Introduction
Infertility is a growing problem worldwide. The term
'infertility' refers to the failure of a couple to conceive
following 12 months of regular intercourse. Infertility affects
10–15% of couples, and a male factor is responsible for ~50% of
infertility cases (1,2). Numerous reports have shown that
greater exposure to electromagnetic radiation (3), smoking (4), heat (5), the consumption of certain drugs
(6), exposure to heavy metals
(7–9), exposure to pesticides (10,11),
and biological hazards may partly explain the mechanisms underlying
male infertility.
The majority of causes of male infertility are
considered to be associated with abnormal spermatogenesis and
failures in sperm function (12).
Mammalian spermatogenesis, which is a complex and highly regulated
process of germ cell proliferation and differentiation, which
occurs within the seminiferous tubules of the testes, involves
dynamic interactions between the developing germ cells and the
Sertoli cells. The Sertoli cells provide structural and nutritional
support for the germ cells. An array of specific and non-specific
molecules that are expressed by the Sertoli cells, including
proteases, protease inhibitors, signaling molecules, growth factors
and cell adhesion molecules, have been identified, which are
responsible for the regulation of spermatogenesis (13–15).
Numerous harmful factors can target the molecules that are
expressed by the Sertoli cells, and disruptions to these molecules
may arrest germ cell production and maturation or dysfunctional
spermatozoa may be produced, resulting in male infertility
(16,17). For example, the dibutyl
phthalate-induced collapse of Sertoli cell vimentin filaments may
lead to the detachment of spermatogenic cells, and the detached
cells may undergo apoptosis since they have lost support from the
Sertoli cells (18). Zhang and Lui
(19) showed that nectin-2 is a
direct molecular target for cadmium and proposed that the
dysregulation of nectin-2 in the Sertoli cells may explain
cadmium-induced male infertility (19).
Dermatopontin (DPT), a 22 kDa non-collagenous
extracellular matrix protein, which promotes cellular adhesion and
extracellular matrix assembly, initially co-purifies with decorin
from bovine dermal extracts (20–22).
Western blotting and northern blot analysis indicates that DPT is
expressed in the skin, skeletal muscle, bone, cartilage and other
tissue types (22,23). DPT reportedly modifies the behavior
of transforming growth factor (TGF)-β on mink lung epithelial cells
via its interaction with decorin within the extracellular matrix
in vivo (24). Furthermore,
a reduction in the expression of DPT is a molecular link between
uterine leiomyomas and keloids. Light microscopy has demonstrated
that Dpt-null corneas from 2-month-old mice exhibit a 24%
reduction in the average stromal thickness compared with the
corneas from wild-type mice, which suggests that DPT is important
in collagen fibril organization (25). Additionally, DPT may be involved in
the pathogenesis and growth of prostate cancer cells (26).
Our previous study demonstrated that DPT is
expressed in the Sertoli cells within the testis (27). An in vitro study showed that
DPT is a novel regulator of the CdCl2-induced reduction
in claudin-11 expression, which suggests that DPT may be associated
with testicular dysfunction. Therefore, the present study aimed to
further investigate the implications of DPT expression for
testicular function.
Materials and methods
Materials
The protein extraction kit and the Beyo-ECL Plus
western blotting reagents were purchased from Beyotime Institute of
Biotechnology (Jiangsu, China). The mouse monoclonal DPT antibody
(cat. no. sc-376863) was purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). The anti-claudin-11 (cat. no. 36-4500) and
anti-zonula occludens (ZO-1; cat. no. 61-7300) antibodies were
purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
All secondary antibodies were purchased from ZsBio (Beijing,
China). The FLAG-tag antibody (cat. no. 20543-1-AP) was purchased
from Proteintech (Wuhan, China). The SYBR®
PrimeScript® RT-PCR kit (Perfect Real Time) was
purchased from Takara Bio, Inc. (Liaoning, China). CdCl2
and busulfan were purchased from Sigma-Aldrich (St. Louis, MO,
USA). The remaining chemicals were purchased from Sangon Biotech
(Shanghai, China).
Animal experiments
A total of 90 C57BL mice, aged between 3 and 4
months (weight, 25–32 g) were obtained from the Experimental Animal
Centre at Chongqing Medical University (Chongqing, China). The
animals were maintained on a 12 h light/dark cycle, with free
access to food and water. The mice were randomly divided into
groups containing 6 mice. The mice received 30 mg/kg−1
busulfan or 3.5 mg/kg−1 CdCl2, which were
administered via intraperitoneal injection. Mice were sacrificed 5,
15, 25, 35 or 70 days following treatment with an overdose of 6%
chloral hydrate. The left testes were removed and were fixed in a
formaldehyde solution at room temperature. The right testes were
removed for protein and RNA extraction. All procedures were
approved by the Animal Care and Use Committee of Chongqing Medical
University (Chongqing, China).
Total RNA extraction and the reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The total RNA was extracted from the testes using
TRIzol® reagent (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. RNA concentrations were
quantified using a Nanodrop 2000 spectrophotometer (Nanodrop
Technologies, Wilmington, DE, USA). Aliquots of the total RNA (1
µg) from each sample were reverse transcribed into cDNA
using the PrimeScript® RT Enzyme (Takara Bio, Inc.),
according to the manufacturer's protocol.
The RT-qPCR was performed in a thermal cycler
(CFX96; Bio-Rad Laboratories, Inc. Hercules, CA, USA) using the
SYBR® PrimeScript® RT-PCR kit (Perfect Real
Time), according to the manufacturer's protocol. β-actin served as
the internal control. The primers used were as follows: DPT,
forward: 5′-GGTGGCTACGGGTACCCATA-3′ and reverse:
5′-GTCAGAGCCTTCCTTCTTGC-3′; β-actin, forward:
5′-TCGTGCGTGACATCAAAGAG-3′ and reverse: 5′-CAAGAAGGAAGGCTGGAAAA-3′.
The primers were synthesized by Sangon Biotech. The reaction
solution (25 µl) contained 1 µl cDNA, 10.5 µl
water, 12.5 µl SYBR® Premix Ex Taq™ and 0.5
µl of 10 µM forward and reverse primers. The PCR
thermal cycling parameters were 1 min at 94°C, followed by 40
cycles of 10 sec at 94°C, 15 sec at 58°C and 15 sec at 72°C. The
relative expression of DPT was normalized against β-actin and was
calculated using the 2−ΔΔCt method.
Western blotting
The total protein was extracted from the testes
using radioimmunoprecipitation assay lysis buffer, and the
concentrations were determined using the bicinchoninic acid assay.
Protein samples (40 µg) were separated on a 10% sodium
dodecyl sulfate-polyacrylamide gel and were transferred onto
polyvinylidene difluoride membranes. The membranes were blocked
with 5% non-fat milk for 2 h at 37°C, then incubated with the
primary antibodies against DPT (1:500, mouse monoclonal),
claudin-11 (1:300; rabbit polyclonal), ZO-1 (1:300, rabbit
polyclonal) and FLAG (1:1000, rabbit polyclonal) overnight at 4°C.
Following incubation, the membranes were washed briefly with
Tris-buffered saline containing 0.1% Tween-20 (100mM Tris, 0.9%
NaCl, 0.1% Tween-20; pH 7.4), and were subsequently incubated for 2
at room temperature with the following corresponding secondary
antibody: Horseradish peroxidase (HRP) conjugated-anti-rabbit
immunoglubilin G (IgG; 1:3,000; cat. no. ZB-2301) and
HRP-anti-mouse IgG (1:3,000; cat. no. ZB-2305). The proteins were
detected using the Beyo-ECL kit, according to the manufacturer's
protocol. The densities of bands were analyzed using Quantity One
(version 4.6.2; Bio-Rad Laboratories, Inc.) and were normalized
against β-actin, which served as the loading control.
Histopathological examination
Routine histology was performed to assess the
statuses of the testes. The formalin-fixed testes were embedded in
paraffin and 4 µm sections were cut using a microtome (Leica
RM2135; Wetzlar, Germany). The sections were de-waxed and were
re-hydrated through a descending series of alcohol concentrations
to distilled water. The tissue sections were subsequently stained
with hematoxylin and eosin. The samples were analyzed for changes
in testicular morphology and structure using light microscopy
(Olympus BX51; Olympus, Tokyo, Japan).
Vector constructs
To generate transgenic mice with a Sertoli
cell-specific overexpression of DPT, a fragment of the
Müllerian-inhibiting substance (MIS) upstream promoter, which was
previously validated to be sufficient for the expression of growth
hormone (28), nuclear receptor
subfamily 0, group B, member 1 (29) and Smad4 (30) in Sertoli cells, was used. The
transgenic construct contained the following components: The
upstream promoter region (−180 to +1) of the MIS, FLAG tags, the
complete open reading frame of mouse DPT and the simian virus
(SV)40 polyadenylation poly (A) sequence. The MIS promoter fragment
was amplified from the C57BL mice genomic deoxyribonucleic acid
(DNA) using the primers MIS-FLAG-F:
5′-GTCGACCTCAGGCCTCTGCAGTTATGGG-3′ and MIS-FLAG-R:
5′-GAGGTCCATCTTGTCATCGTCGTCCTTGTAGTCCATGGTGGTACAGCAAG-3′. To clone
DPT from the testes of C57BL mice and to add the FLAG epitope
(DYKDDDDK) to the DPT protein, the primers FLAG-Dpt-F:
5′-CCACCATGGACTACAAGGACGACGATGACAAGATGGACCTCACTCTTCTG-3′ and
FLAG-Dpt-R:
5′-TGGCTGGCAACTAGAAGGCACAGCTAAACGTTTTCGAATTCGCAGTCGTA-3′ were used.
The SV40 fragment was amplified from the pcDNA3.1 vector using the
primers Dpt-SV40-F1: 5′-GAATTCGAAAACGTTTAGTCTAGATAAGTAATGAT-3′ and
Dpt-SV40-R1: 5′-GATCCTCTGGAGATACAGACATGATAAGATACATTG-3′.
Subsequently, the transgenic cassette, MIS-FLAG-DPT-SV40, was
generated by in-fusion PCR using the primers MIS-Dpt-F1:
5′-ATAATCAATGTCAACCCTCAGGCCTCTGCAGTTA-3′ and Dpt-SV40-R1:
5′-GATCCTCTGGAGATACAGACATGATAAGATACATTG-3′. The produced fragment
was cloned into an 18-T simple vector (Takara Bio, Inc.) for
sequencing.
Generation of mice with overexpression of
DPT
The purified transgenic constructs were
microinjected into the pronuclei of fertilized oocytes from C57BL
mice. To identify the founder transgenic mice, tail biopsies were
collected for genomic DNA isolation using the Universal Genomic DNA
Extraction kit (Takara Bio, Inc.) when the mice were 2 weeks of
age. The resulting genomic DNA samples were screened by PCR using
the primers, MIS-FLAG-F and Dpt-SV40-R1. The amplified products
were resolved using 1.5% agarose gel electrophoresis.
Immunohistochemical detection of the
FLAG-tagged DPT protein
Testicular sections (5 µm) were
deparaffinized in xylene and were hydrated in a descending series
of ethanol concentrations, which was followed by antigen retrieval
in 0.01 mol/l citrate buffer (pH 6.0). Non-specific background was
eliminated by blocking with goat serum buffer (ZsBio) at room
temperature for 1 h, followed by incubation of the slides with
anti-FLAG antibody (1:100; rabbit polyclonal) overnight at 4°C. The
samples were subsequently incubated for 1 h at room temperature
with goat-anti-rabbit immunoglobulin G secondary antibody (1:1,000;
polyclonal; cat. no. ZB-2301). Following washing with
phosphate-buffered saline, containing 0.1% Tween-20, the sections
were incubated with diaminobenzidine and were counterstained with
hematoxylin. The tissue sections were examined and images were
captured using a light microscope (Olympus).
Statistical analysis
The data are expressed as the mean ± standard error.
The statistical differences were evaluated using one-way analysis
of variance and Newman-Keuls test. P<0.05 was considered to
indicate a statistically significant difference.
Results
CdCl2- or busulfan-induced
testicular morphology damage
Routine histology was performed to assess
spermatogenesis in the mice following 30 mg/kg−1
busulfan or 3.5 mg/kg−1 CdCl2 treatment
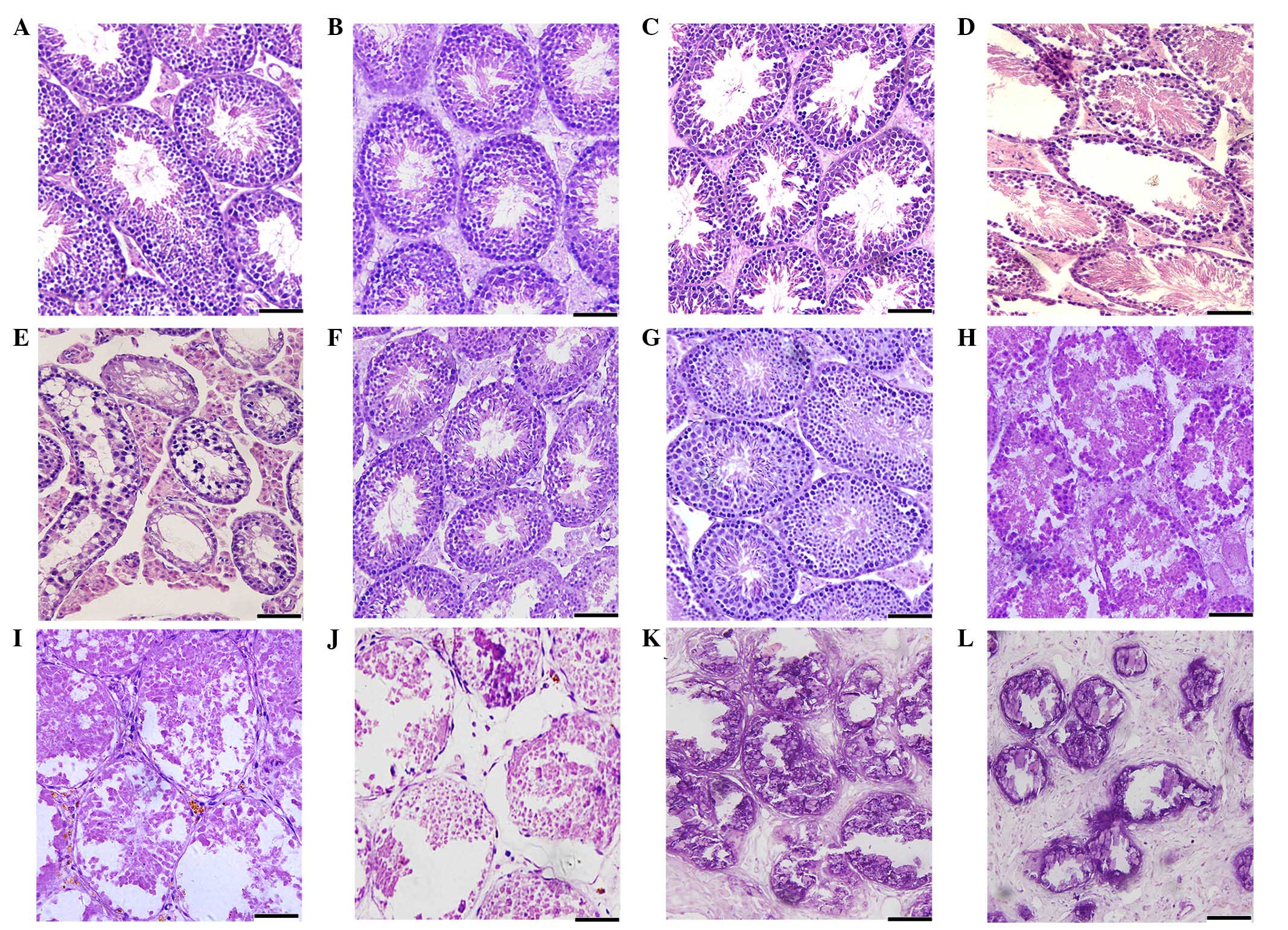
(Fig. 1). No histopathological
changes were observed in the testes of the mice in the control
groups (Fig. 1A and G). After 5
days of treatment with busulfan, the mice exhibited no obvious
changes in the morphology of the epithelium of the seminiferous
tubules compared with the control mice (Fig. 1B). After 15 days treatment with
busulfan, the seminiferous tubule epithelium had become thinner,
and exhibited the detachment of the spermatogenic cells (Fig. 1C). At 25 days following treatment
with busulfan, 15% of the seminiferous tubules contained no germ
cells (Fig. 1D) and 35 days
following treatment with busulfan, 40% of the seminiferous tubules
contained no germ cells (Fig. 1E).
At 70 days following treatment with busulfan, a histological
analysis determined that the majority of the testes contained germ
cells and that regeneration was occurring in the seminiferous
tubules (Fig. 1F).
In the CdCl2-treated mice, the
histological analysis determined that the basement membrane of the
seminiferous tubules was discontinuous, and that secondary
spermatocytes and round spermatids were present in the tubules'
lumens at 5 days post-treatment (Fig.
1H). At 15 days following treatment with CdCl2,
severe necrosis and degeneration of the seminiferous tubules were
apparent, with 20% of the seminiferous tubules exhibiting germ cell
losses (Fig. 1I). At 25 days
following treatment with CdCl2, the majority of the
seminiferous tubules exhibited severe histopathological changes
(Fig. 1J). Between 35 and 70 days
following treatment with CdCl2, the structure of the
testes exhibited more severe disorganization and the degree of
fibrosis within the testes appeared to increase in a time-dependent
manner (Fig. 1K and L).
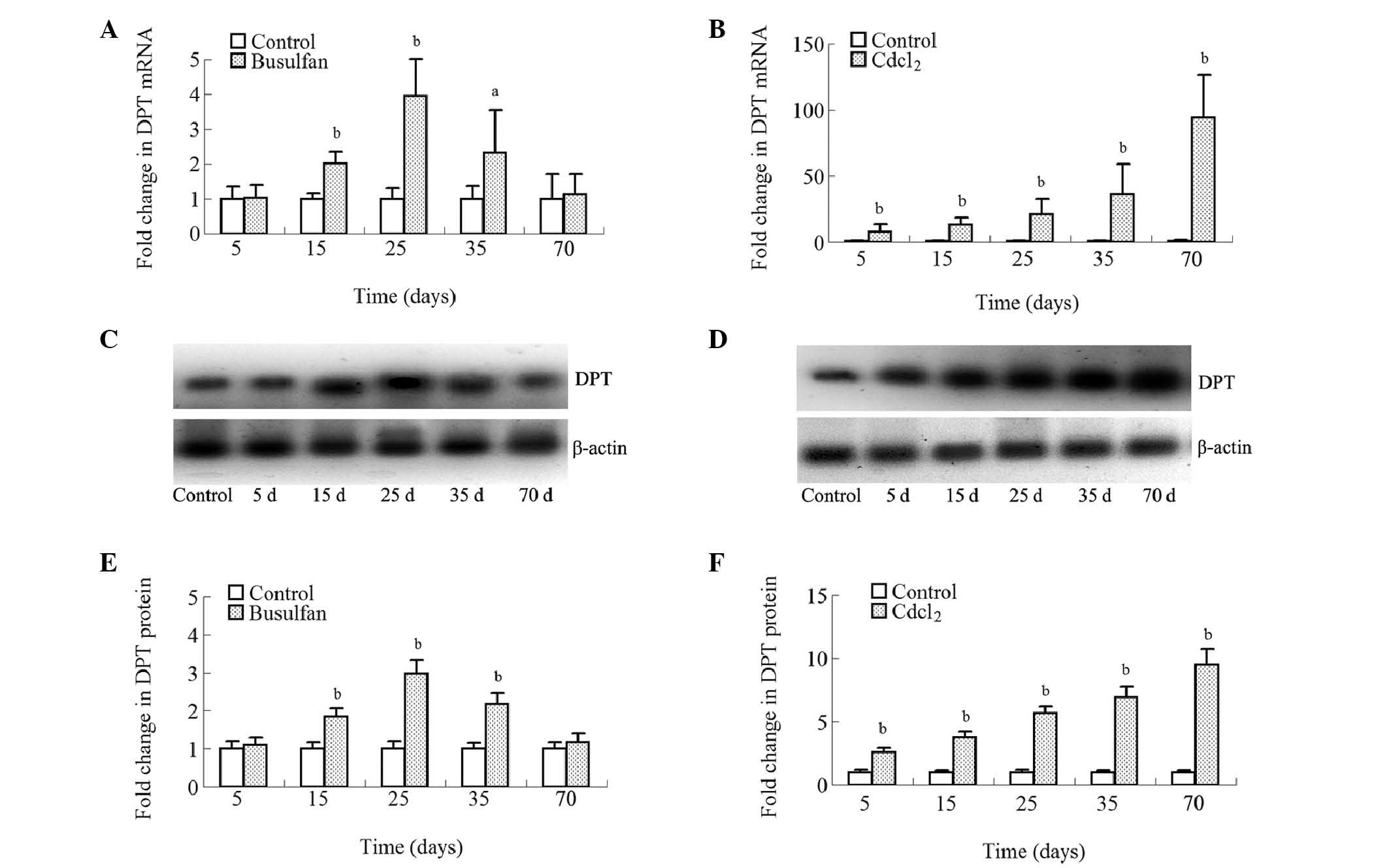
Effects of busulfan or CdCl2
treatment on the expression of DPT in the testes of mice
The effects of busulfan or CdCl2
treatment on the expression levels of DPT in the testes of mice
were determined using PCR and western blotting. At 5 days following
treatment, the busulfan-treated group exhibited no change in the
expression of DPT (P>0.05). Between 15 and 25 days following
treatment with busulfan, the DPT levels gradually increased with
time. Between 35 and 70 days following treatment with busulfan, the
DPT levels gradually normalized (Fig.
2A, C, and E).
The pattern of DPT expression in the mice
administered CdCl2 differed from that observed in the
busulfan-treated mice. Time-dependent changes in the expression of
DPT mRNA (Fig. 2B) and protein
(Fig. 2D and F) were observed in
the mouse testes in response to treatment with
CdCl2.
Establishment of a transgenic mouse with
DPT overexpression
Two males (lines 37 and 45) positive for the mouse
DPT transgene (Fig. 3A) were
selected for further breeding. The 4-month-old mice, originally
derived from the two male founder mice, exhibited an upregulation
of the protein expression of DPT in their testes, however, the
control litter mate did not (Fig. 3B
and C). No apparent differences were observed in the expression
of testicular DPT between the transgenic lines. Since the
transgenic construct contains a FLAG tag, the present study also
tested for the expression of the FLAG epitope in the testes, and
the results revealed that while no expression of the FLAG epitope
occurred in the control mice, its protein was detected in the
testes from the transgenic animals at a molecular weight that was
appropriate for DPT (Fig. 3B).
Histological analysis also immunolocalized the FLAG epitope in the
Sertoli cells that were within the sections of the testicular
tissue from the DPT overexpression mice (Fig. 3D), which further substantiated the
expression of the transgenic construct within the testes.
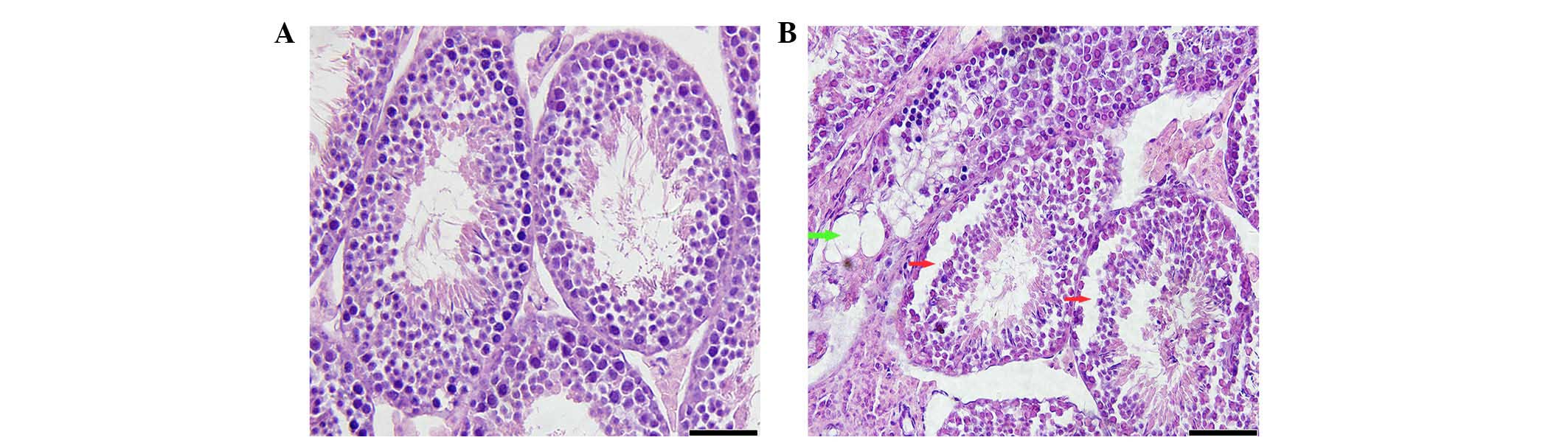
Overexpression of DPT in the Sertoli
cells results in germ cell losses and vacuole formation in the
seminiferous tubules
Testes from the 4-month-old transgenic mice
exhibited histopathological changes in the seminiferous tubules,
which included the desquamation of the spermatogenic cells
(Fig. 4). In addition, a
significant proportion of the tubules within the testes of the
4-month-old transgenic mice exhibited impairments of the
spermatogenesis process, which included the formation of vacuoles,
suggesting that endogenous DPT over-expression in the Sertoli cells
also negatively impacts upon reproductive function.
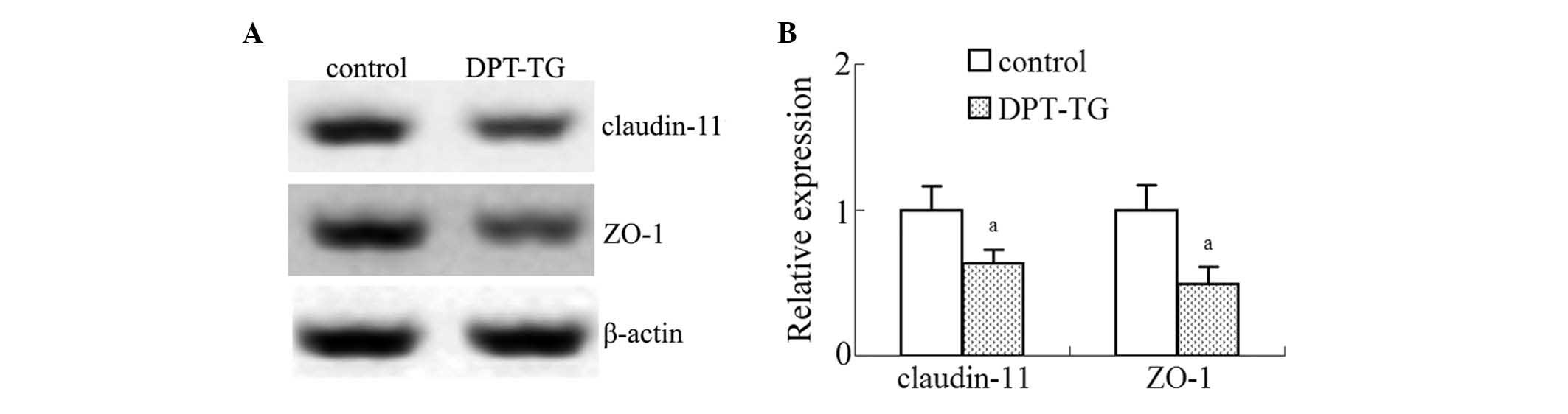
Overexpression of DPT in the Sertoli
cells leads to a decrease in the expression levels of ZO-1 and
claudin-11 in the testes
To determine whether the integrity of the
blood-testis barrier (BTB) is affected by DPT overexpression in
vivo, the present study assessed the steady-state levels of
claudin-11 and ZO-1 by immunoblotting. As shown in Fig. 5, the relative expression levels of
ZO-1 and claudin-11 in the DPT overexpression mice were
significantly reduced as compared with the control mice (Fig. 5), which suggested that DPT may be
associated with injury to the BTB.
Discussion
DPT is a gene with unknown function in male
reproduction, which was previously shown to be expressed in the
testis. The present study demonstrated that increased expression of
DPT has an affect on testicular dysfunction in mice. To investigate
changes in the expression of DPT in mice with testicular
dysfunction, an animal model of testicular dysfunction was
established by treating mice with a single dose of busulfan or
CdCl2. Findings from previous research have demonstrated
that busulfan or CdCl2 can damage reproductive function
in males by disrupting the endogenous balance within the testicular
microenvironment, which leads to germ cell losses and somatic cell
damage (31–33). In the present study, busulfan or
CdCl2 injections damaged the structure of the testicles
of the mice, which concurs with results reported previously. The
histological data suggested that the degree of damage to the testes
was considerably lower in the busulfan-treated mice compared with
the CdCl2-treated mice. At 70 days following busulfan
treatment, the testicular histology appeared to be recovering to an
almost normal profile, while exposure to CdCl2 resulted
in progressive testicular injury. Subsequently, the expression
pattern of DPT in the testis with regard to busulfan or
CdCl2 treatment was characterized. In busulfan treated
groups, the expression of DPT was normal up to day 5, however, was
significantly increased on days 15, 25 and 35, and with recovery up
to day 70. Taking the results from the histological analyses of the
testes following busulfan treatment together with the levels of DPT
expression in the testes at different time points following
busulfan treatment, which were determined using PCR and western
blotting, suggested that an increase in the expression of DPT may
be a good marker for male testicular dysfunction. Therefore, a high
level of DPT expression may indicate a low level of reproductive
function.
Time-dependent changes in the expression of DPT were
observed in the mouse testes in response to treatment with
CdCl2. The pattern of DPT expression in the mice
administered CdCl2 differed from that observed in the
busulfan-treated mice, which further supports the concept that an
increase in the expression of DPT may be indicative of reproductive
dysfunction in male mice.
Since the increases in the expression of DPT in
testis were induced by exogenous detrimental factors, namely
CdCl2 or busulfan administration, the present study
hypothesized that endogenous increases in the levels of DPT
expression were directly associated with testicular function. Our
previous study revealed that DPT is expressed in the Sertoli cells
(27), after which DPT transgenic
mice, which had a Sertoli cell-specific overexpression of DPT, were
established to facilitate studies into the effects of endogenous
DPT overexpression on testicular function in mice. A significant
proportion of the tubules within the testes of the 4-month-old
transgenic mice exhibited impairments of the spermatogenesis
process suggested that just as exogenous factors induce increases
in the levels of DPT expression, endogenous DPT overexpression in
the Sertoli cells also implicates testicular dysfunction.
The present study further investigated the possible
mechanisms underlying testicular injury in mice with Sertoli
cell-specific DPT overexpression.
Alterations in the BTB is associated with male
fertility (34). Sertoli cell
tight junctions (TJ) are an essential component of the BTB, which
create an immunologically balanced microenvironment, and they
provide structural support for spermatogenesis. TJ are
multimolecular membrane specializations comprising several integral
membrane proteins, including occludin, claudins, and ZO-1. The
findings from previous in vitro and in vivo
investigations show that particular factors can affect the
integrity of the BTB by targeting TJ (35). For example, TGF-β3 and tumor
necrosis factor-α, which are secreted by the Sertoli cells and the
germ cells, can induce reversible disruption to the BTB in
vivo by reducing the steady-state levels of occludin and ZO-1
(36).
Our previous data from in vitro
investigations showed that CdCl2-induced downregulation
of claudin-11 is partially counteracted by DPT silencing in 15P-1
Sertoli cells, which suggests that DPT is a novel regulator of
CdCl2-induced BTB damage in vitro (27). In the present study, the
steady-state levels of claudin-11 and ZO-1 were assessed by
immunoblotting to determine whether the integrity of the BTB is
affected by DPT overexpression in vivo. It was revealed that
compared with the control mice, the relative expression levels of
ZO-1 and claudin-11 in the DPT overexpression mice were
significantly reduced, which suggested that DPT overexpression may
be associated with injury to the BTB, and, in turn, testicular
dysfunction.
In conclusion, the expression patterns of DPT in
mice treated with CdCl2 or busulfan indicated that an
increase in DPT expression has implications for testicular
dysfunction in mice. This finding was corroborated by the findings
in the testicles of mice exhibiting endogenous overexpression of
DPT, rather than increases in DPT expression induced by busulfan or
CdCl2. The increase in the expression of DPT may harm
the integrity of the BTB, which may, in turn, be injurious to
testicular function.
Acknowledgments
The present study was supported by the National
Nature Science Foundation of China (nos. 81270687 and
30901062).
References
|
1
|
Irvine DS: Epidemiology and aetiology of
male infertility. Hum Reprod. 13(Suppl 1): 33–44. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gnoth C, Godehardt E, Frank-Herrmann P,
Friol K, Tigges J and Freundl G: Definition and prevalence of
subfertility and infertility. Hum Reprod. 20:1144–1147. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sepehrimanesh M, Kazemipour N, Saeb M and
Nazifi S: Analysis of rat testicular proteome following 30-day
exposure to 900 MHz electromagnetic field radiation.
Electrophoresis. 35:3331–3338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fowler PA, Cassie S, Rhind SM, Brewer MJ,
Collinson JM, Lea RG, Baker PJ, Bhattacharya S and O'Shaughnessy
PJ: Maternal smoking during pregnancy specifically reduces human
fetal desert hedgehog gene expression during testis development. J
Clin Endocrinol Metab. 93:619–626. 2008. View Article : Google Scholar
|
|
5
|
Thonneau P, Bujan L, Multigner L and
Mieusset R: Occupational heat exposure and male fertility: A
review. Hum Reprod. 13:2122–2125. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Benavides-Garcia R, Joachim R, Pina NA,
Mutoji KN, Reilly MA and Hermann BP: Granulocyte colony-stimulating
factor prevents loss of spermatogenesis after sterilizing busulfan
chemotherapy. Fertil Steril. 103:270.e8–280.e8. 2015. View Article : Google Scholar
|
|
7
|
Spiazzi CC, Manfredini V, Barcellos da
Silva FE, Flores EM, Izaguirry AP, Vargas LM, Soares MB and Santos
FW: γ-Oryzanol protects against acute cadmium-induced oxidative
damage in mice testes. Food Chem Toxicol. 55:526–532. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haouem S, Najjar MF, El Hani A and Sakly
R: Accumulation of cadmium and its effects on testis function in
rats given diet containing cadmium-polluted radish bulb. Exp
Toxicol Pathol. 59:307–311. 2008. View Article : Google Scholar
|
|
9
|
Mabrouk A and Cheikh HB: Thymoquinone
supplementation ameliorates lead-induced testis function impairment
in adult rats. Toxicol Ind Health. 2014.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sharma P, Huq AU and Singh R:
Cypermethrin-induced reproductive toxicity in the rat is prevented
by resveratrol. J Hum Reprod Sci. 7:99–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ismail MF and Mohamed HM:
Deltamethrin-induced genotoxicity and testicular injury in rats:
Comparison with biopesticide. Food Chem Toxicol. 50:3421–3425.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Walczak-Jedrzejowska R, Wolski JK and
Slowikowska-Hilczer J: The role of oxidative stress and
antioxidants in male fertility. Cent European J Urol. 66:60–67.
2013. View Article : Google Scholar
|
|
13
|
Desai SS, Roy BS and Mahale SD: Mutations
and polymorphisms in FSH receptor: Functional implications in human
reproduction. Reproduction. 146:R235–R248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smith LB and Walker WH: The regulation of
spermatogenesis by androgens. Semin Cell Dev Biol. 30:2–13. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao X, Mruk DD and Cheng CY:
Intercellular adhesion molecules (ICAMs) and spermatogenesis. Hum
Reprod Update. 19:167–186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eid N, Ito Y and Otsuki Y: Enhanced
mitophagy in Sertoli cells of ethanol-treated rats: Morphological
evidence and clinical relevance. J Mol Histol. 43:71–80. 2012.
View Article : Google Scholar
|
|
17
|
Li MW, Mruk DD, Lee WM and Cheng CY:
Disruption of the blood-testis barrier integrity by bisphenol A in
vitro: Is this a suitable model for studying blood-testis barrier
dynamics? Int J Biochem Cell Biol. 41:2302–2314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alam MS, Ohsako S, Tay TW, Tsunekawa N,
Kanai Y and Kurohmaru M: Di (n-butyl) phthalate induces vimentin
filaments disruption in rat Sertoli cells: A possible relation with
spermatogenic cell apoptosis. Anat Histol Embryol. 39:186–193.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X and Lui WY: Dysregulation of
nectin-2 in the testicular cells: An explanation of cadmium-induced
male infertility. Biochim Biophys Acta. 1839:873–884. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Neame PJ, Choi HU and Rosenberg LC: The
isolation and primary structure of a 22-kDa extracellular matrix
protein from bovine skin. J Biol Chem. 264:5474–5479.
1989.PubMed/NCBI
|
|
21
|
Choi HU, Johnson TL, Pal S, Tang LH,
Rosenberg L and Neame PJ: Characterization of the dermatan sulfate
proteoglycans, DS-PGI and DS-PGII, from bovine articular cartilage
and skin isolated by octyl-sepharose chromatography. J Biol Chem.
264:2876–2884. 1989.PubMed/NCBI
|
|
22
|
Superti-Furga A, Rocchi M, Schäfer BW and
Gitzelmann R: Complementary DNA sequence and chromosomal mapping of
a human proteoglycan-binding cell-adhesion protein (dermatopontin).
Genomics. 17:463–467. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Okamoto O, Suzuki Y, Kimura S and Shinkai
H: Extracellular matrix 22-kDa protein interacts with decorin core
protein and is expressed in cutaneous fibrosis. J Biochem.
119:106–114. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Okamoto O, Fujiwara S, Abe M and Sato Y:
Dermatopontin interacts with transforming growth factor beta and
enhances its biological activity. Biochem J. 337:537–541. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cooper LJ, Bentley AJ, Nieduszynski IA,
Talabani S, Thomson A, Utani A, Shinkai H, Fullwood NJ and Brown
GM: The role of dermatopontin in the stromal organization of the
cornea. Invest Ophthalmol Vis Sci. 47:3303–3310. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takeuchi T, Suzuki M, Kumagai J, Kamijo T,
Sakai M and Kitamura T: Extracellular matrix dermatopontin
modulates prostate cell growth in vivo. J Endocrinol. 190:351–361.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang Q, Hao J, Chen M and Li G:
Dermatopontin is a novel regulator of the CdCl2-induced decrease in
claudin-11 expression. Toxicol In Vitro. 28:1158–1164. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Giuili G, Shen WH and Ingraham HA: The
nuclear receptor SF-1 mediates sexually dimorphic expression of
mullerian inhibiting substance, in vivo. Development.
124:1799–1807. 1997.PubMed/NCBI
|
|
29
|
Jeffs B, Ito M, Yu RN, Martinson FA, Wang
ZJ, Doglio LT and Jameson JL: Sertoli cell-specific rescue of
fertility, but not testicular pathology, in Dax1 (Ahch)-deficient
male mice. Endocrinology. 142:2481–2488. 2001.PubMed/NCBI
|
|
30
|
Narula A, Kilen S, Ma E, Kroeger J,
Goldberg E and Woodruff TK: Smad4 overexpression causes germ cell
ablation and leydig cell hyperplasia in transgenic mice. Am J
Pathol. 161:1723–1734. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zohni K, Zhang X, Tan SL, Chan P and
Nagano MC: The efficiency of male fertility restoration is
dependent on the recovery kinetics of spermatogonial stem cells
after cytotoxic treatment with busulfan in mice. Hum Reprod.
27:44–53. 2012. View Article : Google Scholar
|
|
32
|
Kanatsu-Shinohara M, Toyokuni S, Morimoto
T, Matsui S, Honjo T and Shinohara T: Functional assessment of
self-renewal activity of male germline stem cells following
cytotoxic damage and serial transplantation. Biol Reprod.
68:1801–1807. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Siu ER, Mruk DD, Porto CS and Cheng CY:
Cadmium-induced testicular injury. Toxicol Appl Pharmacol.
238:240–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Koksal IT, Ishak Y, Usta M, Danisman A,
Guntekin E, Bassorgun IC and Ciftcioglu A: Varicocele-induced
testicular dysfunction may be associated with disruption of
blood-testis barrier. Arch Androl. 53:43–48. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Erkanlı Şentürk G, Ersoy Canillioglu Y,
Umay C, Demiralp-Eksioglu E and Ercan F: Distribution of Zonula
Occludens-1 and Occludin and alterations of testicular morphology
after in utero radiation and postnatal hyperthermia in rats. Int J
Exp Pathol. 93:438–449. 2012. View Article : Google Scholar
|
|
36
|
Xia W, Wong EW, Mruk DD and Cheng CY:
TGF-beta3 and TNFalpha perturb blood-testis barrier (BTB) dynamics
by accelerating the clathrin-mediated endocytosis of integral
membrane proteins: A new concept of BTB regulation during
spermatogenesis. Dev Biol. 327:48–61. 2009. View Article : Google Scholar :
|