Introduction
Hepatocellular carcinoma (HCC) is one of the most
malignant types of tumor worldwide, and is the third most common
cause of cancer-associated mortality (1,2). Due
to the limitations of surgery and liver transplantation, including
inoperable tumors and tissue matching, chemotherapy remains the
major treatment method for HCC (3). Previous studies have shown that the
metastasis of cancer cells involves complex processes, in which the
cancer cells invade the surrounding tissue, enter the bloodstream
or lymph circulation, and form new tumors (4,5). The
degradation of the extracellular matrix (ECM) is crucial in cancer
cell migration and invasion, and a series of proteinases are
involved in this process, including matrix metalloproteinases
(MMPs) (6).
Diosmetin (3′,5,7-trihydroxy-4′-methoxyflavone
(C16H12O6; DIOS; Fig. 1) is found in the legume, Acacia
farnesiana, and in the leaves of Olea europaea L., and
is the aglycone of the lavonoid glycoside, diosmin (7). It has been confirmed that DIOS has
several medicinal properties, including antibacterial (8), antimicrobial (9), anti-inflammatory (10) and antioxidant (11) activities. It has also been
confirmed that DIOS exerts cytostatic effects in MDA-MB 468 cells,
a breast cancer cell line, by inducing cell cycle arrest (12). However, the effect of DIOS on the
invasion and metastasis of HCC cells, and the antimetastatic
mechanisms of DIOS remain to be fully elucidated. The aim of the
present study was to investigate the anti-metastasis effect of DIOS
on HCC cells and the underlying mechanisms.
Materials and methods
Reagents and antibodies
Diosmetin (Sigma-Aldrich, St. Louis, MO, USA) was
dissolved in dimethyl sulfoxide (DMSO; MP Biomedicals, Santa Ana,
CA, USA) at a stock solution concentration of 5 mg/ml, and was
diluted as a working fluid for cell culture medium prior to use.
Concentrations of DIOS used in the MTT assay were 0, 2, 5, 10, 20,
30, 40, 50 and 100 µg/ml; whereas 0, 10, 20 and 40
µg/ml DIOS was used in the other assays. The 3-(4,
5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was
purchased from Sigma-Aldrich. Matrigel was purchased from BD
Biosciences (Franklin Lakes, NJ, USA). SYBR Premix Ex Taq™ II kits
were purchased from Takara Bio, Inc. (Shiga, Japan). Antibodies
against GAPDH, MMP-2, MMP-9, c-Jun N-terminal kinase (JNK),
phosphorylated (p)-JNK, extracellular signal-regulated kinase
(ERK)1/2, p-ERK1/2 and protein kinase C (PKC)-δ were purchased from
Cell Signal Technology, Inc. (Boston, MA, USA). Horseradish
peroxidase-(HRP) conjugated goat anti-rabbit immunoglobulin G
secondary antibody was purchased from EarthOx Life Sciences
(Millibrae, CA, USA).
Cell culture
The MHcc97H and SK-HEP-1 HCC cell lines were
purchased from the Shanghai Cell Bank of Chinese Academy of Science
(Shanghai, China). The cells were maintained in Dulbecco's modified
Eagle's medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.), and were cultured in a 37°C, 5%
CO2 incubator. The cells were passaged at 90%
confluence.
Cell proliferation assay
Cell proliferation rates were detected using an MTT
assay. The cells were seeded into a 96-well plate at a density of
104 per well in 100 µl culture medium. Following
24 h adhesive culture at 37°C, the medium was removed and replaced
with the same volume of medium containing either 2, 5, 10, 20, 30,
40, 50 and 100 µg/ml DIOS, with cells cultured in normal
medium as a control group. After 24 h incubation at 37°C, 20
µl MTT stock solution, at a concentration of 5 mg/ml, was
added to each well of the plate and, following 3 h incubation at
37°C, the medium was removed gently and 200 µl DMSO was
added per well. The absorbance was then detected using a microplate
reader (PerkinElmer, Waltham, MA, USA) at a wavelength of 570 nm.
These experiments were performed independently in triplicate.
Wound healing assay
Migration capacities of the HCC cell lines under
DIOS treatment were detected using a wound healing assay. The cells
were seeded in a 24-well plate in DMEM containing 10% FBS for 24 h,
when the cells were at 100% confluence. A wound was then created in
the cell layer using a pipette tip. Following washing twice with
phosphate-buffered saline (PBS) to remove cellular debris, the
cells were cultured in the absence or presence of 5, 10 or 20
µg/ml DIOS in DMEM containing 1% FBS for 24 h at 37°C. The
cells were observed under a microscope, and images of the cells
were captured when the wound was created and at 24 h-post wounding.
Migration rates were calculated using the following formula:
Migration rate = [width of (0–24 h)/width of 24 h] × 100%. The
experiments were performed in triplicate independently.
Cell motility assay
Cells were seeded in Transwell chambers, comprising
porous polycarbonate membranes with a pore size of 8.0 µm
(Corning, Corning, NY, USA), at a concentration of 1×105
cells/chamber in the absence or presence of 100 µl 5, 10 or
20 µg/ml DIOS in DMEM. Chambers were then fitted into the
lower wells of the Transwell system in a 24-well plate (BD
Biosciences), which contained 600 µl DMEM containing 10%
FBS. Following incubation for 24 h at 37°C, the cells that passed
through the membrane were fixed in 70% ethanol, and then stained
with 0.1%crystal violet (Amresco, Solon, OH, USA). The cells were
observed under a an Olympus IX70 microscope (Olympus Corporation,
Tokyo, Japan) and counted in the last four fields of each group.
Three independent experiments were performed in triplicate.
Cell invasion assay
The methods used for the cell invasion assay were
similar to those of the cell motility assay, with the exception
that each Transwell chamber was pretreated with Matrigel (1:10
diluted in DMEM), of which 100 µl per chamber was added. The
chambers were placed into a 37°C incubator for 2 h prior to
use.
Cell adhesion assay
A cell adhesion assay were performed, as previously
reported (3). Briefly, each well
of a 96-well plate was coated with 10 µl fibronectin
(R&D systems, Minneapolis, MN, USA), and the plates were placed
in to a 37°C incubator for 2 days. The plates were washed twice
with DMEM prior to use. The cells were pretreated with 5, 10 or 20
µg/ml DIOS for 24 h at 37°C, following which the cells were
harvested and seeded into the 96-well plate coated with
fibreonectin, at a density of 5×105 cells/ml for 100
µl. After 2 h, the medium and non-adhesive cells were
removed, and the adhered cells were detected using an MTT
assay.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The expression levels of the MMPs and tissue
inhibitors of MMPs were detected using RT-qPCR. The total RNA of
the cells were extracted using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), and reverse transcription to cDNA was
performed using a PrimeScript™ RT reagent kit with gDNA Eraser
(Takara Bio, Inc.). qPCR reactions were performed using a Roche
LightCycler 480 II (Roche Diagnostics, Basel, Switzerland),
according to the instructions of the SYBR® Premix Ex
Taq™ II, ROX plus (Takara Bio, Inc.). Specific primers for each
gene were designed as follows: GAPDH, forward
5′-TGCACCACCAACTGCTTAG-3′ and reverse 5′-AGTAGAGGCAGGGATGATGTTC-3′
as internal control; MMP2, forward 5′-CCACAGGAGGAGAAGGCTGT-3′ and
reverse 5′-CTCCAGTTAAAGGCGGCATC-3′; and MMP9, forward
5′-ACGACGTCTTCCAGTACCGA-3′ and reverse 5′-TTGGTCCACCTGGTTCAACT-3′.
Thermal cycling conditions were 95°C for 30 sec, followed by 40
cycles of amplification at 95°C for 5 sec and 60°C for 30 sec. Data
are representative of three independent assays and expression
levels were calculated according to the ΔΔCq method and expressed
as 2–ΔΔCq (13).
Western blot analysis
The protein expression levels of MMP-2, MMP-9, JNK,
p-JNK, ERK1/2, p-ERK1/2 and PKC-δ were detected using western
blotting, the protocol of which was as reported previously
(14). Briefly, cells were seeded
in 100 mm culture dishes at a density of 105 cells/ml in
10 ml culture media and subsequently cultured at 37°C for 24 h in
an atmosphere containing 5% CO2. Subsequently, cells
were exposed to various concentrations of DIOS (0, 10, 20 and 40
µg/ml and were washed in PBS twice and suspended in lysis
buffer for 30 min on ice. Lysates were then centrifuged at 13,000 ×
g at 4°C for 10 min, separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were then blocked in Tris-buffered saline
(Beyotime Institute of Biotechnology, Haimen, China) with 0.1%
Tween 20 (Sangon Biotech Co., Ltd., Shanghai, China) (TBST),
containing 5% bovine serum albumin for 1 h. The membranes were then
incubated with the following rabbit anti-human primary antibodies
at 4°C overnight: Anti-MMP-2 monoclonal antibody (mAb; 13132),
anti-MMP-9 mAb (13667), anti-JNK polyclonal antibody (pAb; 9258),
anti-p-JNK mAb (4668), anti-ERK1/2 mAb (4695), anti-p-ERK1/2 mAb
(8544) and anti-PKC-δ pAb (all 1:1,000; 2058). Following washing
three times with TBST supplemented with 150 mM NaCl for 10 min, the
membranes were incubated with HRP-conjugated goat anti-rabbit
immunoglobulin G secondary antibody (1:1,000; E030120-02) for 2 h
at room temperature. Membranes were washed three times with TBST
for 10 min and the bands were exposed in a dark room and analyzed
using Alpha-view gradation analyzing system (Alpha View SA 3.4.0,
ProteinSimple, Santa Clara, CA, USA).
Statistical analysis
Data were obtained from ≥3 independent experiments
and all results are presented as the mean ± standard error of the
mean. Between-group differences were assessed via Student's t-test
using SPSS 18.0 (SPSS, Inc., Chicago, IL, USA). Comparisons were
relative to untreated controls. P<0.05 was considered to
indicate a statistically significant difference.
Results
DIOS does not inhibit cell proliferation
at a low concentration
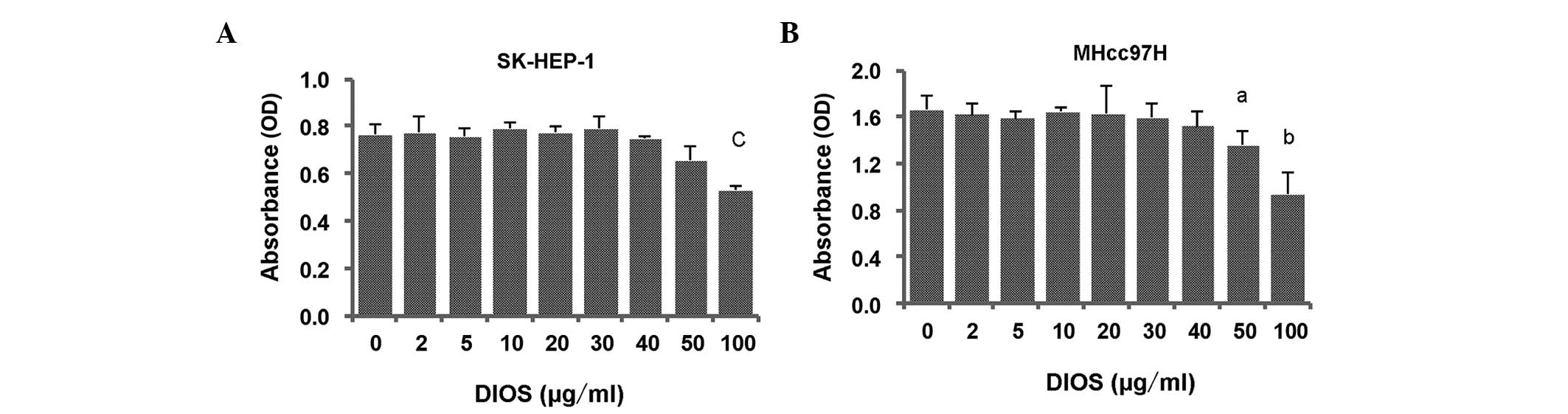
MTT assays were performed to investigate the
inhibitory ability of DIOS on the proliferation of SK-HEP-1 and
MHcc97H cells. As shown in Fig. 2,
the SK-HEP-1 and MHcc97H cells were treated with various
concentrations of DIOS for 24 h, however, cell proliferation was
not affected by DIOS until the concentration reached 50
µg/ml for the MHcc97H cells (P<0.05) and 100 µg/ml
for the SK-HEP-1 cells (P<0.001).
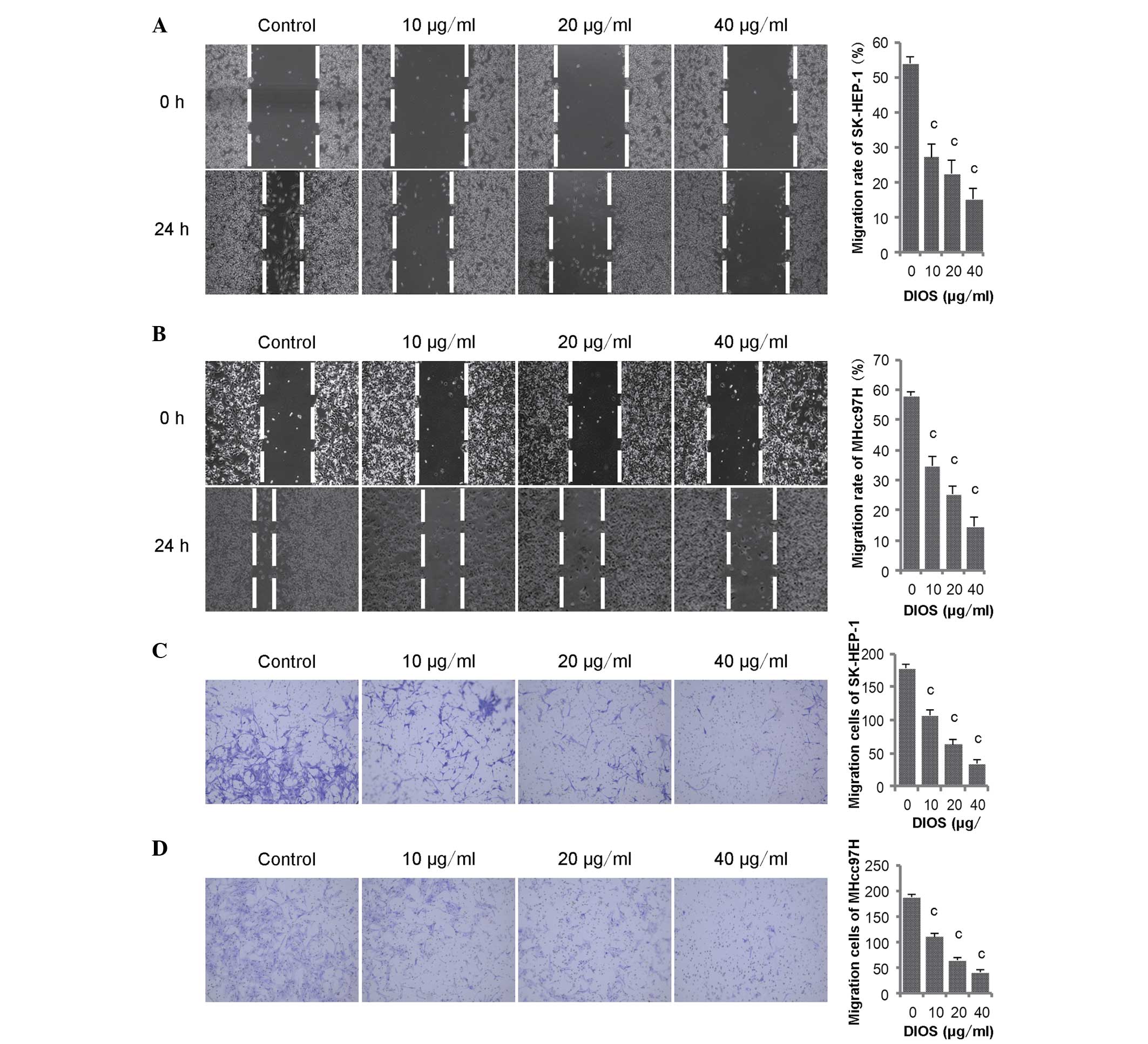
DIOS inhibits the migration of SK-HEP-1
and MHcc97H cells
The role of DIOS in HCC metastasis was also
investigated using a wound healing assay and cell motility assay.
Initially, to identify whether the effect of DIOS on HCC occurred
due the inhibition of cell proliferation or metastatic suppression,
the SK-HEP-1 and MHcc97H cells were treated with 10, 20 and 40
mg/ml DIOS. As described above, the results of the previous MTT
assay showed that these concentrations had no effect on cell
proliferation. In the wound healing assay, the width of the wounds
were measured at 0 h and at 24 h post-DIOS treatment and migration
rates were calcuated. The results are shown in Fig. 3, in which the migration rates of
the SK-HEP-1 and MHcc97H cells were >50% higher, compared with
those in the control groups 24 h post-wounding. However, the
migration rate was significantly reduced in the groups treated with
DIOS, which occurred in a dose-dependent manner (Fig. 3A and B; P<0.001). In the cell
motility assay, the cells which passed though the porous
polycarbonate membranes were counted to assess whether DIOS
affected the migration of the SK-HEP-1 and MHcc97H. The results
showed that fewer cells passed though the membranes of the
Transwell chambers following DIOS treatment (Fig. 3C and D; P<0.001). The results of
the wound healing and cell motility assays indicated that DIOS
significantly inhibited the migration of the SK-HEP-1 and MHcc97H
HCC cells.
DIOS inhibits the invasion of SK-HEP-1
and MHcc97H cells
In the present study, tumor aggressiveness was
evaluated using a basement membrane invasion assay. The cells
observed to degrade the Matrigel and pass through the porous
polycarbonate membranes were counted, and the results revealed that
DIOS efficiently inhibited the invasion of the SK-HEP-1 and MHcc97H
cells across the membranes pretreated with Matrigel. The numbers of
cells on the lower surface of the membranes decreased in a
dose-dependent manner following DIOS treatment (Fig. 4A and D; P<0.001). These results
indicated that DIOS significantly inhibited HCC cell invasion.
DIOS reduces the adherence abilities of
SK-HEP-1 and MHcc97H cells
Cancer cell metastasis involves multiple processes,
including migration, adhesion and invasion, and the adherence of
cells to the ECM or the basement membrane is a crucial step during
cancer invasiveness (3). In the
present study, the adherence abilities of the SK-HEP-1 and MHcc97H
cell lines were significantly reduced following DIOS pretreatment
(Fig. 4E and F; P<0.001). These
results indicated that DIOS effectively inhibited the adherence
ability of the SK-HEP-1 and MHcc97H cells.
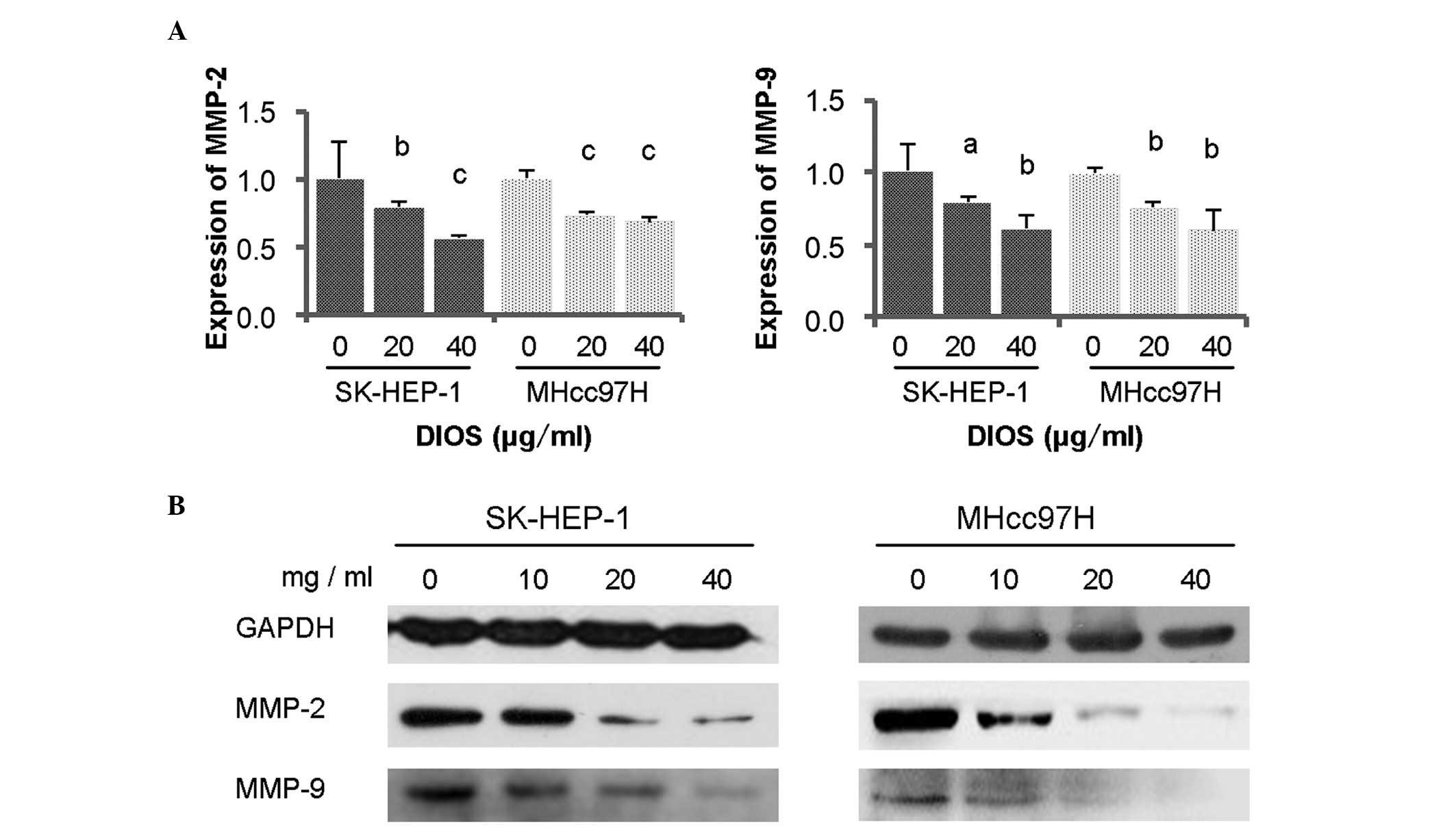
DIOS downregulates the expression levels
of MMP-2 and MMP-9 in SK-HEP-1 and MHcc97H cells
It is known that MMPs are key enzymes involved in
degradation of the ECM, and that MMP-2 and MMP-9 are important in
cancer invasion and metastasis (3). To determine the effect of DIOS on HCC
cell metastasis, the expression levels of MMP-2/9 were detected
using RT-qPCR and Western blot analyses. The results demonstrated
that the expression levels of MMP-2/9 were significantly reduced
following DIOS treatment (Fig. 5A and
B; P<0.05).
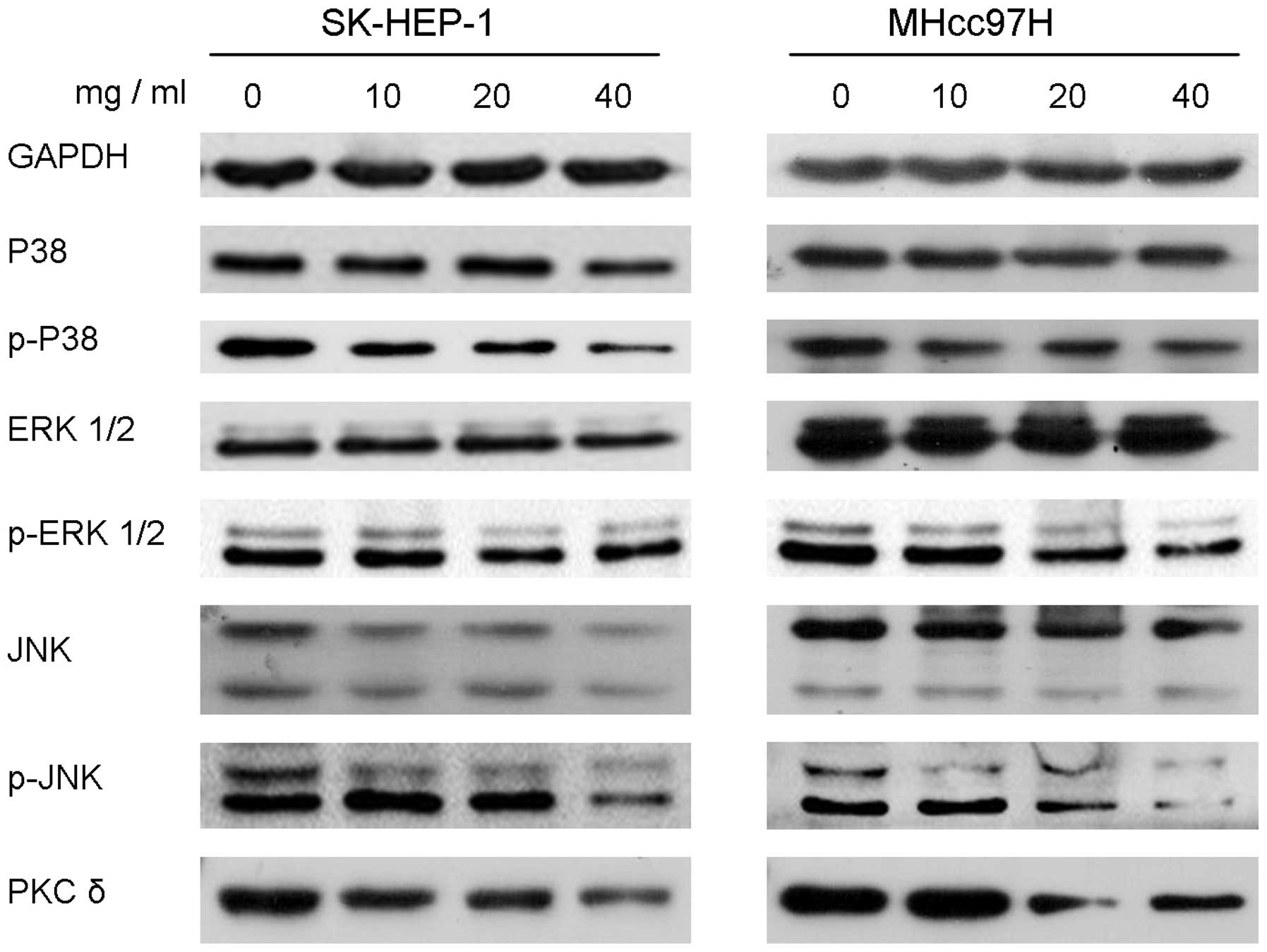
Downregulation of MMP-2/9 by DIOS is
associated with the MAPK and PKC-δ pathways
The present study further investigated the mechanism
underlying the inhibitory effect of DIOS on HCC metastasis. MAPK
and PKC-δ pathway proteins, including P38, ERK 1/2, JNK, PKC-δ and
their phosphorylated forms, were measured using Western blotting.
DIOS treatment for 24 h had no effect on the protein levels of P38
in the SK-HEP-1 or MHcc97H cell lines, however, the levels of the
phosphorylated form, p-P38, were markedly decreased. DIOS treatment
for 24 h markedly reduced the protein expression levels of ERK 1/2
and JNK, and markedly reduced the protein levels of p-ERK 1/2 and
p-JNK. The results showed that DIOS also downregulated the protein
levels of PKC-δ (Fig. 6).
Discussion
HCC is one of most malignant types of tumor and a
major common cause of cancer-associated mortality. Several
traditional Chinese medicines have been reported for their
antitumor properties, including baicalein (15), dihydromyricetin (2,7,16)
and resveratrol (17). As a
flavonoids compound, DIOS has several medicinal properties,
including antibacterial, antimicrobial anti-inflammatory and
antioxidant effects (12).
Although a previous study demonstrated that DIOS induces cell cycle
arrest of HCC cells (18), the
role of DIOS on the metastasis of HCC cells remains to be fully
elucidated. The metastasis of cancer involves complex processes,
including migration and invasion through the tumor stroma,
intravasation, tumor cell dissemination, extravasation and cell
growth at metastatic sites (19).
The present study showed that DIOS inhibited the migration,
invasion and adhesion of SK-HEP-1 and MHcc97H cells, which
demonstrated that DIOS effectively suppressed the metastasis of
SK-HEP-1 and MHcc97H cells.
MMPs are a family of proteolytic enzymes, which have
a number of important physiological roles, including ECM
modification, accelerating cell migration and cleaving cytokines
(20). In the MMP family, MMP-2/9
are reported as substrate-specific gelatinases, which are critical
in ECM degradation (12,21). Elevated levels of MMP-2/9 correlate
with invasion, metastasis and poor prognosis in various types of
cancer (22,23). Therefore, the suppression of
MMP-2/9, is an important strategy to prevent cancer cell invasion
(24). In the present study, MMP-2
and MMP-9 were downregulated following DIOS treatment for 24 h, in
the SK-HEP-1 and MHcc97H cells. This result indicated that DIOS
suppressed the metastasis of the SK-HEP-1 and MHcc97H cells through
inhibition of the expression of MMP-2/9.
It has been observed previously that members of the
MAPK family, including p38 MAPK, ERK 1/2 and JNK, are activated in
several types of cancer (25,26).
There is increasing evidence that the MAPK family is involved in
the migration and invasion of cancer, and that all three of the
proteins mentioned above regulate the expression of MMPs (23,27,28).
To investigate whether the downregulation of MMP-2 and MMP-9 is
associated with the MAPK family, the expression levels of p38, ERK
1/2 and JNK, and their phosphorylated forms, were detected using
Western blotting. The data demonstrated no significant change in
the the expression levels of p38 and ERK 1/2, however, the
phosphorylations of p38 and ERK decreased significantly in the
SK-HEP-1 and MHcc97H cells. The expression levels of JNK and p-JNK
were downregulated significantly in the SK-HEP-1 and MHcc97H cells.
These results suggested that the MAPK family (p38 MAPK, ERK 1/2 and
JNK) was important in the DIOS-mediated cell metastasis.
The upstream protein of ERK is PKC, which is a
family of intracellular protein kinases that activate
serine/threonine kinases, including MAPK, nuclear factor κB and
phosphati-dylinositol-3-kinase by controlling the growth, migration
and death of cells, and several PKCs are considered to be
associated with tumor progression (23). It has been reported that PKC-δ is
overexpressed in human ductal carcinoma (29). The inhibition of PKC-δ may suppress
the migration of cells through the PKC/ERK/MMP-9 pathways (30–32).
In the present study, PKC-δ was downregulated following DIOS
treatment for 24 h in the SK-HEP-1 and MHcc97H cells, which
indicated that DIOS inhibited the metastasis of the SK-HEP-1 and
MHcc97H cell via the PKC/MAPK/MMPs pathways.
In conclusion, the results of the present study
showed that DIOS inhibited the migration, invasion and adhesion of
HCC cells by decreasing the gene and protein expression levels of
MMP-2/9. Furthermore, the decreased expression of MMP-2/9 was
regulated by the PKC-δ/MAPK/MMPs pathways. These results suggested
that DIOS has a potent antimetastatic effect on HCC cells.
Acknowledgments
This study was supported, in part, by grants from
the Special Funds from Education Department of Guangdong Province
(grant no. JB1212), the Chinese NSFC grants (grant no. 31370824),
the Yangfan Plan of Talents Recruitment Grant, Guangdong, China
(grant no. YueRenCaiBan [2014] 1), the University Talents
Recruitment Grant of Guangdong, China (grant no. YueCaiJiao [2012]
328) and The Excellent Postgraduate Essay Development Project of
Guangdong Medical College (grant no. 2014-18).
References
|
1
|
Feng M, Gao W, Wang R, Chen W, Man YG,
Figg WD, Wang XW, Dimitrov DS and Ho M: Therapeutically targeting
glypican-3 via a conformation-specific single-domain antibody in
hepatocellular carcinoma. Proc Natl Acad Sci USA. 110:E1083–E1091.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Q, Liu J, Liu B, Xia J, Chen N, Chen
X, Cao Y, Zhang C, Lu C, Li M and Zhu R: Dihydromyricetin promotes
hepatocellular carcinoma regression via a p53 activation-dependent
mechanism. Sci Rep. 4:46282014.PubMed/NCBI
|
|
3
|
Zhang QY, Li R, Zeng GF, Liu B, Liu J, Shu
Y, Liu ZK, Qiu ZD, Wang DJ, Miao HL, et al: Dihydromyricetin
inhibits migration and invasion of hepatoma cells through
regulation of MMP-9 expression. World J Gastroenterol.
20:10082–10093. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hsieh MJ, Lin CW, Yang SF, Chen MK and
Chiou HL: Glabridin inhibits migration and invasion by
transcriptional inhibition of matrix metalloproteinase 9 through
modulation of NF-κB and AP-1 activity in human liver cancer cells.
Br J Pharmacol. 171:3037–3050. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen TY, Li YC, Liu YF, Tsai CM, Hsieh YH,
Lin CW, Yang SF and Weng CJ: Role of MMP14 gene polymorphisms in
susceptibility and pathological development to hepatocellular
carcinoma. Ann Surg Oncol. 18:2348–2356. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alexander SP, Benson HE, Faccenda E,
Pawson AJ, Sharman JL, McGrath JC, Catterall WA, Spedding M, Peters
JA, Harmar AJ, et al: The Concise Guide to PHARMACOLOGY 2013/14:
Overview. Br J Pharmacol. 170:1449–1458. 2013. View Article : Google Scholar
|
|
7
|
Spanakis M, Kasmas S and Niopas I:
Simultaneous determination of the flavonoid aglycones diosmetin and
hesperetin in human plasma and urine by a validated GC/MS method:
In vivo metabolic reduction of diosmetin to hesperetin. Biomed
Chromatogr. 23:124–131. 2009. View
Article : Google Scholar
|
|
8
|
Chan BC, Ip M, Gong H, Lui SL, See RH,
Jolivalt C, Fung KP, Leung PC, Reiner NE and Lau CB: Synergistic
effects of diosmetin with erythromycin against ABC transporter
over-expressed methicillin-resistant Staphylococcus aureus1 (MRSA)
RN4220/pUL5054 and inhibition of MRSA pyruvate kinase.
Phytomedicine. 20:611–614. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meng JC, Zhu QX and Tan RX: New
antimicrobial mono-and sesquiterpenes from Soroseris hookeriana
subsp. erysimoides. Planta Med. 66:541–544. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chandler D, Woldu A, Rahmadi A, Shanmugam
K, Steiner N, Wright E, Benavente-García O, Schulz O, Castillo J
and Münch G: Effects of plant-derived polyphenols on TNF-alpha and
nitric oxide production induced by advanced glycation endproducts.
Mol Nutr Food Res. 54(Suppl 2): S141–S150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liao W, Ning Z, Chen L, Wei Q, Yuan E,
Yang J and Ren J: Intracellular antioxidant detoxifying effects of
diosmetin on 2, 2-azobis (2-amidinopropane) dihydrochloride
(AAPH)-induced oxidative stress through inhibition of reactive
oxygen species generation. J Agric Food Chem. 62:8648–8654. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Androutsopoulos VP, Mahale S, Arroo RR and
Potter G: Anticancer effects of the flavonoid diosmetin on cell
cycle progression and proliferation of MDA-MB 468 breast cancer
cells due to CYP1 activation. Oncol Rep. 21:1525–1528.
2009.PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2–ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
14
|
Liu J, Shu Y, Zhang Q, Liu B, Xia J, Qiu
M, Miao H, Li M and Zhu R: Dihydromyricetin induces apoptosis and
inhibits proliferation in hepatocellular carcinoma cells. Oncol
Lett. 8:1645–1651. 2014.PubMed/NCBI
|
|
15
|
Chen H, Gao Y, Wu J, Chen Y, Chen B, Hu J
and Zhou J: Exploring therapeutic potentials of baicalin and its
aglycone baicalein for hematological malignancies. Cancer Lett.
354:5–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeng G, Liu J, Chen H, Liu B, Zhang Q, Li
M and Zhu R: Dihydromyricetin induces cell cycle arrest and
apoptosis in melanoma SK-MEL-28 cells. Oncol Rep. 31:2713–2719.
2014.PubMed/NCBI
|
|
17
|
Liu B, Zhou Z, Zhou W, Liu J, Zhang Q, Xia
J, Liu J, Chen N, Li M and Zhu R: Resveratrol inhibits
proliferation in human colorectal carcinoma cells by inducing
G1/S-phase cell cycle arrest and apoptosis through
caspase/cyclin-CDK pathways. Mol Med Rep. 10:1697–1702.
2014.PubMed/NCBI
|
|
18
|
Androutsopoulos VP and Spandidos DA: The
flavonoids diosmetin and luteolin exert synergistic cytostatic
effects in human hepatoma HepG2 cells via CYP1A-catalyzed
metabolism, activation of JNK and ERK and P53/P21 up-regulation. J
Nutr Biochem. 24:496–504. 2013. View Article : Google Scholar
|
|
19
|
Kwon M, Lee SJ, Wang Y, Rybak Y, Luna A,
Reddy S, Adem A, Beaty BT, Condeelis JS and Libutti SK: Filamin A
interacting protein 1-like inhibits WNT signaling and MMP
expression to suppress cancer cell invasion and metastasis. Int J
Cancer. 135:48–60. 2014. View Article : Google Scholar :
|
|
20
|
Elkington PT and Friedland JS: Matrix
metalloproteinases in destructive pulmonary pathology. Thorax.
61:259–266. 2006. View Article : Google Scholar
|
|
21
|
Chen T, Li M, Zhang R and Wang H:
Dihydroartemisinin induces apoptosis and sensitizes human ovarian
cancer cells to carboplatin therapy. J Cell Mol Med. 13:1358–1370.
2009. View Article : Google Scholar
|
|
22
|
Hurst DR and Welch DR: Metastasis
suppressor genes at the interface between the environment and tumor
cell growth. Int Rev Cell Mol Biol. 286:107–180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin CW, Shen SC, Chien CC, Yang LY, Shia
LT and Chen YC: 12-O-tetradecanoylphorbol-13-acetate-induced
invasion/migration of glioblastoma cells through activating
PKCalpha/ERK/NF-kappaB-dependent MMP-9 expression. J Cell Physiol.
225:472–481. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hidalgo M and Eckhardt SG: Development of
matrix metal-loproteinase inhibitors in cancer therapy. J Natl
Cancer Inst. 93:178–193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hwang YP, Yun HJ, Kim HG, Han EH, Lee GW
and Jeong HG: Suppression of PMA-induced tumor cell invasion by
dihydroartemisinin via inhibition of PKCalpha/Raf/MAPKs and
NF-kappaB/AP-1-dependent mechanisms. Biochem Pharmacol.
79:1714–1726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He Q, Zhou X, Li S, Jin Y, Chen Z, Chen D,
Cai Y, Liu Z, Zhao T and Wang A: MicroRNA-181a suppresses salivary
adenoid cystic carcinoma metastasis by targeting MAPK-Snai2
pathway. Biochim Biophys Acta. 1830:5258–5266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Woo JH, Lim JH, Kim YH, Suh SI, Min DS,
Chang JS, Lee YH, Park JW and Kwon TK: Resveratrol inhibits phorbol
myristate acetate-induced matrix metalloproteinase-9 expression by
inhibiting JNK and PKC delta signal transduction. Oncogene.
23:1845–1853. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reunanen N, Westermarck J, Häkkinen L,
Holmström TH, Elo I, Eriksson JE and Kähäri VM: Enhancement of
fibroblast collagenase (matrix metalloproteinase-1) gene expression
by ceramide is mediated by extracellular signal-regulated and
stress-activated protein kinase pathways. J Biol Chem.
273:5137–5145. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mauro LV, Grossoni VC, Urtreger AJ, Yang
C, Colombo LL, Morandi A, Pallotta MG, Kazanietz MG, Bal de Kier
Joffé ED and Puricelli LL: PKC Delta (PKCdelta) promotes tumoral
progression of human ductal pancreatic cancer. Pancreas.
39:e31–e41. 2010. View Article : Google Scholar
|
|
30
|
Hu CT, Cheng CC, Pan SM, Wu JR and Wu WS:
PKC mediates fluctuant ERK-paxillin signaling for hepatocyte growth
factor-induced migration of hepatoma cell HepG2. Cell Signal.
25:1457–1467. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu CT, Wu JR, Cheng CC, Wang S, Wang HT,
Lee MC, Wang LJ, Pan SM, Chang TY and Wu WS: Reactive oxygen
species-mediated PKC and integrin signaling promotes tumor
progression of human hepatoma HepG2. Clin Exp Metastasis.
28:851–863. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsieh HL, Wu CY and Yang CM: Bradykinin
induces matrix metalloproteinase-9 expression and cell migration
through a PKC-delta-dependent ERK/Elk-1 pathway in astrocytes.
Glia. 56:619–632. 2008. View Article : Google Scholar : PubMed/NCBI
|