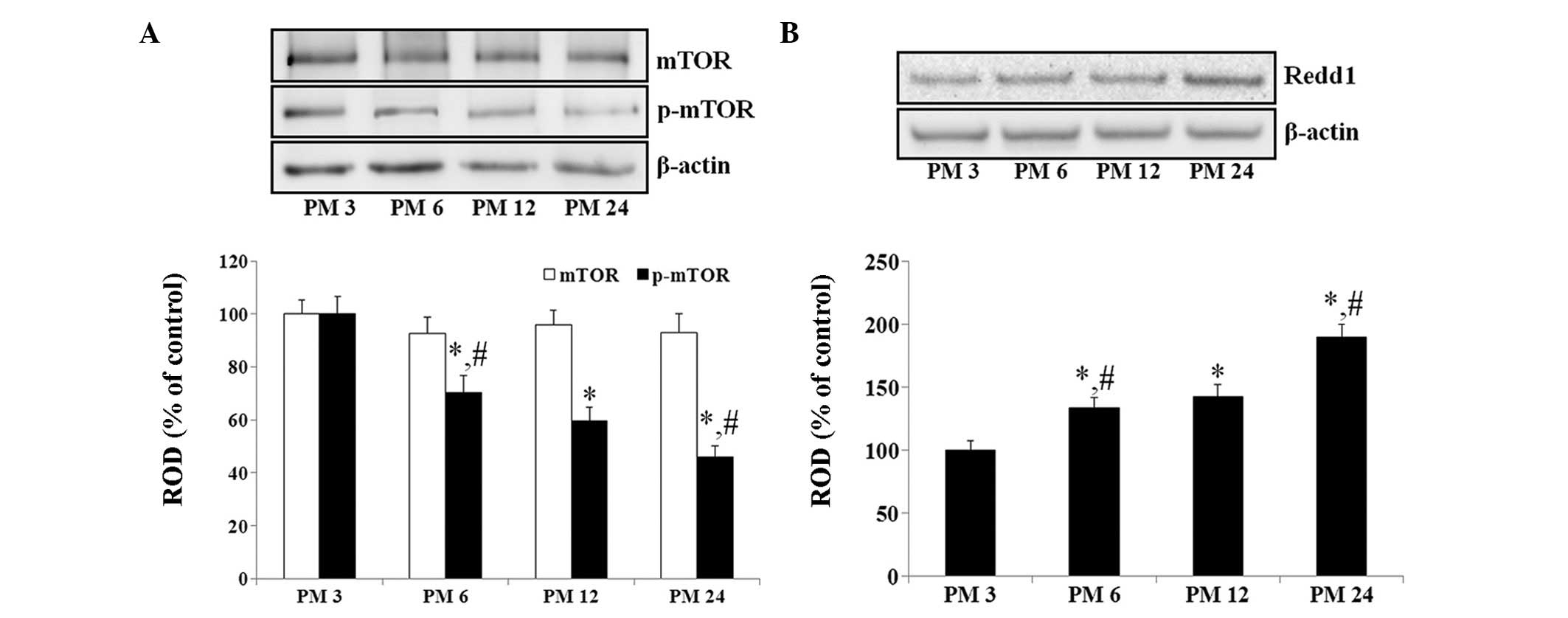
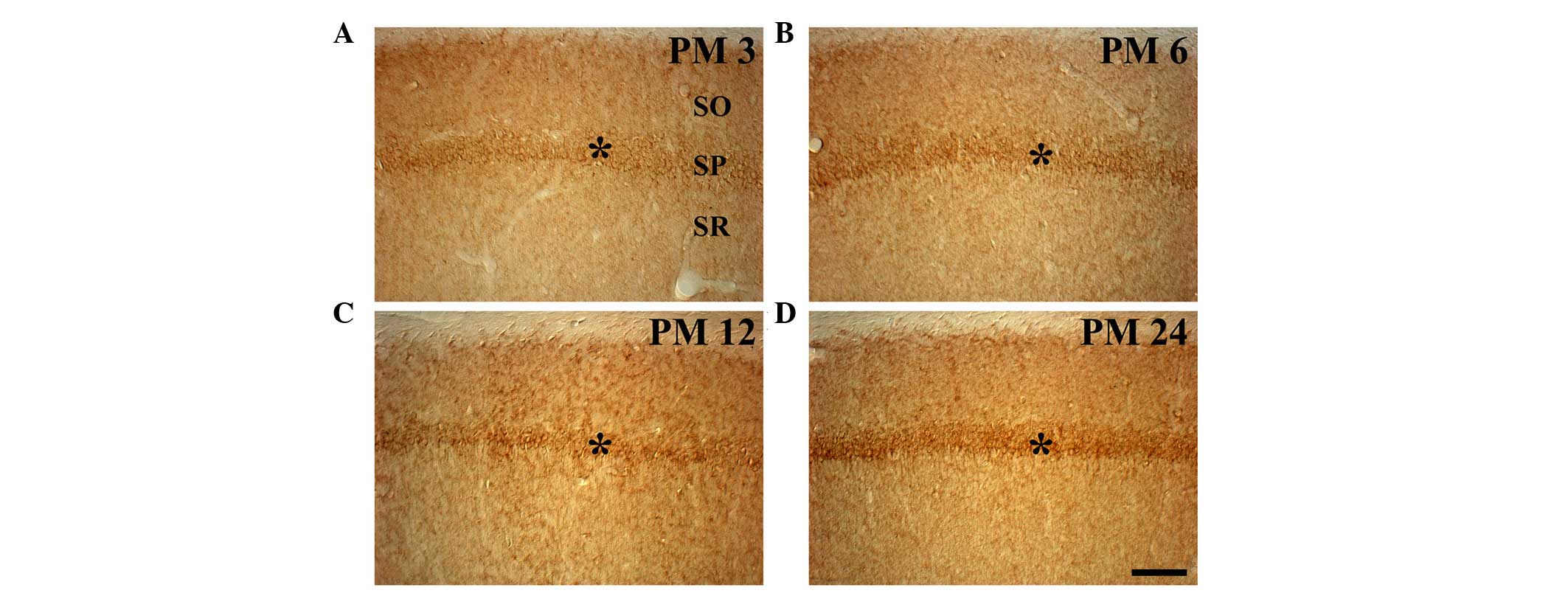
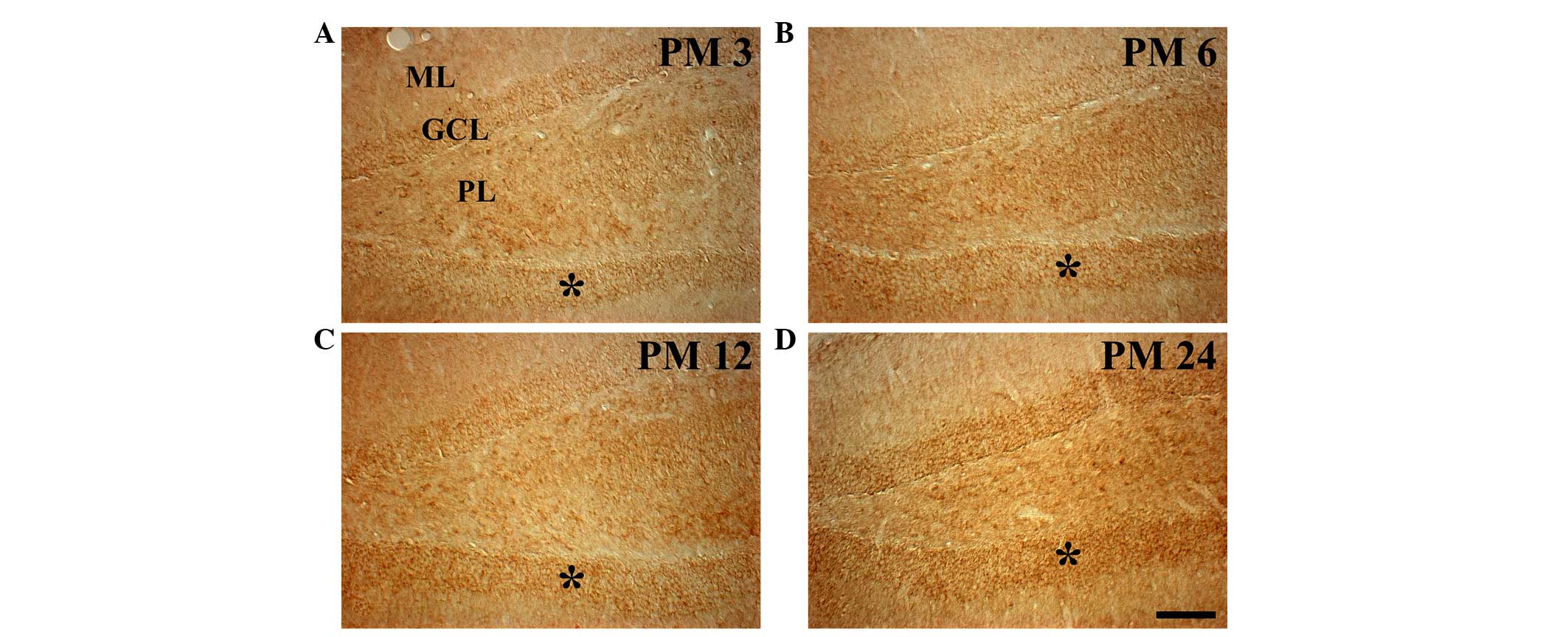
|
1
|
Hou XQ, Wu DW, Zhang CX, Yan R, Yang C,
Rong CP, Zhang L, Chang X, Su RY, Zhang SJ, et al: Bushen-Yizhi
formula ameliorates cognition deficits and attenuates oxidative
stress-related neuronal apoptosis in scopolamine-induced senescence
in mice. Int J Mol Med. 34:429–439. 2014.PubMed/NCBI
|
|
2
|
Lister JP and Barnes CA: Neurobiological
changes in the hippocampus during normative aging. Arch Neurol.
66:829–833. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang X, Wang JG, Ma DB, Ma XF, Zhu GJ,
Zhou H, Yu CJ, Qian XY and Gao X: Myosin light chain kinase
regulates hearing in mice by influencing the F-actin cytoskeleton
of outer hair cells and cochleae. Int J Mol Med. 33:905–912.
2014.PubMed/NCBI
|
|
4
|
Zou L, Yang R, Zhang P and Dai Y: The
enhancement of amyloid precursor protein and beta-site amyloid
cleavage enzyme 1 interaction: Amyloid-beta production with aging.
Int J Mol Med. 25:401–407. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cardozo-Pelaez F, Brooks PJ, Stedeford T,
Song S and Sanchez-Ramos J: DNA damage, repair, and antioxidant
systems in brain regions: A correlative study. Free Radic Biol Med.
28:779–785. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee CH, Park JH, Choi JH, Yoo KY, Ryu PD
and Won MH: Heat shock protein 90 and its cochaperone, p23, are
markedly increased in the aged gerbil hippocampus. Exp Gerontol.
46:768–772. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rutten BP, Korr H, Steinbusch HW and
Schmitz C: The aging brain: Less neurons could be better. Mech
Ageing Dev. 124:349–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shigenaga MK, Hagen TM and Ames BN:
Oxidative damage and mitochondrial decay in aging. Proc Natl Acad
Sci USA. 91:10771–10778. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang F, Chu X, Yin M, Liu X, Yuan H, Niu Y
and Fu L: MTOR and autophagy in normal brain aging and caloric
restriction ameliorating age-related cognition deficits. Behav
Brain Res. 264:82–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang R, Kadar T, Sirimanne E, MacGibbon A
and Guan J: Age-related memory decline is associated with vascular
and microglial degeneration in aged rats. Behav Brain Res.
235:210–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jacobson L, Zhang R, Elliffe D, Chen KF,
Mathai S, McCarthy D, Waldvogel H and Guan J: Correlation of
cellular changes and spatial memory during aging in rats. Exp
Gerontol. 43:929–938. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rosenzweig ES and Barnes CA: Impact of
aging on hippocampal function: Plasticity, network dynamics, and
cognition. Prog Neurobiol. 69:143–179. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Laplante M and Sabatini DM: MTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harrison DE, Strong R, Sharp ZD, Nelson
JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter
CS, et al: Rapamycin fed late in life extends lifespan in
genetically heterogeneous mice. Nature. 460:392–395.
2009.PubMed/NCBI
|
|
15
|
Maiese K, Chong ZZ, Shang YC and Wang S:
MTOR: On target for novel therapeutic strategies in the nervous
system. Trends Mol Med. 19:51–60. 2013. View Article : Google Scholar :
|
|
16
|
Selman C, Tullet JM, Wieser D, Irvine E,
Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D,
Ramadani F, et al: Ribosomal protein S6 kinase 1 signaling
regulates mammalian life span. Science. 326:140–144. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jaworski J, Spangler S, Seeburg DP,
Hoogenraad CC and Sheng M: Control of dendritic arborization by the
phosphoinositide-3′-kina se-Akt-mammalian target of rapamycin
pathway. J Neurosci. 25:11300–11312. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kumar V, Zhang MX, Swank MW, Kunz J and Wu
GY: Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and
Ras-MAPK signaling pathways. J Neurosci. 25:11288–11299. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee CC, Huang CC and Hsu KS: Insulin
promotes dendritic spine and synapse formation by the PI3K/Akt/mTOR
and Rac1 signaling pathways. Neuropharmacology. 61:867–879. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ellisen LW: Growth control under stress:
MTOR regulation through the REDD1-TSC pathway. Cell Cycle.
4:1500–1502. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shoshani T, Faerman A, Mett I, Zelin E,
Tenne T, Gorodin S, Moshel Y, Elbaz S, Budanov A, Chajut A, et al:
Identification of a novel hypoxia-inducible factor 1-responsive
gene, RTP801, involved in apoptosis. Mol Cell Biol. 22:2283–2293.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ellisen LW, Ramsayer KD, Johannessen CM,
Yang A, Beppu H, Minda K, Oliner JD, McKeon F and Haber DA: REDD1,
a developmentally regulated transcriptional target of p63 and p53,
links p63 to regulation of reactive oxygen species. Mol Cell.
10:995–1005. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee CH, Park JH, Cho JH, Ahn JH, Yan BC,
Lee JC, Shin MC, Cheon SH, Cho YS, Cho JH, et al: Changes and
expressions of Redd1 in neurons and glial cells in the gerbil
hippocampus proper following transient global cerebral ischemia. J
Neurol Sci. 344:43–50. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Malagelada C, Jin ZH and Greene LA: RTP801
is induced in Parkinson's disease and mediates neuron death by
inhibiting Akt phosphorylation/activation. J Neurosci.
28:14363–14371. 2008. View Article : Google Scholar
|
|
25
|
Horak P, Crawford AR, Vadysirisack DD,
Nash ZM, DeYoung MP, Sgroi D and Ellisen LW: Negative feedback
control of HIF-1 through REDD1-regulated ROS suppresses
tumorigenesis. Proc Natl Acad Sci USA. 107:4675–4680. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Malagelada C, López-Toledano MA, Willett
RT, Jin ZH, Shelanski ML and Greene LA: RTP801/REDD1 regulates the
timing of cortical neurogenesis and neuron migration. J Neurosci.
31:3186–3196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mansfield KD, Guzy RD, Pan Y, Young RM,
Cash TP, Schumacker PT and Simon MC: Mitochondrial dysfunction
resulting from loss of cytochrome c impairs cellular oxygen sensing
and hypoxic HIF-alpha activation. Cell Metab. 1:393–399. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang L, Zhang J, Zheng K, Shen H and Chen
X: Long-term ginsenoside Rg1 supplementation improves age-related
cognitive decline by promoting synaptic plasticity associated
protein expression in C57BL/6J mice. J Gerontol A Biol Sci Med Sci.
69:282–294. 2014. View Article : Google Scholar
|
|
29
|
Cheal ML: The gerbil: A unique model for
research on aging. Exp Aging Res. 12:3–21. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Institute of Laboratory Animal Research,
Committee for the Update of the Guide for the Care and Use of
Laboratory Animals and National Research Council: Guide For The
Care And Use Of Laboratory Animals. 8th edition. National Academies
Press; Washington, DC: pp. 2202011
|
|
31
|
Lee CH and Won MH: Increased dynamin-1 and
-2 protein expression in the aged gerbil hippocampus. Cell Mol
Neurobiol. 34:791–796. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lazic SE: Statistical evaluation of
methods for quantifying gene expression by autoradiography in
histological sections. BMC Neuroscience. 10:1–15. 2009. View Article : Google Scholar
|
|
33
|
Varghese F, Bukhari AB, Malhotra R and De
A: IHC Profiler: an open source plugin for the quantitative
evaluation and automated scoring of immunohistochemistry images of
human tissue samples. PloS one. 9:e968012014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Murakami M, Ichisaka T, Maeda M, Oshiro N,
Hara K, Edenhofer F, Kiyama H, Yonezawa K and Yamanaka S: MTOR is
essential for growth and proliferation in early mouse embryos and
embryonic stem cells. Mol Cell Biol. 24:6710–6718. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Halloran J, Hussong SA, Burbank R,
Podlutskaya N, Fischer KE, Sloane LB, Austad SN, Strong R,
Richardson A, Hart MJ and Galvan V: Chronic inhibition of mammalian
target of rapamycin by rapamycin modulates cognitive and
non-cognitive components of behavior throughout lifespan in mice.
Neuroscience. 223:102–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Majumder S, Caccamo A, Medina DX,
Benavides AD, Javors MA, Kraig E, Strong R, Richardson A and Oddo
S: Lifelong rapamycin administration ameliorates age-dependent
cognitive deficits by reducing IL-1β and enhancing NMDA signaling.
Aging Cell. 11:326–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kolosova NG, Vitovtov AO, Muraleva NA,
Akulov AE, Stefanova NA and Blagosklonny MV: Rapamycin suppresses
brain aging in senescence-accelerated OXYS rats. Aging (Albany NY).
5:474–484. 2013.
|
|
38
|
Jinno S and Kosaka T: Stereological
estimation of numerical densities of glutamatergic principal
neurons in the mouse hippocampus. Hippocampus. 20:829–840.
2010.
|