Introduction
Atrial fibrillation (AF) is one of the most common
types of arrhythmia encountered in clinical practice (1). The main characteristics of AF are the
alterations in atrial electrophysiology and structure, which is
termed electrophysiological and structural remodeling and is
important in the development, maintenance and recurrence of AF
(2,3). Ion influx via calcium and potassium
channels is necessary for cellular neurochemical changes.
Alterations to the expression of ion channels, particularly L-type
calcium channels (LTCCs) and Kv4.3 potassium channels, form the
possible basis of early remodeling during rapid pacing (4–6).
However, the pathogenetic mechanisms underlying atrial structural
remodeling remain poorly understood.
Alterations to cytoplasmic Ca2+
concentration have a critical role in the occurrence and
maintenance of AF (7).
Stimulation, such as the arrival of an action potential, increases
Ca2+ entry from the extracellular space through LTCCs,
and the resultant intracellular Ca2+ elevation (calcium
overload) mediates atrial electrical remodeling (8). Increased cytoplasmic Ca2+
can not only exert feedback inhibition, which curtails further
Ca2+ entry (9), but
also activates other signaling molecules, including calmodulin and
mitogen-activated protein kinase (MAPK) (10,11).
A previous study demonstrated that patients with permanent AF
exhibit severe alterations in cardiac tissue architecture,
accompanied by increased atrial expression of extracellular
signal-regulated kinase 1/2 (ERK-1/2), one of the three major
members of the MAPK family (12).
In addition, L-type CaV1.2 channels have previously been
demonstrated to activate Ca2+-mediated second messenger
pathways, including the MAPK pathway (13). These studies suggest that the MAPK
pathway may consist of attractive candidate kinases for the
regulation and mediation of Ca2+-induced ion channel
remodeling. The present study aimed to investigate alterations to
the ion channel-MAPK axis, and to determine its influence on ion
channel remodeling during AF.
Materials and methods
Isolation and culture of rat atrial
myocytes
The present study and all experimental protocols
involved were approved by the Institutional Animal Care and Use
Committee of the Third Military Medical University (Chongqing,
China). A total of 20 female Wistar rats (2-week-old) were
purchased from the Experimental Animal Center of the Third Military
Medical University (Chongqing, China). All animals were housed in
the same temperature (22±2°C) and humidity-controlled holding
facility with free access to food and water, and were exposed to a
regular 12/12-h light/dark cycle. Rats were anesthetized with
over-dosed CO2 (Chongqing Qunhe Medical Instrument Co.,
Ltd., Chongqing, China) and then fixed in a supine position. After
sterilization, an incision was made along the right edge of the
sternum and the chest wall was removed. The heart was retrieved and
then washed in cold phosphate-buffered saline. Subsequently, the
left and right atria were isolated and washed in serum-free
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Under aseptic conditions, the
right atrial appendage was trimmed with scissors into small
sections of ~1 mm3, which were digested with 0.08%
trypsin at 37°C for 5 min. The digestion was inactivated by the
addition of medium containing 5% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.). The solution was maintained at room
temperature for 5 min and the supernatant was subsequently filtered
through a 100-mesh filter. Digestion was performed twice. Finally,
suspensions of single cells were prepared by treatment of the
digested product with 0.1% type II collagenase (Sigma-Aldrich,
Steinheim, Germany) at 37°C for 15 min. The cells were then seeded
into flasks at a density of ~1×108/l, followed by
incubation with DMEM supplemented with 10% fetal bovine serum at
37°C in an atmosphere containing 5% CO2. In the control
group, after 72 h of routine culture, the medium was replaced with
serum-free DMEM for 24 h, and rapid pacing was performed for up to
24 h. In the experimental groups, after 72 h of routine culture,
the medium was replaced with serum-free DMEM for 24 h, and the
following reagents were added to the media of the various groups:
Verapamil (Sigma-Aldrich, St. Louis, MO, USA), PD98058
(Sigma-Aldrich), SB203580 (Sigma-Aldrich), or PD98058 + SB203580 at
the same concentration (10 µmol/l) for 30 min.
Rapid pacing of atrial myocardial
cells
Once cell confluence reached ~80%, the culture
dishes were placed in an electric field. The cells were stimulated
with 10 Hz, 1.5 V/cm using the BL-420E+ Biological and Functional
Experimental system (PCLab, Chengdu, China) at 3, 6, 12 and 24
h.
Monitoring of intracellular
Ca2+
The intracellular Ca2+ signals were
recorded in the rat atrial myocytes using the Fluo-3/AM
Ca2+ indicator (Invitrogen; Thermo Fisher Scientific,
Inc.) at a concentration of 2 µmol/l. Briefly, the growth
medium was removed from the cells and was replaced with dye-loading
medium (100 µl/well) containing 2 µM Fluo-3/AM
Ca2+ indicator and 20% Pluronic acid F127 in Locke's
buffer (154 mM NaCl, 5.6 mM KCl, 1.0 mM MgCl2, 2.3 mM
CaCl2, 8.6 mM HEPES, 5.6 mM glucose and 0.1m M glycine;
pH 7.4). The cells were incubated at 37°C in an atmosphere
containing 5% CO2 for 30 min, and were washed four times
with fresh DMEM. Calcium imaging was carried out using a Leica TCS
SP5 confocal microscope (Leica Microsystems, Wetzlar, Germany).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
The mRNA expression levels of LTCC-α1c and Kv4.3
potassium channels were detected by RT-PCR. Total cellular RNA was
extracted from the rat atrial myocytes of each group using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), and cDNA was prepared using a Superscript II First-Strand
cDNA Synthesis kit (Invitrogen; Thermo Fisher Scientific, Inc.),
according to manufacturer's protocol. RT-PCR was performed and the
primer sequences were designed as follows: α1c, forward
5′-ATGGAGGCTGGAGCCCAGATTGA-3′, reverse
5′-GACATTGAGGTCCGCACCGAAGG-3′ (annealing temperature, 61.3°C);
Kv4.3, forward 5′-GCAGCAACCTGAAATCTGAAACT-3′, reverse
5′-GATAAGCAATGAACCCATCTCCA-3′ (annealing temperature, 56.1°C); and
β-actin, forward 5′-TGAGAGGGAAATCGTGCGTGAC-3′ and reverse
5′-ATCTGCTGGAAGGTGGACAGTGAG-3′ (annealing temperature: 53.9°C).
These primers were synthesized by Sangon Biotech Co., Ltd.
(Shanghai, China). PCR was run on the Veriti Thermal Cycler (Thermo
Fisher Scientific, Inc.) with each sample at 20 µl,
including 1 µl primer, 10 µl Taq Master mix (2X,
E00019, Genscript Biotech Co., Nanjing, China) and 9 µl
deionized/distilled water. The amplification process was performed
for 25 cycles: Initial denaturation at 94°C for 45 sec, annealing
for 30 sec at the indicated temperature, and final extension for 5
min at 72°C. PCR products were separated by 1% agarose gel
electrophoresis and were stained with ethidium bromide.
Western blot analysis
A total of 1.5×106 rat atrial myocytes
from each group were lysed in 0.5 ml radioimmunoprecipitation assay
[50 mM Tris-HCl (pH 7.2), 150 mM NaCl, 1% NP40, 0.1% SDS, 0.5% DOC,
1 mM PMSF, 25 mM MgCl2 and phosphatase inhibitor
cocktail (Shanghai Qcbio Science & Technologies Co., Ltd.,
Shanghai, China)] buffer and 5 µl phenylmethylsulfonyl
fluoride. Cell homogenates were centrifuged at 12,000 × g for 30
min and the resulting supernatant (total tissue homogenate) was
stored at −80°C for further analysis. Protein concentration was
quantified using a BCA Protein Quantification kit (Abcam,
Cambridge, MA, USA) and a total of 15 µg protein from each
group was separated by 10% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis and transferred to polyvinylidene difluoride
membranes (EMD Millipore, Temecula, CA, USA). The membranes were
blocked by incubation with 5% BSA for 1 h and then incubated
overnight at 4°C for 1 h with primary antibodies, followed by
incubation with horseradish peroxidase (HRP)-conjugated secondary
antibodies for 2 h at room temperature. The following primary
antibodies were used: Rabbit polyclonal anti-rat ERK (cat. no.
sc-94; 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
rabbit polyclonal anti-rat phosphorylated (p)-ERK (cat. no.
sc-16982; 1:500; Santa Cruz Biotechnology, Inc.), rabbit polyclonal
anti-rat p38MAPK (cat. no. sc-535; 1:500; Santa Cruz Biotechnology,
Inc.), rabbit anti-rat p-p38MAPK (1:500; cat. no. sc-101759; Santa
Cruz Biotechnology, Inc.), rabbit anti-rat α1c (cat. no. AB5150;
1:2,000; Chemicon; EMD Millipore, Billerica, MA, USA), rabbit
anti-rat Kv4.3 (cat. no. AB5194; 1:1,000; Chemicon; EMD Millipore)
and rabbit polyclonal anti-GAPDH antibody (cat. no. ABS16; EMD
Millipore) was used as internal control. The secondary antibodies
used were as follows: HRP-conjugated goat anti-rabbit
immunoglobulin G (cat. no. ANT-178; 1:5,000 dilution; Beijing
Dingguo Biotechnology Co., Ltd., Beijing, China). HRP
chemiluminescent substrate was used and stained blots were
visualized using an Odyssey Imaging System (Li-Cor Biosciences,
Lincoln, NE, USA). Gel quantification was conducted using ImageJ
software (version 1.2; National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
Statistical analysis was performed using SPSS
version 17.0 (SPSS, Inc., Chicago, IL, USA). Group comparison was
performed using one-factor analysis of variance. Data are presented
as the mean ± standard deviation. P<0.05 was considered to
indicate a statistically significant difference.
Results
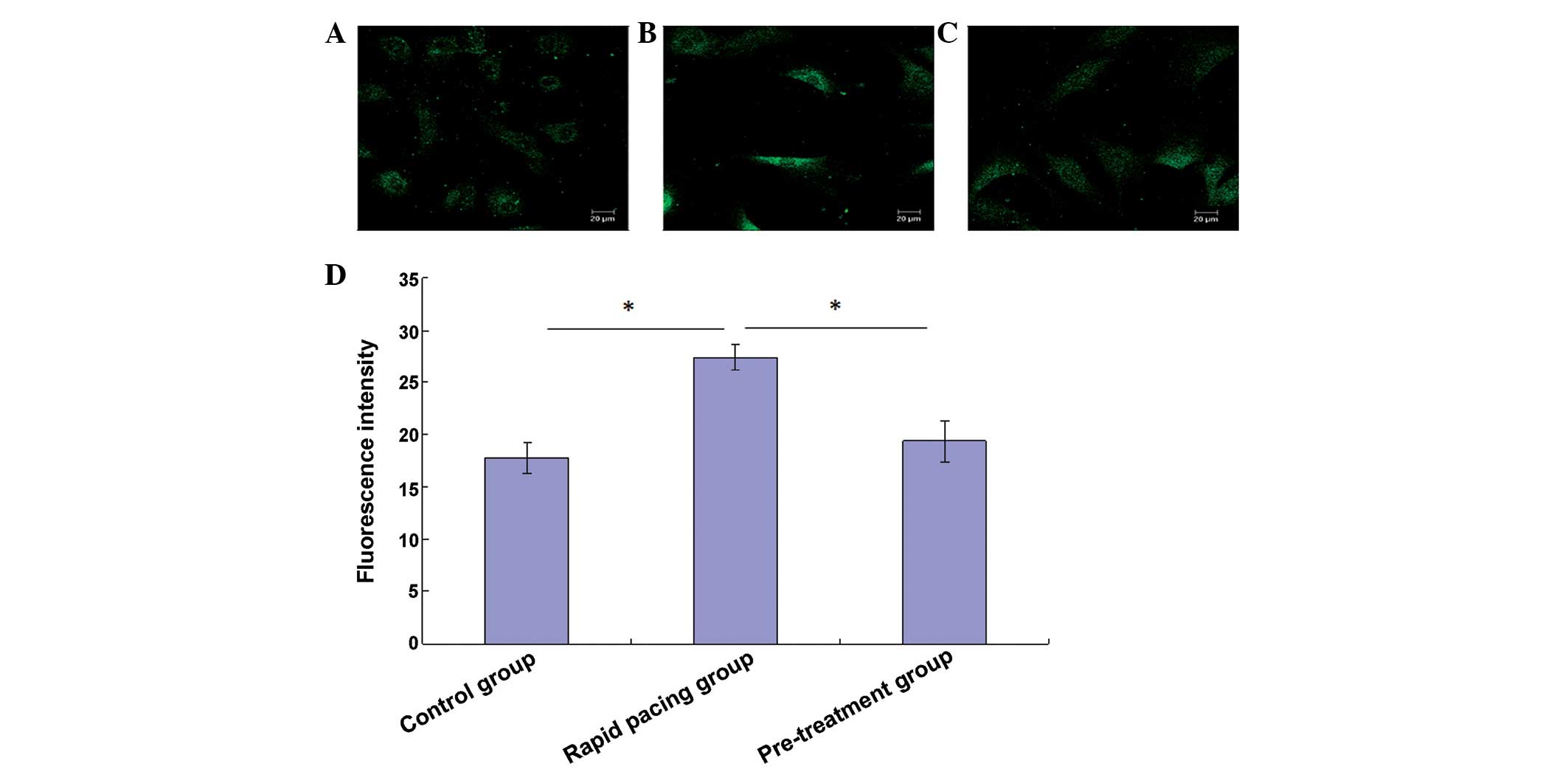
Verapamil pretreatment inhibits fast
pacing-induced increases in intracellular Ca2+
concentration
The Fluo-3/AM Ca2+ indicator was used to
monitor Ca2+ concentration (Fig. 1). Fast pacing of rat atrial
myocytes significantly increased intracellular Ca2+
concentrations, which was reflected by increases in intracellular
calcium fluorescence intensity 24 h after rapid pacing (Fig. 1A, B and D). Conversely, verapamil
pretreatment prior to pacing significantly reduced the increase in
intracellular calcium fluorescence intensity (Fig. 1C and D).
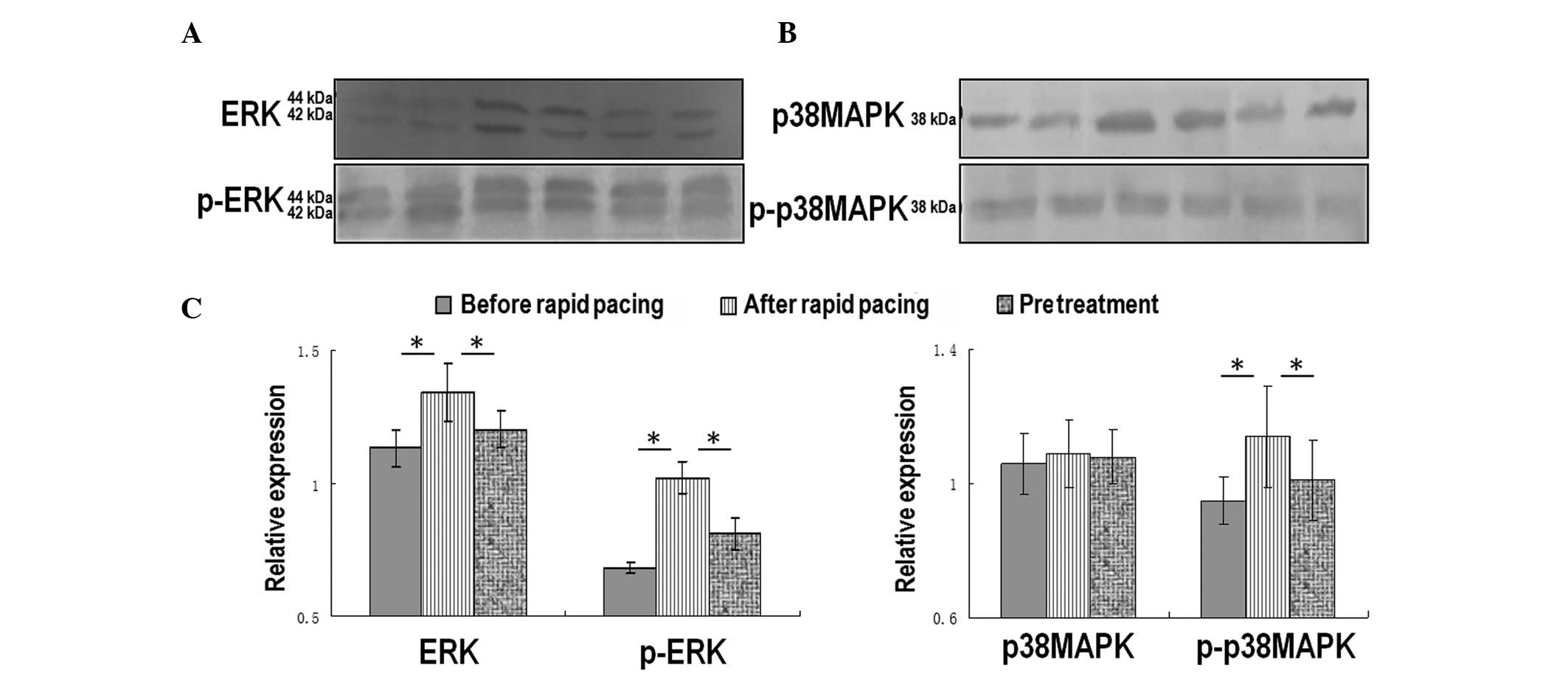
Alterations to ERK and p38MAPK protein
expression during fast pacing
The total and phosphorylated forms of two subtypes
of the MAPK family: ERK and p38MAPK were detected by western
blotting (Fig. 2). The expression
levels of total and p-ERK protein were significantly increased 24 h
after fast pacing (Fig. 2A and C).
The expression levels of phosphorylated p38MAPK were upregulated 24
h after fast pacing; however, total p38MAPK expression remained
changed (Fig. 2B and C). Notably,
verapamil pretreatment significantly inhibited the upregulation of
both phosphorylated proteins (Fig. 2A
and C).
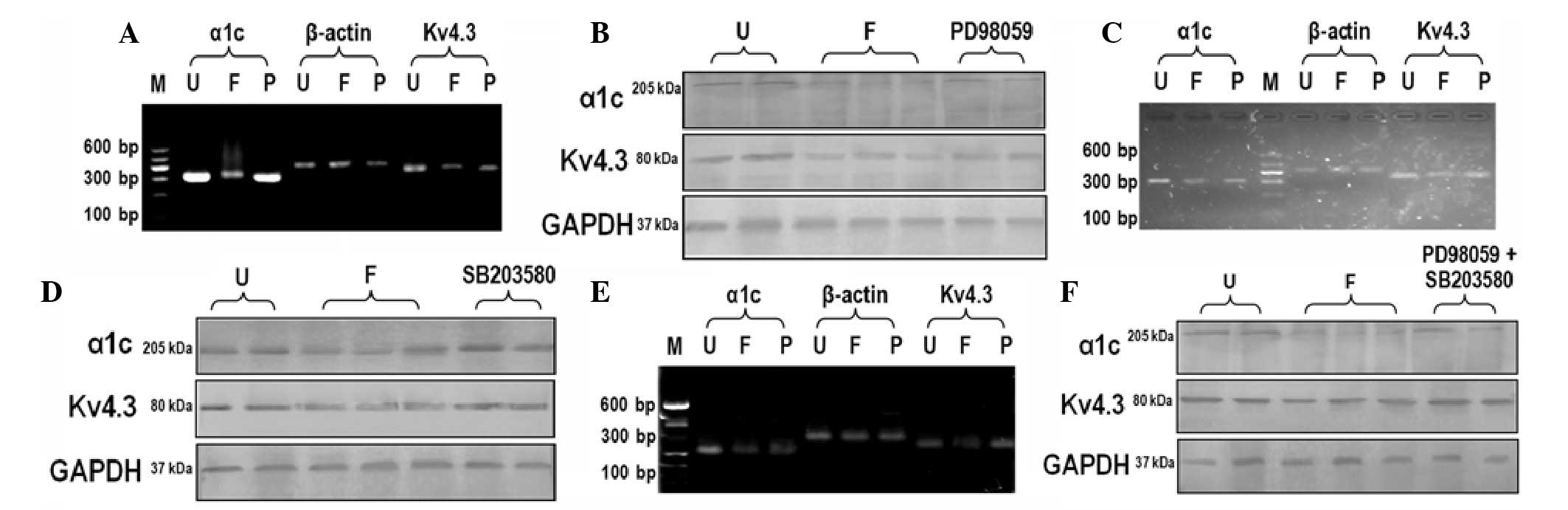
Effects of PD98059 on the expression of
ion channel proteins
The mRNA expression levels of LTCC-α1c and Kv4.3
potassium channels were significantly lower 24 h after fast pacing,
as compared with those prior to pacing (Fig. 3A). This downregulation was
diminished by pretreatment with the ERK1/2-specific inhibitor
PD98059. However, compared with the untreated group, the expression
levels of α1c and Kv4.3 were lower in the PD98059 group. The
results of western blotting indicated that the protein expression
levels corroborated the PCR results (Fig. 3B).
Effects of SB203580 on the expression of
ion channel proteins
Similar to the effects of PD98059, treatment with
SB203580 could rescue the fast pacing-induced downregulation of α1c
and Kv4.3 expression (Fig. 3C and
D). However, the effects were not as noticeable as the effects
of PD98059.
Effects of PD98059 and SB203580
co-treatment on the expression of ion channel proteins
Simultaneous administration with PD98059 and
SB203580 prior to fast pacing was able to inhibit the
downregulation of α1c and Kv4.3 expression (Fig. 3E and F). However, these reagents
could not totally rescue the expression of α1c and Kv4.3 to normal
levels.
Discussion
Intracellular calcium overload has been reported as
a key factor for electrical remodeling in AF (14). LTCCs, which are a member of the
voltage-dependent calcium channel family, act as a voltage sensor
for excitation-contraction coupling, and are a major pathway for
calcium influx in cardiac and skeletal muscle (15). LTCCs function as macromolecular
complexes, which are involved in the ubiquitous regulation of
intracellular signaling (16). The
Ca2+ influx through LTCCs forms the basis of the
characteristic prolonged 'plateau' phase of the cardiac action
potential (17). LTCC function is
multifaceted, however in cardiac muscle cells it has an essential
role in coupling excitation to contraction. In cardiac myocytes,
LTCCs increase Ca2+ entry and trigger calcium release
from the sarcoplasmic reticulum by activating intracellular calcium
channel ryanodine receptors (18).
In addition, calcium is an important second messenger, which has a
key role in calmodulin-dependent activation of signaling cascades
that convey local calcium signals to the nucleus (19). Therefore, opening of LTCCs may be
considered an important basis of multiple cardiac arrhythmias,
which may result in sudden death. The present study demonstrated
that fast pacing may significantly increase intracellular
Ca2+ concentration in atrial myocytes, and verapamil
treatment distinctly inhibited this increase, thus suggesting that
LTCCs have an important role in Ca2+ influx in the early
phase of fast pacing. That verapamil could significantly antagonize
rapid pacing-induced increased activation of ERK and p38MAPK
indicated that the opening of LTCCs had a close relationship with
alterations to the MAPK pathway.
MAPKs are evolutionarily highly conserved protein
kinases specific to serine, threonine and tyrosine (20). The MAPK family consists of three
major members: p38, ERK and c-Jun N-terminal kinase (JNK). p38MAPK
and JNK mediate cell responses to various stressors, and lead to
cell death when activated (21).
ERK predominantly mediates the proliferation response, with ERK1/2
as the hub of MAPK signaling pathways (22). In the present study, the expression
levels of total ERK1/2, p-ERK1/2 and p-p38MAPK were upregulated 24
h after fast pacing. MAPK is mainly activated by the G
protein-coupled receptor agonist, and can also be activated by
angiotensin II and endothelin-1 via a c-Src dependent mechanism
(23). Other factors, such as high
osmotic stress, hypoxia/reoxygenation injury, free radicals and
reactive oxygen species can also stimulate MAPK activities
(24–26). In a previous study, short-term
rapid electrical stimulation resulted in a significant upregulation
of connexin 43 and conduction velocity in cardiomyocytes, via an
increased autocrine action of angiotensin II that activated ERK and
p38MAPK (27). Pretreatment with
losartan, an antagonist of angiotensin type 1 receptor, inhibited
vasoconstriction, as well as the activation of ERK1/2 and p38MAPK
(28). It may be hypothesized that
electric field stimulation results in Ca2+ influx and
overload, followed by conformational changes of calmodulin upon
Ca2+ binding, and subsequent activation of MAPK
signaling molecules by calmodulin binding.
Several nuclear targets for MAPK pathways have been
identified, including Elk-1, Ets-1, c-Myc, Sap1a, Tal, signal
transducer and activator of transcription, c-Jun and activating
transcription factor 2 (29).
Following mitogen stimulation, MAPK rapidly phosphorylates Elk-1.
ERK activation stimulates activator protein (AP)-1 activity via
induction of c-Fos, which translocates to the nucleus and combines
with pre-existing Jun proteins to form AP-1 dimers that are more
stable than those formed by Jun proteins alone (30). In addition, activated ERK1/2 is
able to phosphorylate numerous substrates, including various
membrane proteins such as CD120a, Syk and calnexin, and cytoplasmic
proteins such as MAPK-activated protein kinase and cytosolic
phospholipase A2 (31). Activation
of the ERK/MAPK signaling cascades may participate in the early
structural remodeling of ion channels in AF. The present study
demonstrated that inhibition of ERK1/2 and/or p38MAPK was able to
rescue the downregulation of α1c and Kv4.3 expression caused by
fast pacing. This is necessary and sufficient to demonstrate the
above hypothesis. In addition, neither single nor combined use of
PD98059 and SB203580 could totally block the fast pacing-induced
downregulation of α1c and Kv4.3 expression, thus suggesting the
existence of other possible regulatory pathways. In addition, a
previous study reported that the function of verapamil to block
LTCC did not emerge if fast pacing lasted for over 2 weeks
(14); therefore, other factors
may participate, such as T-type calcium channels.
In conclusion, the present study demonstrated that
the MAPK pathway had an important role in the opening of LTCCs, and
interruption of MAPK molecules could affect the expression of ion
channels. The reciprocal interaction of the MAPK pathway and ion
channels offers therapeutic potential against AF.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 30600252).
References
|
1
|
Nattel S, Burstein B and Dobrev D: Atrial
remodeling and atrial fibrillation: Mechanisms and implications.
Circ Arrhythm Electrophysiol. 1:62–73. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Allessie M, Ausma J and Schotten U:
Electrical, contractile and structural remodeling during atrial
fibrillation. Cardiovasc Res. 54:230–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krogh-Madsen T, Abbott GW and Christini
DJ: Effects of electrical and structural remodeling on atrial
fibrillation maintenance: A simulation study. PLoS Comput Biol.
8:e10023902012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bosch RF, Scherer CR, Rüb N, Wöhrl S,
Steinmeyer K, Haase H, Busch AE, Seipel L and Kühlkamp V: Molecular
mechanisms of early electrical remodeling: Transcriptional
downregulation of ion channel subunits reduces I(Ca,L) and I(to) in
rapid atrial pacing in rabbits. J Am Coll Cardiol. 41:858–869.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cutler MJ, Jeyaraj D and Rosenbaum DS:
Cardiac electrical remodeling in health and disease. Trends
Pharmacol Sci. 32:174–180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao Z, Xie Y, Wen H, Xiao D, Allen C,
Fefelova N, Dun W, Boyden PA, Qu Z and Xie LH: Role of the
transient outward potassium current in the genesis of early
afterdepolarizations in cardiac cells. Cardiovasc Res. 95:308–316.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Voigt N, Li N, Wang Q, Wang W, Trafford
AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, et al:
Enhanced sarcoplasmic reticulum Ca2+ leak and increased
Na+–Ca2+ exchanger function underlie delayed
afterdepolarizations in patients with chronic atrial fibrillation.
Circulation. 125:2059–2070. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kho C, Lee A and Hajjar RJ: Altered
sarcoplasmic reticulum calcium cycling - targets for heart failure
therapy. Nat Rev Cardiol. 9:717–733. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Parekh AB: Slow feedback inhibition of
calcium release-activated calcium current by calcium entry. J Biol
Chem. 273:14925–14932. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Millon-Frémillon A, Brunner M, Abed N,
Collomb E, Ribba AS, Block MR, Albigès-Rizo C and Bouvard D:
Calcium and calmodulin-dependent serine/threonine protein kinase
type II (CaMKII)-mediated intramolecular opening of integrin
cytoplasmic domain-associated protein-1 (ICAP-1α) negatively
regulates β1 integrins. J Biol Chem. 288:20248–20260. 2013.
View Article : Google Scholar
|
|
11
|
Wurzinger B, Mair A, Pfister B and Teige
M: Cross-talk of calcium-dependent protein kinase and MAP kinase
signaling. Plant Signal Behav. 6:8–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goette A, Staack T, Röcken C, Arndt M,
Geller JC, Huth C, Ansorge S, Klein HU and Lendeckel U: Increased
expression of extracellular signal-regulated kinase and
angiotensin-converting enzyme in human atria during atrial
fibrillation. J Am Coll Cardiol. 35:1669–1677. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rajadhyaksha A, Husson I, Satpute SS,
Küppenbender KD, Ren JQ, Guerriero RM, Standaert DG and Kosofsky
BE: L-type Ca2+ channels mediate adaptation of
extracellular signal-regulated kinase 1/2 phosphorylation in the
ventral tegmental area after chronic amphetamine treatment. J
Neurosci. 24:7464–7476. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goette A, Honeycutt C and Langberg JJ:
Electrical remodeling in atrial fibrillation. Time course and
mechanisms Circulation. 94:2968–2974. 1996.
|
|
15
|
Catterall WA: Excitation-contraction
coupling in vertebrate skeletal muscle: A tale of two calcium
channels. Cell. 64:871–874. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wehrens XH, Lehnart SE and Marks AR:
Intracellular calcium release and cardiac disease. Annu Rev
Physiol. 67:69–98. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
George AL Jr: Molecular and genetic basis
of sudden cardiac death. J Clin Invest. 123:75–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Priori SG and Chen SR: Inherited
dysfunction of sarcoplasmic reticulum Ca2+ handling and
arrhythmogenesis. Circ Res. 108:871–883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hagenston AM and Bading H: Calcium
signaling in synapse-to-nucleus communication. Cold Spring Harb
Perspect Biol. 3:a0045642011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Runchel C, Matsuzawa A and Ichijo H:
Mitogenactivated protein kinases in mammalian oxidative stress
responses. Antioxid Redox Signal. 15:205–218. 2011. View Article : Google Scholar
|
|
22
|
Kolch W: Coordinating ERK/MAPK signalling
through scaffolds and inhibitors. Nat Rev Mol Cell Biol. 6:827–837.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yogi A, Callera GE, Montezano AC, Aranha
AB, Tostes RC, Schiffrin EL and Touyz RM: Endothelin-1, but not Ang
II, activates MAP kinases through c-Src independent Ras-Raf
dependent pathways in vascular smooth muscle cells. Arterioscler
Thromb Vasc Biol. 27:1960–1967. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rosette C and Karin M: Ultraviolet light
and osmotic stress: Activation of the JNK cascade through multiple
growth factor and cytokine receptors. Science. 274:1194–1197. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Laderoute KR and Webster KA:
Hypoxia/reoxygenation stimulates Jun kinase activity through redox
signaling in cardiac myocytes. Circ Res. 80:336–344. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takano H, Zou Y, Hasegawa H, Akazawa H,
Nagai T and Komuro I: Oxidative stress-induced signal transduction
pathways in cardiac myocytes: Involvement of ROS in heart diseases.
Antioxid Redox Signal. 5:789–794. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakashima T, Ohkusa T, Okamoto Y, Yoshida
M, Lee JK, Mizukami Y and Yano M: Rapid electrical stimulation
causes alterations in cardiac intercellular junction proteins of
cardiomyocytes. Am J Physiol Heart Circ Physiol. 306:H1324–H1333.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Z, Carter JD, Dailey LA and Huang YC:
Pollutant particles produce vasoconstriction and enhance MAPK
signaling via angiotensin type I receptor. Environ Health Perspect.
113:1009–1014. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Plotnikov A, Zehorai E, Procaccia S and
Seger R: The MAPK cascades: Signaling components, nuclear roles and
mechanisms of nuclear translocation. Biochim Biophys Acta.
1813:1619–1633. 2011. View Article : Google Scholar
|
|
30
|
Karin M: The regulation of AP-1 activity
by mitogen-activated protein kinases. J Biol Chem. 270:16483–16486.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roux PP and Blenis J: ERK and p38
MAPK-activated protein kinases: A family of protein kinases with
diverse biological functions. Microbiol Mol Biol Rev. 68:320–344.
2004. View Article : Google Scholar : PubMed/NCBI
|