes in the hippocampus during normative aging. Arch Neurol.
66:829–833. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang X, Wang JG, Ma DB, Ma XF, Zhu GJ,
Zhou H, Yu CJ, Qian XY and Gao X: Myosin light chain kinase
regulates hearing in mice by influencing the F-actin cytoskeleton
of outer hair cells and cochleae. Int J Mol Med. 33:905–912.
2014.PubMed/NCBI
|
|
4
|
Zou L, Yang R, Zhang P and Dai Y: The
enhancement of amyloid precursor protein and beta-site amyloid
cleavage enzyme 1 interaction: Amyloid-beta production with aging.
Int J Mol Med. 25:401–407. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cardozo-Pelaez F, Brooks PJ, Stedeford T,
Song S and Sanchez-Ramos J: DNA damage, repair, and antioxidant
systems in brain regions: A correlative study. Free Radic Biol Med.
28:779–785. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee CH, Park JH, Choi JH, Yoo KY, Ryu PD
and Won MH: Heat shock protein 90 and its cochaperone, p23, are
markedly increased in the aged gerbil hippocampus. Exp Gerontol.
46:768–772. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rutten BP, Korr H, Steinbusch HW and
Schmitz C: The aging brain: Less neurons could be better. Mech
Ageing Dev. 124:349–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shigenaga MK, Hagen TM and Ames BN:
Oxidative damage and mitochondrial decay in aging. Proc Natl Acad
Sci USA. 91:10771–10778. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang F, Chu X, Yin M, Liu X, Yuan H, Niu Y
and Fu L: MTOR and autophagy in normal brain aging and caloric
restriction ameliorating age-related cognition deficits. Behav
Brain Res. 264:82–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang R, Kadar T, Sirimanne E, MacGibbon A
and Guan J: Age-related memory decline is associated with vascular
and microglial degeneration in aged rats. Behav Brain Res.
235:210–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jacobson L, Zhang R, Elliffe D, Chen KF,
Mathai S, McCarthy D, Waldvogel H and Guan J: Correlation of
cellular changes and spatial memory during aging in rats. Exp
Gerontol. 43:929–938. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rosenzweig ES and Barnes CA: Impact of
aging on hippocampal function: Plasticity, network dynamics, and
cognition. Prog Neurobiol. 69:143–179. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Laplante M and Sabatini DM: MTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harrison DE, Strong R, Sharp ZD, Nelson
JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter
CS, et al: Rapamycin fed late in life extends lifespan in
genetically heterogeneous mice. Nature. 460:392–395.
2009.PubMed/NCBI
|
|
15
|
Maiese K, Chong ZZ, Shang YC and Wang S:
MTOR: On target for novel therapeutic strategies in the nervous
system. Trends Mol Med. 19:51–60. 2013. View Article : Google Scholar :
|
|
16
|
Selman C, Tullet JM, Wieser D, Irvine E,
Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D,
Ramadani F, et al: Ribosomal protein S6 kinase 1 signaling
regulates mammalian life span. Science. 326:140–144. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jaworski J, Spangler S, Seeburg DP,
Hoogenraad CC and Sheng M: Control of dendritic arborization by the
phosphoinositide-3′-kina se-Akt-mammalian target of rapamycin
pathway. J Neurosci. 25:11300–11312. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kumar V, Zhang MX, Swank MW, Kunz J and Wu
GY: Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and
Ras-MAPK signaling pathways. J Neurosci. 25:11288–11299. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee CC, Huang CC and Hsu KS: Insulin
promotes dendritic spine and synapse formation by the PI3K/Akt/mTOR
and Rac1 signaling pathways. Neuropharmacology. 61:867–879. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ellisen LW: Growth control under stress:
MTOR regulation through the REDD1-TSC pathway. Cell Cycle.
4:1500–1502. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shoshani T, Faerman A, Mett I, Zelin E,
Tenne T, Gorodin S, Moshel Y, Elbaz S, Budanov A, Chajut A, et al:
Identification of a novel hypoxia-inducible factor 1-responsive
gene, RTP801, involved in apoptosis. Mol Cell Biol. 22:2283–2293.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ellisen LW, Ramsayer KD, Johannessen CM,
Yang A, Beppu H, Minda K, Oliner JD, McKeon F and Haber DA: REDD1,
a developmentally regulated transcriptional target of p63 and p53,
links p63 to regulation of reactive oxygen species. Mol Cell.
10:995–1005. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee CH, Park JH, Cho JH, Ahn JH, Yan BC,
Lee JC, Shin MC, Cheon SH, Cho YS, Cho JH, et al: Changes and
expressions of Redd1 in neurons and glial cells in the gerbil
hippocampus proper following transient global cerebral ischemia. J
Neurol Sci. 344:43–50. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Malagelada C, Jin ZH and Greene LA: RTP801
is induced in Parkinson's disease and mediates neuron death by
inhibiting Akt phosphorylation/activation. J Neurosci.
28:14363–14371. 2008. View Article : Google Scholar
|
|
25
|
Horak P, Crawford AR, Vadysirisack DD,
Nash ZM, DeYoung MP, Sgroi D and Ellisen LW: Negative feedback
control of HIF-1 through REDD1-regulated ROS suppresses
tumorigenesis. Proc Natl Acad Sci USA. 107:4675–4680. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Malagelada C, López-Toledano MA, Willett
RT, Jin ZH, Shelanski ML and Greene LA: RTP801/REDD1 regulates the
timing of cortical neurogenesis and neuron migration. J Neurosci.
31:3186–3196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mansfield KD, Guzy RD, Pan Y, Young RM,
Cash TP, Schumacker PT and Simon MC: Mitochondrial dysfunction
resulting from loss of cytochrome c impairs cellular oxygen sensing
and hypoxic HIF-alpha activation. Cell Metab. 1:393–399. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang L, Zhang J, Zheng K, Shen H and Chen
X: Long-term ginsenoside Rg1 supplementation improves age-related
cognitive decline by promoting synaptic plasticity associated
protein expression in C57BL/6J mice. J Gerontol A Biol Sci Med Sci.
69:282–294. 2014. View Article : Google Scholar
|
|
29
|
Cheal ML: The gerbil: A unique model for
research on aging. Exp Aging Res. 12:3–21. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Institute of Laboratory Animal Research,
Committee for the Update of the Guide for the Care and Use of
Laboratory Animals and National Research Council: Guide For The
Care And Use Of Laboratory Animals. 8th edition. National Academies
Press; Washington, DC: pp. 2202011
|
|
31
|
Lee CH and Won MH: Increased dynamin-1 and
-2 protein expression in the aged gerbil hippocampus. Cell Mol
Neurobiol. 34:791–796. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lazic SE: Statistical evaluation of
methods for quantifying gene expression by autoradiography in
histological sections. BMC Neuroscience. 10:1–15. 2009. View Article : Google Scholar
|
|
33
|
Varghese F, Bukhari AB, Malhotra R and De
A: IHC Profiler: an open source plugin for the quantitative
evaluation and automated scoring of immunohistochemistry images of
human tissue samples. PloS one. 9:e968012014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Murakami M, Ichisaka T, Maeda M, Oshiro N,
Hara K, Edenhofer F, Kiyama H, Yonezawa K and Yamanaka S: MTOR is
essential for growth and proliferation in early mouse embryos and
embryonic stem cells. Mol Cell Biol. 24:6710–6718. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Halloran J, Hussong SA, Burbank R,
Podlutskaya N, Fischer KE, Sloane LB, Austad SN, Strong R,
Richardson A, Hart MJ and Galvan V: Chronic inhibition of mammalian
target of rapamycin by rapamycin modulates cognitive and
non-cognitive components of behavior throughout lifespan in mice.
Neuroscience. 223:102–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Majumder S, Caccamo A, Medina DX,
Benavides AD, Javors MA, Kraig E, Strong R, Richardson A and Oddo
S: Lifelong rapamycin administration ameliorates age-dependent
cognitive deficits by reducing IL-1β and enhancing NMDA signaling.
Aging Cell. 11:326–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kolosova NG, Vitovtov AO, Muraleva NA,
Akulov AE, Stefanova NA and Blagosklonny MV: Rapamycin suppresses
brain aging in senescence-accelerated OXYS rats. Aging (Albany NY).
5:474–484. 2013.
|
|
38
|
Jinno S and Kosaka T: Stereological
estimation of numerical densities of glutamatergic principal
neurons in the mouse hippocampus. Hippocampus. 20:829–840.
2010.
|