Introduction
Hepatoblastoma is the most common type of malignant
liver tumor in children, accounting for ~62% of primary hepatic
malignant tumors. The etiology and pathogenetic mechanisms of
hepatoblastoma have remained to be fully elucidated; however, it
has been indicated that chromosomal abnormalities, genetic factors,
low birth weight and the adverse external factors during pregnancy
may be factors associated with the occurrence of hepatoblastoma
(1–3). Surgery is currently the most
effective method for treating hepatoblastoma. However, as it is
difficult to detect hepatoblastoma in early stages, approximately
half of the patients with hepatoblastoma present with an
unresectable tumor at the time-point of diagnosis (4). Therefore, it is urgently required to
develop potential diagnostic biomarkers and therapeutic targets for
hepatoblastoma.
Enhancer of zeste homolog 2 (EZH2) is a sub-unit of
the polycomb-repressive complex 2, which also contains EED and
SUZ12, catalyzes the trimethylation of histone H3 on Lys 27 and
mediates transcriptional silencing (5). EZH2 is overexpressed in a variety of
cancer types, including prostate, breast, gastric and bladder
carcinomas (6). Overexpression of
EZH2 has been associated with the invasion and progression of
malignant cancers, particularly with the progression of prostate
cancer (7). EZH2 has also been
reported to be overexpressed in hepatocellular carcinomas, in which
it has an oncogenic function (8).
Of note, EZH2 was found to be a promising biomarker and associated
with the progression and aggressive biological behavior of
hepatocellular carcinoma (9).
Hajósi-Kalcakosz et al (10) previously demonstrated that EZH2 is
positively expressed in hepatoblastoma using immunohistochemistry.
However, the roles of EZH2 protein in hepatoblastoma, the most
common malignant liver cancer in children, have remained largely
elusive.
The present study therefore investigated the
expression and the roles of EZH2 protein in the proliferation of
hepatoblastoma. It was revealed that EZH2 was overexpressed in
hepatoblastoma tissues; furthermore, loss-of-function studies using
small hairpin (sh)RNA-mediated knockdown of EZH2 indicated a
potential oncogenic function of this gene by driving the
proliferation of hepatoblastoma cell lines.
Materials and methods
Tissue samples
Seven surgical specimens of hepatoblastoma were
collected and analyzed in the present study. The peri-tumor tissues
were used as controls. Patient details are listed in Table I. Written informed consent was
obtained from the patients' guardians. The present study was
approved by the ethics committee of Xinhua Hospital, Affiliated to
the School of Medicine, Shanghai Jiaotong University (Shanghai,
China).
 | Table ICharacteristics of patients diagnosed
with hepatoblastoma. |
Table I
Characteristics of patients diagnosed
with hepatoblastoma.
| Patient No. | Gender (M/F) | Age (M) | Presenting
symptom | Clinical stage | Metastasis (Y/N) | Preoperative
chemotherapy (Y/N) |
|---|
| 1 | M | 8 | Abdominal mass | III | N | Y |
| 2 | M | 96 | Abdominal pain | IV | Y | N |
| 3 | M | 11 | Jaundice | III | N | N |
| 4 | M | 5 | Abdominal mass | III | N | Y |
| 5 | M | 14 | Abdominal mass | III | N | Y |
| 6 | F | 18 | Abdominal mass | III | N | Y |
| 7 | F | 21 | Abdominal mass | IV | Y | Y |
Cell culture
The human embryonic kidney (HEK)293T cell line and
the Huh6 and HepG2 hepatoblastoma cell lines were obtained from the
Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and
cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.). All
cultures were maintained in a humidified atmosphere containing 5%
CO2 at 37°C.
shRNA, lentiviral construction and
transduction
The shRNA targeting of human EZH2 was performed as
described previously (8). Briefly,
the synthesized sequence, 5′-GAC TCT GAA TGC AGT TGC TTC AGT
ACCC-3′, against EZH2 (GeneChem Co., Ltd., Shanghai, China) were
inserted into the pGCSIL-enhanced green fluorescent protein (EGFP)
vector (GeneChem Co., Ltd.) via its AgeI and EcoRI
sites (New England BioLabs, Inc., Ipswich, MA, USA). A negative
sequence used as a control was inserted into the identical plasmid
at the identical site. The resulting lentiviral vector expressing
the EZH2 siRNA or negative control siRNA was co-transfected into
HEK293T cells with Lipofectamine 2000 (Invitrogen, Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. The
viral titer was determined by counting EGFP-positive cells under a
MicroPublisher 3.3 RTV fluorescence microscope (Olympus
Corporation, Tokyo, Japan). The supernatant of the transfected
HEK293T cells, which contained the lentiviral vector, was used to
transduce 1×105/cm2 Huh6 and HepG2 cells in
the presence of polybrene (10 µg/ml; GeneChem Co., Ltd.).
Cells and supernatants were harvested at different time-points (24,
48, 72 and 96 h) after transduction.
Cell proliferation assay
The stably transfected cells were plated into
96-well plates at a density of 1×104 per well. The
medium in each well was replaced daily. Numbers of cells were
detected at 0, 48 or 96 h post-transfection using a Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies, Kumamoto, Japan)
according to the manufacturer's instructions. At 2 h following
addition of the CCK-8 stain, the absorbance values of the wells
were measured at 450 nm using a microplate reader (Synergy™ H1
Multi-Mode Reader; BioTek Instruments, Inc., Winooski, VT, USA).
Each experiment was performed in triplicate.
Protein extraction and western blot
analysis
Western blotting was used to determine EZH2 and p27
expression levels in extracts from Huh6 and HepG2 cells grown in a
six-well plate. The cells were washed twice with cold
phosphate-buffered saline (PBS) prior to addition of
radioimmunoprecipitation assay lysis buffer containing protease
inhibitors (Thermo Fisher Scientific, Inc.). Extracts were
centrifuged at 12,000 × g for 15 min at 4°C and the supernatant was
collected. The protein concentration was determined using a
bicinchoninic acid protein assay (Thermo Fisher Scientific, Inc.).
Forty micrograms of extracted protein were purified by 12% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (Invitrogen;
Thermo Fisher Scientific, Inc.) and electrophoretically transferred
onto nitrocellulose membranes. The membranes were blocked overnight
in 5% bovine serum albumin for 2 h, washed 3 times for 5 min in PBS
containing 0.1% Tween 20 (Shanghai Yeasen Biotechnology Co., Ltd.,
Shanghai, China). The membranes were then incubated with primary
antibodies at 4°C overnight. The primary antibodies used were all
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA)
and were as follows: Rabbit monoclonal anti-EZH2 (1:1,000; cat. no.
5246), rabbit monoclonal anti-p27 (1:1,000; cat. no. 3686) and
rabbit polyclonal anti-α-tubulin (1:1,000; cat. no. 2148)
antibodies. Subsequently, membranes were incubated with a goat
anti-rabbit IgG coupled with horseradish peroxidase (1:2,000; Cell
Signaling Technology, Inc.; cat. no. 7074) for 2 h at room
temperature. Immunoreactivity was visualized using enhanced
chemiluminescence reagents (SuperSignal West Pico; Thermo Fisher
Scientific, Inc.) and Kodak X-OMAT film (Kodak, Rochester, NY, USA)
with a ChemiDoc XRS molecular imager (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) used to capture the images.
Flow cytometric analysis of cell cycle
distribution
Equal numbers of Huh6 and HepG2 cells were seeded in
a 12-well plate. Seventy-two hours after transfection, cells were
trypsinized and fixed with 70% ethanol overnight and stained with
100 µg/ml propidium iodide (PI; R&S Biotech, Shanghai,
China) containing 100 µg/ml RNase (DNase free; Shanghai
Yeasen Biotechnology Co., Ltd.). After incubation at 37°C for 30
min, the samples were analyzed on BD FACSCanto II flow cytometer
(BD Biosciences, Franklin Lakes, NJ, USA). The average percentages
of cells in G1, S or G2 phases of the cell cycle were quantified
and the standard error was calculated for three experiments.
Statistical analysis
All data were reported as the mean ± standard error
of the mean. Statistical analysis was performed using SPSS version
19.0 (International Business Machines, Armonk, NY, USA). Student's
t-tests (two-tailed, unpaired) was performed for comparison between
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
EZH2 is overexpressed in hepatoblastoma
tissues
Seven patients with hepatoblastoma were enrolled in
the present study (Table I). Their
age ranged from five months to eight years and five of the patients
were male. Their presenting symptoms included a palpable abdominal
mass, abdominal pain and jaundice. Five of the patients had
received four courses of pre-operative chemotherapy to enable their
tumors to be resected. The remaining patients received primary
surgery with post-operative chemotherapy.
The hepatoblastoma tissues and adjacent normal
tissues were assessed for the expression of EZH2 using western blot
analysis. As shown in Fig. 1, EZH2
was found to be overexpressed in hepatoblastoma tissues compared
with that in normal peri-tumor tissues. This upregulation of EZH2
in hepatoblastoma tissues indicated its potential oncogenic roles
in hepatoblastoma. Therefore, the function of EZH2 was further
assessed in hepatoblastoma cell lines in vitro by using
shRNA-mediated depletion of EZH2.
shRNA-mediated suppression of EZH2 in
hepatoblastoma cell lines
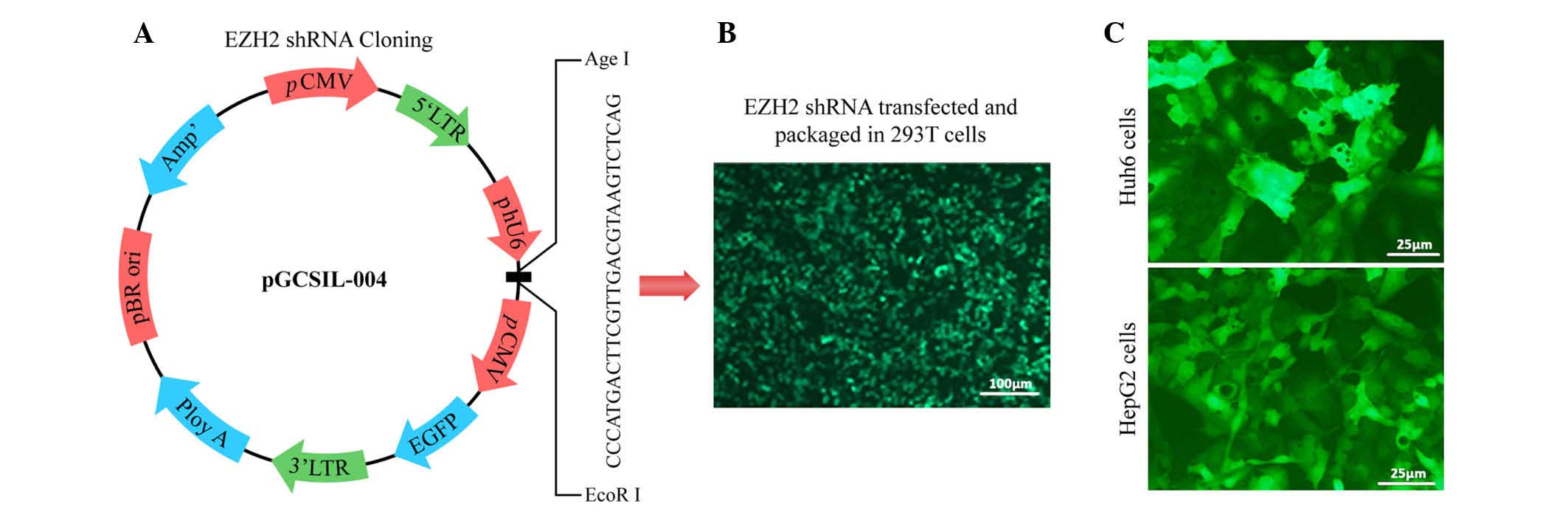
To further study the biological roles of EZH2 in
hepatoblastoma, a lentiviral shRNA system was applied (Fig. 2A and B). Huh6 and HepG2 cells were
transduced with lentivirus expressing shRNA targeting EZH2 or empty
vector (Fig. 2C). A marked
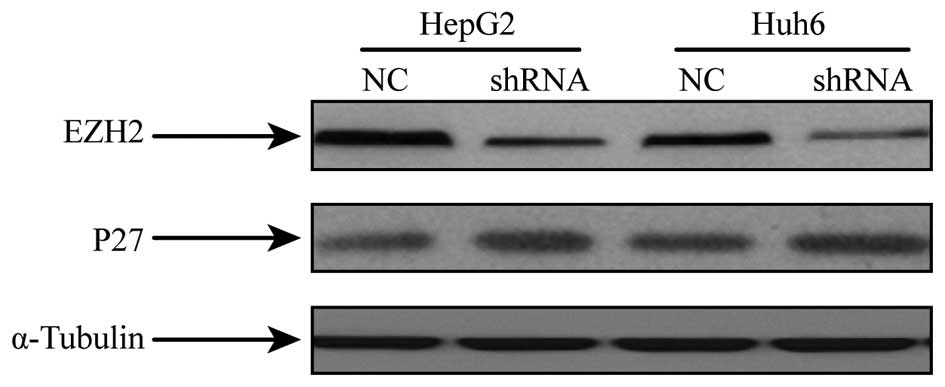
knockdown of EZH2 protein expression was detected at 48 h after
transfection (Fig. 3).
EZH2 deletion inhibits hepatoblastoma
cell proliferation
To assess the potential effects of shRNA-mediated
EZH2 silencing on the proliferation of hepatoblastoma cells, a
CCK-8 assay was performed. As shown in Fig. 4, transfection with shRNA targeting
EZH2 caused a significant reduction in the proliferation of the
Huh6 and HepG2 cell lines compared with that in the NC groups
(P<0.05). Huh6, NC, 0.36±0.05 vs. shEZH2, 0.50±0.01 and HepG2,
NC, 1.17±0.02 vs. 1.49±0.01. These results suggested that EZH2
participates in the regulation of signaling cascades that
positively influence hepatoblastoma cancer cell proliferation.
Inhibition of EZH2 results in G1 phase
arrest in hepatoblastoma cells
To further explore the effects of EZH2 repression on
the cell cycle, flow cytometric cell cycle analysis following PI
staining was performed. The results clearly showed that EZH2
repression significantly increased the G1 populations, accompanied
by a decrease in the S-phase populations, in Huh6 and HepG2 cells
(Fig. 5). These results indicated
that EZH2 depletion inhibited the proliferation of hepatoblastoma
cells by blocking G1-to-S-phase transition.
Inhibition of EZH2 increases p27
Western blot analysis of p27 in hepatoblastoma
specimens revealed a reduced expression compared to that in normal
tissues (Fig. 1). To assess the
effects of EZH2 on p27 expression, hepatoblastoma cells subjected
to shRNA-mediated EZH2 silencing were analyzed for p27 expression
by western blot analysis. It was revealed that the protein
expression of p27 was increased in cells with EZH2 repression
compared to that in the NC group (Fig.
3).
Discussion
The present study initially assessed the protein
levels of EZH2 in hepatoblastoma tissues compared with those in
adjacent normal liver tissues, revealing that EZH2 was
significantly overexpressed in hepatoblastoma tissues. This result
indicated that EZH2 is a potential oncogene and tumor-specific
marker in hepatoblastoma. To further study the role of EZH2 in
hepatoblastoma, EZH2 was silenced in the Huh6 and HepG2
hepatoblastoma cell lines by using an effective lentiviral shRNA
system. A significant inhibition of cell growth was observed in
vitro following knockdown of EZH2 expression, which was
consistent with previous observations in hepatocellular carcinoma
(8,11).
Next, the present study examined the role of EZH2 in
hepatoblastoma cells by observing the effects of EZH2 knockdown on
the cell cycle. Silencing of EZH2 was found to inhibit
G1-to-S-phase transition in vitro. Previous studies
suggested that EZH2 is crucial for regulating the cell cycle by
repressing several tumor suppressor genes, including p16, p27 and
RUNX3 (12–15). As p27 is known to be a regulator of
the G1/S-phase checkpoint (15),
the present study investigated the protein levels of p27 in
hepatoblastoma tissues as well as in cell lines following EZH2
knockdown. Compared to the normal tissues, p27 was shown to be
downregulated in hepatoblastoma tissues, along with overexpression
of EZH2. Of note, p27 was significantly upregulated following EZH2
knockdown in the Huh6 and HepG2 hepatoblastoma cell lines.
P27, a cyclin-dependent kinase inhibitor, has been
reported as a target gene of EZH2 in several carcinoma types
(15,16). In line with these findings, the
present study confirmed that in hepatoblastoma tissues with EZH2
overexpression, p27 expression was downregulated, and that
depletion of EZH2 in hepatoblastoma cell lines increased the
expression of p27. These results suggested that EZH2 contributed to
hepatoblastoma cell proliferation in part through epigenetic
silencing of p27.
In conclusion, the present study demonstrated that
EZH2 is upregulated in hepatoblastoma. It exerts its oncogenic
function at least in part by inhibiting p27 expression and thereby
reducing the proliferation of hepatoblastoma cells by blocking
G1-to-S-phase transition. These results indicated that EXH2 may
represent a diagnostic biomarker and a potential therapeutic target
for the treatment of hepatoblastoma.
References
|
1
|
Yang SS, Brough AJ and Bernstein J: Tumors
of the liver in early infancy: Hepatoblastoma. Mich Med.
71:539–543. 1972.PubMed/NCBI
|
|
2
|
Rowland JM: Hepatoblastoma: Assessment of
criteria for histologic classification. Med Pediatr Oncol.
39:478–483. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malogolowkin MH, Katzenstein HM, Krailo M
and Meyers RL: Treatment of hepatoblastoma: The North American
cooperative group experience. Front Biosci (Elite Ed). 4:1717–1723.
2012. View Article : Google Scholar
|
|
4
|
Warmann SW and Fuchs J: Drug resistance in
hepatoblastoma. Curr Pharm Biotechnol. 8:93–97. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao R, Wang L, Wang H, Xia L,
Erdjument-Bromage H, Tempst P, Jones RS and Zhang Y: Role of
histone H3 lysine 27 methylation in Polycomb-group silencing.
Science. 298:1039–1043. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xiao Y: Enhancer of zeste homolog 2: A
potential target for tumor therapy. Int J Biochem Cell Biol.
43:474–477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Varambally S, Dhanasekaran SM, Zhou M,
Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt
RG, Otte AP, et al: The polycomb group protein EZH2 is involved in
progression of prostate cancer. Nature. 419:624–629. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Y, Lin MC, Yao H, Wang H, Zhang AQ,
Yu J, Hui CK, Lau GK, He ML, Sung J and Kung HF:
Lentivirus-mediated RNA interference targeting enhancer of zeste
homolog 2 inhibits hepatocellular carcinoma growth through
down-regulation of stathmin. Hepatology. 46:200–208. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cai MY, Tong ZT, Zheng F, Liao YJ, Wang Y,
Rao HL, Chen YC, Wu QL, Liu YH, Guan XY, et al: EZH2 protein: A
promising immunomarker for the detection of hepatocellular
carcinomas in liver needle biopsies. Gut. 60:967–976. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hajósi-Kalcakosz S, Dezső K, Bugyik E,
Bödör C, Paku S, Pávai Z, Halász J, Schlachter K, Schaff Z and Nagy
P: Enhancer of zeste homologue 2 (EZH2) is a reliable
immunohistochemical marker to differentiate malignant and benign
hepatic tumors. Diagn Pathol. 7:862012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yonemitsu Y, Imazeki F, Chiba T, Fukai K,
Nagai Y, Miyagi S, Arai M, Aoki R, Miyazaki M, Nakatani Y, et al:
Distinct expression of polycomb group proteins EZH2 and BMI1 in
hepatocellular carcinoma. Hum Pathol. 40:1304–1311. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ougolkov AV, Bilim VN and Billadeau DD:
Regulation of pancreatic tumor cell proliferation and
chemoresistance by the histone methyltransferase enhancer of zeste
homologue 2. Clin Cancer Res. 14:6790–6796. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kotake Y, Cao R, Viatour P, Sage J, Zhang
Y and Xiong Y: pRB family proteins are required for H3K27
trimethylation and Polycomb repression complexes binding to and
silencing p16INK4alpha tumor suppressor gene. Genes Dev. 21:49–54.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fujii S, Ito K, Ito Y and Ochiai A:
Enhancer of zeste homologue 2 (EZH2) down-regulates RUNX3 by
increasing histone H3 methylation. J Biol Chem. 283:17324–17332.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakagawa S, Okabe H, Sakamoto Y, Hayashi
H, Hashimoto D, Yokoyama N, Sakamoto K, Kuroki H, Mima K, Nitta H,
et al: Enhancer of zeste homolog 2 (EZH2) promotes progression of
cholangiocarcinoma cells by regulating cell cycle and apoptosis.
Ann Surg Oncol. 20(Suppl 3): S667–S675. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sakurai T, Bilim VN, Ugolkov AV, Yuuki K,
Tsukigi M, Motoyama T and Tomita Y: The enhancer of zeste homolog 2
(EZH2), a potential therapeutic target, is regulated by miR-101 in
renal cancer cells. Biochem Biophys Res Commun. 422:607–614. 2012.
View Article : Google Scholar : PubMed/NCBI
|