Introduction
Hypoxia is a prominent feature of solid tumor types
resulting in a broad range of effects on a number of cellular
pathways and is one of the major contributors to the development of
resistance to anticancer drugs (1). The limited supply of nutrition and
oxygen creates hypoxic regions, which affect cancer progression and
induce changes in cellular metabolism (2). Melanoma, one of the most aggressive
and treatment-resistant solid tumor types, is also affected by
hypoxia. The predominant transcriptional regulator of the hypoxic
response is the heterodimeric hypoxia-inducible factor (HIF)1
(3), which serves an essential
role in the maintenance of oxygen homeostasis (4). Oxygen levels affect protein
stability, subcellular localization and transcriptional potency of
the HIF1α subunit. Under normoxic conditions, HIF1α is hydroxylated
on two conserved proline residues and is subsequently degraded via
the proteasome (3). Hypoxia
prevents the hydroxylation of HIF1α, leading to its accumulation
and translocation into the nucleus where it dimerizes with HIF1β,
and together they regulate the expression of hundreds of genes
(3). One of these genes encodes
carbonic anhydrase (CA)IX, which is involved in the regulation of
pH, tumor cell survival, adhesion and migration (2). CAIX exhibits only limited expression
in normal tissues, however, its expression is highly elevated in
various cancer types, including colorectal and lung carcinomas
(5,6). CAIX has not been shown to be
expressed in melanomas (7). HIF1
is upregulated by mutated Ras and Braf, as well as by
loss-of-function mutations of
phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase, and it has
been reported that mutant BRAF-V600 increases the expression of
HIF1α in melanoma cells (8). BRAF
is a key regulator of cell growth and proliferation in melanoma and
when mutated can act as an oncogene (9).
Approximately 60% of melanomas display a mutation in
the gene encoding the serine/threonine protein kinase, BRAF
(10). The mutated protein can be
inhibited by specific inhibitors (11). Vemurafenib, a specific BRAF-V600
kinase inhibitor, already in clinical use, arrests the cell-cycle
and induces apoptosis in melanoma cells (12,13).
However, inhibition of BRAF often provides short and incomplete
responses, followed by relapses (14). Hypoxia contributes to the relapse
since it markedly influences the phenotype of melanoma cells, their
aggressiveness and treatment sensitivity, leading to cancer
progression. Hypoxia can trigger a dynamic, adaptive phenotypic
response, where cells switch from a highly proliferative, poorly
invasive phenotype to a highly invasive, less proliferative one,
through changes in receptors involved in the non-canonical
wingless-type MMTV integration site family, member 5A signaling
pathway, tyrosine-protein kinase transmembrane receptor (ROR)1 and
ROR2 (15).
The present study investigated the role of hypoxia
on the treatment with vemurafenib in human melanoma cells. In a
panel of different cell lines, hypoxia altered the metabolism and
increased the cell motility of A375 cells exposed to vemurafenib.
Further experiments revealed that vemurafenib had no affect on the
protein expression levels of HIF1α and CAIX in hypoxic A375 cells,
suggesting a cell-type specific pattern of melanoma
progression.
Materials and methods
Cell lines and reagents
The A375 human melanoma cell line was purchased from
American Type Culture Collection (Rockville, MD, USA). The 518A2
and M14 melanoma cell lines were described previously (16). All cells were cultured in RPMI-1640
medium (Lonza, Inc., Verviers, Belgium), supplemented with 10%
fetal calf serum (FCS; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), at 37°C in a humidified 5% CO2
incubator. Routine tests to exclude mycoplasma and to characterize
the origin of the cells (short tandem repeat analysis) were
performed. Genotyping for a BRAF-V600 mutation was performed using
the BRAF StripAssay (ViennaLab, Vienna, Austria). Vemurafenib
(PLX4032), a selective inhibitor of BRAF-V600, was purchased from
Selleckchem (Houston, TX, USA). Exposure to hypoxia was performed
in an anaerobic work station (Ruskin Technologies, Bridgend, UK) in
2% O2, 5% CO2, 10% H2, and 83%
N2, at 37°C.
Real-time cell proliferation assays in
normoxic and hypoxic conditions
In order to track the cell growth of melanoma cells
treated with vemurafenib, a real-time characterization was
performed using the xCELLigence system (ACEA Bioscience, Inc., San
Diego, CA, USA). The impedance-based xCELLigence system®
was placed at 37°C in a humidified 5% CO2 incubator. A
total of 5×103 melanoma cells/well were seeded and
placed in the xCELLigence system®. The proliferation was
measured for 24 h prior to the addition of the following compounds:
i) 1 or 5 µM vemurafenib, ii) 1 mM dimethyloxalylglycine
(DMOG; Sigma-Aldrich, St. Louis, MO, USA), iii) 1 or 5 µM
vemurafenib plus 1 mM DMOG. The plates were placed back in the
xCELLigence system® and cell proliferation was measured
for 100 h. DMOG was used to induce hypoxia in cells when it was
technically not possible to use a hypoxia chamber.
Determination of extracellular pH (pHe)
and oxygen consumption in melanoma cells
Since hypoxia-induced changes in metabolism can
affect pHe and oxygen consumption, these two parameters were
measured by a non-invasive SDR optical sensor system (PreSens
Precision Sensing GmbH, Regensburg, Germany) embedded in the
hypoxic chamber. A total of 0.2×106 melanoma cells were
seeded into 24-well OxoDish® plates (PreSens Precision
Sensing GmbH) for the determination of oxygen consumption, and in
24-well HydroDish® plates (PreSens Precision Sensing
GmbH) for the determination of pHe, for 24 h. The cells were
subsequently incubated with 1 µM vemurafenib. Oxygen and pH
kinetics were visualized in real-time using the SDR optical sensor
system. The pHe was measured every 25 min for 112 h and the oxygen
consumption was measured every 5 min for 5 h.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.,) from normoxic and
hypoxic melanoma cells treated with vemurafenib (1 µM). The
RNA (2 µg) was reverse-transcribed into cDNA using the
High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems,
Foster City, CA, USA) using random heptameric primers. RT-qPCR
analysis of HIF1α and CA9, and β-actin as an
internal standard, were performed on a StepOneTM Real-Time PCR
system (Applied Biosystems) using the Power SYBR® Green
PCR Master mix (Applied Biosystems). The primers used were as
follows: HIF1α, sense: 5′-GCTTGGTGCTGATTTGTGAACC-3′ and
antisense: 5′-GCATCCTGTACTGTCCTGTGGTG-3′; CA9, sense:
5′-CCGAGCGACGCAGCCTTTGA-3′ and antisense:
5′-GGCTCCAGTCTCGGCTACCT-3′; β-actin, sense:
5′-TCCTCCCTGGAGAAGAGCTA-3′ and antisense:
5′-ACATCTGCTGGAAGGTGGAC-3′. The results were analyzed using the
Applied Biosystems 7500 system v1.4.0 software (Applied
Biosystems).
HIF1α knockdown induced by small
interfering (si)RNA in A375 melanoma cells
A total of 9×105 A375 cells were plated
into 6-well tissue culture plates, containing antibiotic-free
growth medium. The cells were grown up to 60–80% confluence and
were subsequently transfected with HIF1α specific siRNA
oligonucleotides (7 and 10 µg/ml; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) or with control siRNAs (7 and 10
µg/ml) using the siRNA Reagent System (Santa Cruz
Biotechnology, Inc.), according to the manufacturer's protocol. The
transfected cells were grown in medium containing vemurafenib (1
µM) for 24 h, harvested and immunoblotting were
performed.
Immunoblotting
A total of 1×105 melanoma cells
were incubated with vemurafenib (1 µM) and placed in
normoxia or hypoxia for 24 h. The cells were subsequently lysed in
lysis buffer, as described previously (16), and equal quantities of protein (60
µg per lane) were separated on sodium dodecyl
sulphate-polyacrylamide gel electrophoresis gels (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) under reducing conditions.
The proteins were transferred onto polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). and blocked with
Tris-buffered saline (TBS; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 3% bovine serum albumin (BSA; Roth, Karlsruhe,
Germany) for 2 h at room temperature. Subsequently, the membranes
were incubated wit the following primary mouse anti-human
immunoglobulin (Ig) G antibodies overnight at 4°C: Anti-HIF1α
(1:8,000; clone 54; 565924; BD Biosciences, San Jose, CA, USA),
anti-CAIX (1:8,000; clone M75) (17), anti-phosphorylated (p-)focal
adhesion kinase (FAK; Tyr397; 1:500; clone M121; ab24781), anti-FAK
(1:5,000; clone 63D5; ab72140), anti-protein kinase C (PKC) α
(1:1,000; clone M237; ab86715; all Abcam, Cambridge, UK),
anti-p-protein kinase B (AKT; Ser473; 1:1,000; clone 587F11; 4051),
anti-AKT (1:2,000; clone 40D4; 2920), anti-p-signal transducer and
activator of transcription (STAT)3 (Tyr705; 1:2,000; clone M9C6;
4113), anti-STAT3 (1:1,000; clone 124H6; 9139),
anti-p-extracellular-signal-regulated kinases (ERK)1/2
(Thr202/Tyr204; 1:2,000; clone E10; 9106), anti-ERK1/2 (1:2,000;
clone 3A7; 9107) or anti-β-actin (1:1,000; clone 8H10D10; 3700; all
Cell Signaling Technology, Inc., Danvers, MA, USA). Following
washing three times for 5 min at room temperature with TBS
supplemented with 0.5% BSA and 0.05% Tween 20 (Sigma-Aldrich), the
membranes were incubated with corresponding peroxidase-conjugated
horse anti-mouse IgG monoclonal secondary antibody (1:2,000; 7076;
Cell Signaling Technology, Inc.) for 2 h at room temperature. Blots
were developed with LumiGLO chemiluminescent substrate (Cell
Signaling Technology, Inc.) and the bands were visualized using the
Filmentwickler CP1000 Processor (AGFA, Mortsel, Belgium).
Wound-healing assay
To analyze 2D-cell migration behavior, a
wound-healing assay was performed, as previously described
(18). Melanoma cells seeded to
confluence, were starved overnight in medium containing 0.5% FCS
and treated with vemurafenib (1 µM). A wound was made with a
sterile micropipette tip. Images of the cells were captured
immediately following wound initiation and then every 15 min for up
to 48 h using an inverted Zeiss microscope (Axiovert40-CFL; Carl
Zeiss, Oberkochen, Germany). Wound-healing was quantified using
ImageJ software (version 2; National Institutes of Health,
Bethesda, MA, USA) (19) and the
results were analyzed by unpaired Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Hypoxia influences the cell growth of
BRAF-V600E mutant melanoma cells treated with vemurafenib
All three melanoma cell lines tested harbor the
BRAF-V600E mutation (data not shown). The influence of hypoxia on
the cell growth of melanoma cell lines treated with vemurafenib was
assessed in a real-time setting using the impedance-based
xCELLigence system® for up to 140 h under normoxic and
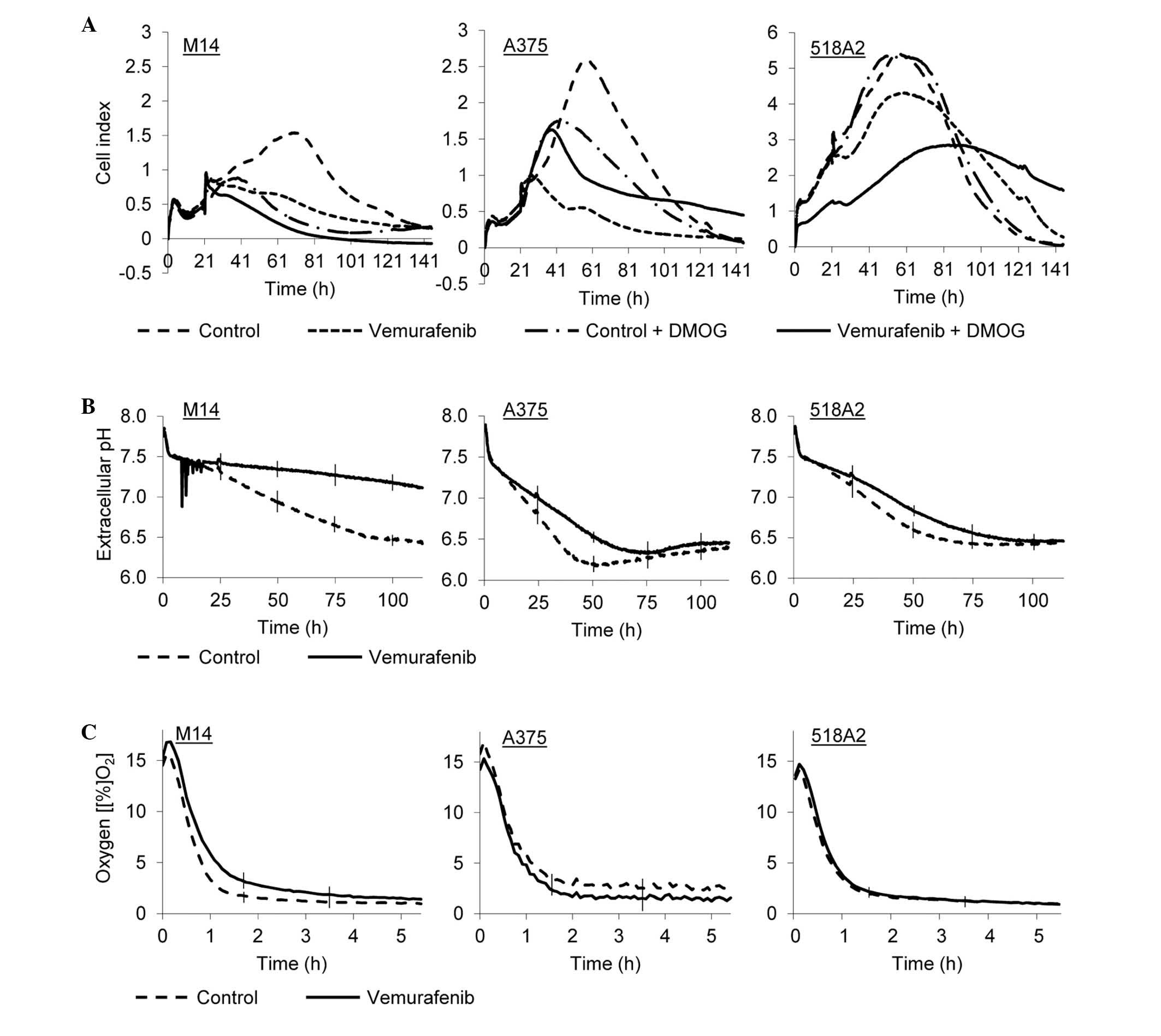
hypoxic conditions (Fig. 1A).
Normoxic, vemurafenib-treated M14, A375 and 518A2 cells exhibited
reduced proliferation after 65 h by 67, 80 and 20%, respectively,
compared with normoxic, untreated cells. At this time point,
hypoxic M14 and A375 cells reduced their growth by 83 and 44%,
respectively, compared with the normoxic counterparts. The 518A2
cells were not susceptible to hypoxic conditions and the
proliferation rate revealed no change. However, hypoxic
vemurafenib-treated A375 cells exhibited an unexpected increase in
the cell proliferation rate (cell index, 1) when compared with
normoxic, vemurafenib-treated A375 cells (cell index, 0.5).
Hypoxic, vemurafenib-treated M14 and 518A2 cells reduced growth by
an additional 26 and 34, respectively, compared with normoxic,
vemurafenib-treated M14 and 518A2 cells.
Modification of pHe and oxygen
consumption in hypoxic, vemurafenib-treated melanoma cells
Cultivation of melanoma cells under hypoxia for 130
h in the presence of vemurafenib resulted in reduced acidification
of the extracellular environment. Incubation with vemurafenib
markedly reduced the pHe of the growth medium of hypoxic M14 cells.
For the other two cell lines, pHe dropped at a reduced rate,
reaching 6.3±0.02 for A375 cells after 70 h and slightly increased
to 6.5±0.02. For 518A2 cells, the pHe dropped to 6.4±0.04 after 75
h (Fig. 1B). No change was
observed in oxygen consumption between treated and untreated 518A2
cells. A decrease of oxygen consumption was observed in M14 cells,
whereas a slight increase was observed in the A375 cells (Fig. 1C).
Effect of hypoxia on HIF1α and CAIX in
vemurafenib-treated melanoma cells
Relative mRNA expression of HIF1α under
hypoxic conditions were between 0.7 and 0.8 for all three cell
lines. In normoxic M14 cells, the mRNA expression of HIF1α
was 1±0.3 and it decreased following vemurafenib treatment under
both hypoxic and normoxic conditions to 0.4±0.03 and 0.2±0.07,
respectively. The relative mRNA expression of HIF1α in
normoxic A375 cells was 1±0.2 and decreased to 0.7±0.2 following
vemurafenib treatment. However, under hypoxic conditions,
vemurafenib treatment increased the mRNA expression of HIF1α
to 1.4±0.4 in A375 cells. The relative mRNA expression of
HIF1α in 518A2 revealed no significant changes between the
different treatment and culture conditions. The relative mRNA
expression of CA9 under normoxic conditions were between 1
and 2 in all three cell lines, and were reduced following
treatment. The mRNA expression levels of CA9 were 2.8±0.08
for M14, 49.2±5.3 for A375 and 12.1±0.9 for 518A2 cell lines under
hypoxia. Following treatment with vemurafenib, all three cell lines
exhibited increased mRNA expression levels of CA9 (5.8±0.7,
90.0±2.7 and 15.5±1.2 for M14, A375 and 518A2, respectively). All
mRNA expression levels were normalized against the housekeeping
gene coding for β-actin (Fig.
2A).
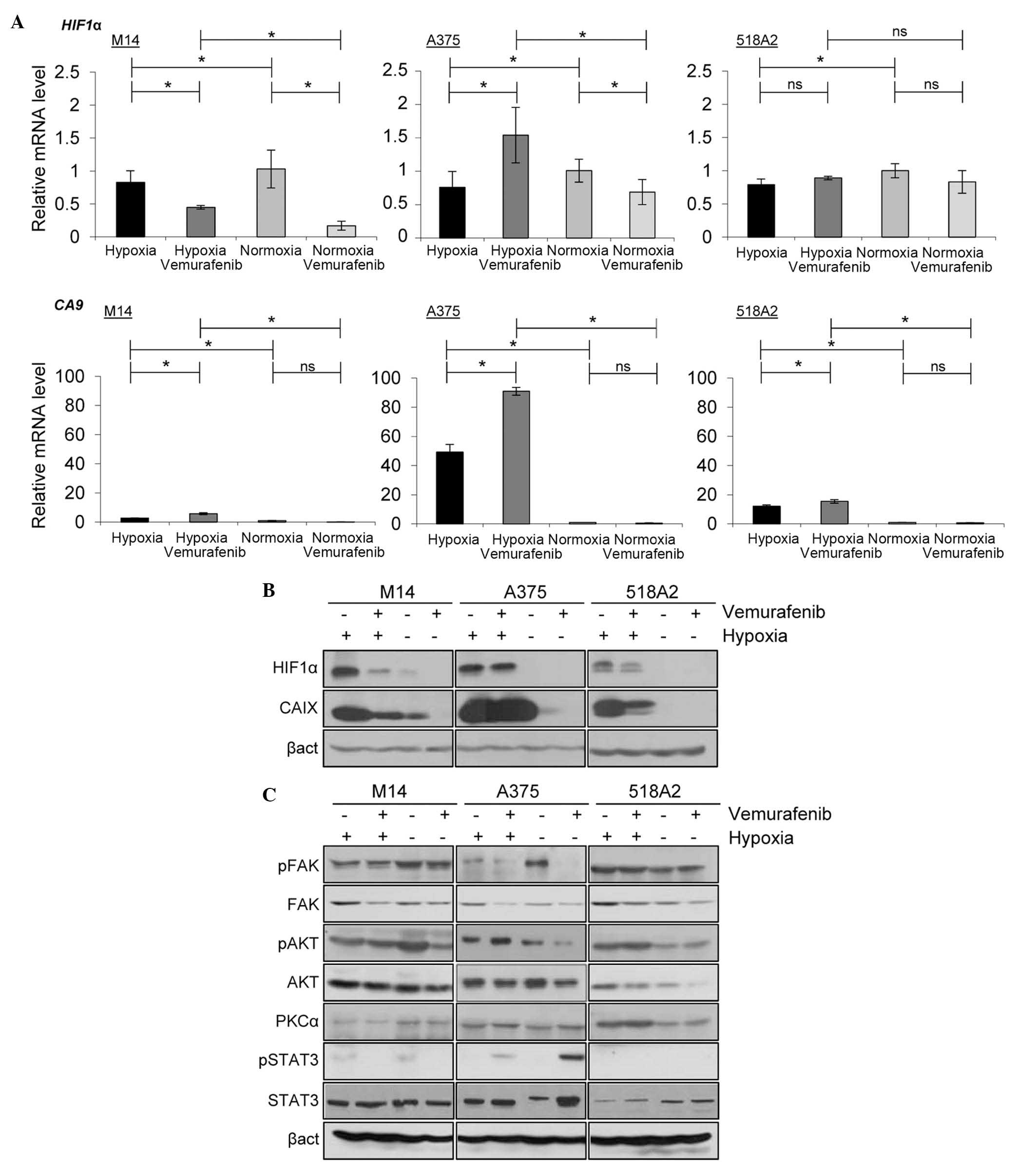 | Figure 2Effect of hypoxia on HIF1α, CAIX and
multiple signaling pathway proteins in melanoma cells treated with
vemurafenib. (A) Reverse transcription-quantitative polymerase
chain reaction analysis of the mRNA expression levels of HIF1α and
CA9 normalized against β-act. The data are presented as the mean ±
standard deviation of three independent experiments
(*P<0.05; ns, not significant). (B) The protein
expression levels of HIF1α and CAIX in vemurafenib-treated melanoma
cells were assessed by immunoblotting. (C) Immunoblotting of
proteins involved in the phosphatidylinositol-4,5-bisphosphate
3-kinase and Janus kinase-STAT pathways under normoxic and hypoxic
conditions after vemurafenib treatment. STAT, signal transducer and
activator of transcription; p-, phosphorylated; HIF,
hypoxia-inducible factor; CA, carbonic anhydrase; act, actin; FAK,
focal adhesion kinase; AKT, protein kinase B; PKC, protein kinase
C. |
The protein expression levels of HIF1α and CAIX were
evaluated by immunoblotting. HIF1α and CAIX were expressed in all
three cell lines under hypoxia. However, the expression level of
each protein was reduced in vemurafenib-treated M14 and 518A2
cells; this was not observed in A375 cells (Fig. 2B).
Hypoxia affects multiple signaling
pathways in vemurafenib-treated melanoma cells
To further assess how hypoxia modifies the
expression of proteins involved in cell growth, migration and
survival, an extended analysis of signaling pathways by
immunoblotting was performed. Melanoma cell lines were treated with
vemurafenib and exposed to hypoxia or normoxia. Under hypoxia, the
expression of p-FAK was downregulated in A375 and M14 cells, and
was upregulated in 518A2 cells. An increase in the expression
levels of p-AKT and PKCα was observed in hypoxic A375 and 518A2
cells compared with normoxic cells. Exposure to hypoxia resulted in
an upregulation of p-STAT3 in vemurafenib-treated A375 cells
(Fig. 2C).
HIF1α knockdown affects mitogen-activated
protein kinase (MAPK) and phosphatidylinositol-4,5-bisphosphate
3-kinase (PI3K)/AKT pathway proteins in A375 cells
To assess whether knockdown of HIF1α affected
signaling pathway proteins in A375 cells, an siRNA-based approach
was used. This resulted in a significant reduction of HIF1α
(Fig. 3A). Additionally, the
silencing of HIF1α decreased the protein expression of CAIX
(Fig. 3A) and affected the
expression levels of numerous MAPK and PI3K/AKT pathway proteins.
The knockdown of HIF1α decreased the expression of p-AKT,
which was restored following the treatment with vemurafenib. By
contrast, the expression of p-ERK was increased following the
knockdown of HIF1α and decreased following treatment with
vemurafenib. No change was observed in the protein expression
levels of p-FAK and PKCα following the silencing of HIF1α
(Fig. 3B).
 | Figure 3Effect of HIF1α silencing on the
expression of multiple signaling pathway proteins in A375 melanoma
cells treated with vemurafenib. (A) Silencing of HIF1α in A375
cells and its effects on the expression of CAIX. (B) Immunoblotting
of proteins involved in the phosphatidylinositol-4,5-bisphosphate
3-kinase and mitogen-activated protein kinases pathways following
silencing of HIF1α in A375 cells after vemurafenib treatment. si,
small interfering; p-, phosphorylated; HIF, hypoxia-inducible
factor; CA, carbonic anhydrase; act, actin; FAK, focal adhesion
kinase; AKT, protein kinase B; PKC, protein kinase C; ERK,
extracellular-signal-regulated kinases. |
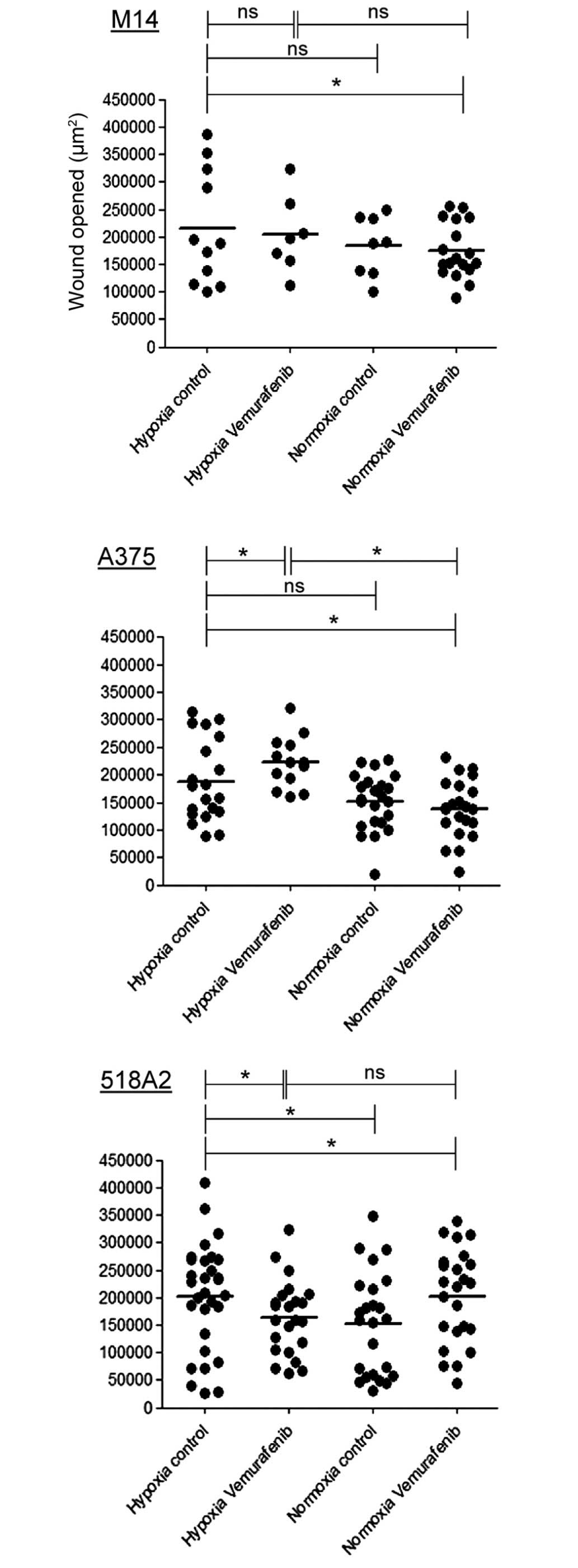
Hypoxia enhances cell migration of
vemurafenib-treated A375 melanoma cells
To assess directional cell migration in
vitro, a wound-healing assay was performed. Migration was
induced in all three cell lines by hypoxia. When vemurafenib was
added, hypoxic cells behaved differently. After 8 h, 518A2 cells
reduced the migration by 34%, M14 cells revealed no changes and
A375 cells increased migration by 20% compared with hypoxic
untreated cells (Fig. 4).
Discussion
The present study explored the role of hypoxia as a
cause of melanoma heterogeneity. The characterization of different
subsets of melanoma cells in a tumor lesion is of major clinical
relevance for a successful therapeutic intervention (15,20).
Hypoxia not only promotes the growth of a tumor by enhancing
glycolytic flux and increasing angiogenesis, but also allows tumors
to acquire resistance to anticancer drugs (3,15).
Vemurafenib is a specific inhibitor of the mutated BRAF-kinase and
is used to treat patients with metastatic melanoma presenting the
BRAF-V600 activating mutation (13). All cell lines harboring the
BRAF-V600E-mutation tested were sensitive to vemurafenib under
normoxic conditions in a real-time setting using the
impedance-based xCELLigence system® (Fig. 1A). However, a different
proliferative response was observed when these cell lines were
treated with vemurafenib in a hypoxic microenvironment. M14 and
518A2 cells continued to respond to the drug and decreased cell
proliferation rates, whereas hypoxic, vemurafenib-treated A375
cells demonstrated an increase of cell proliferation rate by 20%
compared with normoxic, vemurafenib-treated A375 cells (Fig. 1A). Hypoxia induces acidification of
the tumor microenvironment, which promotes cell migration and
invasion (2). Low pHe is a
consequence of the abnormal metabolism in the tumor and a
supportive factor for its progression (2). Hypoxic M14 cells displayed less
pronounced extracellular acidosis, reaching a pHe value of 6.5±0.03
following 90 h (Fig. 1B).
Vemurafenib prevented the acidosis and caused a shift in pHe from
acidic to alkaline for this cell line, suggesting that the
metabolism of the treated cells was significantly reduced. This was
accompanied by decreased oxygen consumption (Fig. 1B and C). Altogether, this indicated
that M14 cells retained their sensitivity to vemurafenib even under
hypoxia. In hypoxic A375 and 518A2 cells, pHe values were rendered
acidic (<6.5) in a relatively short period of time. Shifts
following vemurafenib treatment were smaller and reached pHe values
comparable to untreated cells after ~80 h (Fig. 1B). These data provided
pathophysiological evidence of a diminished response to vemurafenib
under hypoxia, which was further supported by a minor change in
oxygen consumption of these cells (Fig. 1C). The survival of resistant A375
and 518A2 cell pools was also reflected in equalized pHe values,
whereas the pHe for M14 cells was increased due to the rapid
die-off of vemurafenib-sensitive M14 cells.
The main transcriptional regulator of the hypoxic
response, HIF1, has been shown to be associated with melanomas
(3). HIF1 serves an essential role
in the maintenance of oxygen homeostasis and controls the
expression of hundreds of genes, including CAIX, mediating
developmental and physiological pathways. All melanoma cell lines
tested expressed the hypoxic factors, HIF1α and CAIX, and
vemurafenib decreased their expression levels in hypoxic 518A2 and
M14 cells (Fig. 2B). Notably, in
hypoxic A375 cells, the protein expression levles of HIF1α and CAIX
remained unchanged following vemurafenib treatment (Fig. 2B), suggesting that vemurafenib had
no affect on the hypoxic fraction of this cell line. This was not
reflected in the mRNA expression levels of HIF1α and CAIX,
suggesting that the transcription of HIF1α and CAIX was not
influenced by vemurafenib (Fig.
2A). It has been previously shown that the BRAF-V600E mutation
increased HIF1α, suggesting that the expression of HIF1α is
partially regulated via the MAPK/ERK pathway (8).
The present study subsequently focused its attention
on the effect of hypoxia and vemurafenib on proteins involved in
the PI3K, MAPK and Janus kinase-STAT signaling pathways. It was
determined that hypoxia and vemurafenib differently influenced
signaling pathway proteins, including p-FAK, p-AKT, PKCα and
p-STAT3, in the three cell lines. The expression of p-FAK was
downregulated in hypoxic A375 and M14 cells, and was upregulated in
hypoxic 518A2 cells. An increase in the expression levels of p-AKT
and PKCα was observed in hypoxic A375 and 518A2 cells. Hypoxia
induced an upregulation in the expression of p-STAT3 in
vemurafenib-treated A375 cells (Fig.
2C). Since the expression levels of HIF1α and CAIX were not
decreased in hypoxic A375 cells following treatment with
vemurafenib, the present study silenced HIF1α using an
siRNA-based approach (Fig. 3A) and
evaluated the effects of silencing on the PI3K and MAPK signaling
pathways. Silencing of HIF1α in hypoxic A375 cells restored
the protein expression of p-ERK and the expression decreased again
following treatment with vemurafenib. By contrast, the expression
of p-AKT decreased after silencing of HIF1α and resumed
following the treatment with vemurafenib (Fig. 3B). The data highlighted the
complexity of the hypoxic melanoma phenotype and the challenge of
optimizing BRAF-targeted therapy in this disease. Hypoxia was
suggested as a possible facilitator of migration, switching between
a proliferative to invasive phenotype in melanoma cells (3,20).
All three cell lines increased the migration under hypoxic
conditions compared with normoxic conditions (Fig. 4). However, following treatment with
vemurafenib, hypoxic A375 cells increased their migratory capacity,
whereas hypoxic 518A2 cells exhibited reduced migration (Fig. 4).
The present findings supported the assumption that
melanoma cell populations are not homogenous, however, consist of
subpopulations with differing migrative, proliferative and
tumor-initiating potentials. The development of resistance to
vemurafenib can be explained, only in part, by the activation of
other signaling pathways. A major challenge in cancer research is
therefore to develop methods to characterize cell heterogeneity,
and novel strategies to overcome resistance and target alternative
pathways, which are not hampered by hypoxia.
Acknowledgments
The present study was a part of the EU Marie Curie
Initial Training Network Biomedical engineering for cancer and
brain disease diagnosis and therapy development (no.
PITN-GA-2010-264417).
Abbreviations:
|
HIF1α
|
hypoxia-inducible factor 1α
|
|
CAIX
|
carbonic anhydrase IX
|
References
|
1
|
Rohwer N and Cramer T: Hypoxia-mediated
drug resistance: Novel insights on the functional interaction of
HIFs and cell death pathways. Drug Resist Update. 14:191–201. 2011.
View Article : Google Scholar
|
|
2
|
Pastorek J and Pastorekova S:
Hypoxia-induced carbonic anhydrase IX as a target for cancer
therapy: From biology to clinical use. Semin Cancer Biol. 31:52–64.
2015. View Article : Google Scholar
|
|
3
|
Hanna SC, Krishnan B, Bailey ST, Moschos
SJ, Kuan PF, Shimamura T, Osborne LD, Siegel MB, Duncan LM, O'Brien
ET III, et al: HIF1 alpha and HIF2α independently activate SRC to
promote melanoma metastases. J Clin Invest. 123:2078–2093. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Semenza GL: Hypoxia-inducible factors:
Mediators of cancer progression and targets for cancer therapy.
Trends Pharmacol Sci. 33:207–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ivanov S, Liao SY, Ivanova A,
Danilkovitch-Miagkova A, Tarasova N, Weirich G, Merrill MJ,
Proescholdt MA, Oldfield EH, Lee J, et al: Expression of
hypoxia-inducible cell-surface transmembrane carbonic anhydrases in
human cancer. Am J Pathol. 158:905–919. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chrastina A, Závada J, Parkkila S, Kaluz
T, Kaluzová M, Rajcáni J, Patorek J and Pastoreková S:
Biodistribution and pharmacokinetics of I-125I-labeled monoclonal
antibody M75 specific for carbonic anhydrase IX, an intrinsic
marker of hypoxia, in nude mice xenografted with human colorectal
carcinoma. Int J Cancer. 105:873–881. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Syrjänen L, Luukkaala T, Leppilampi M,
Kallioinen M, Pastorekova S, Pastorek J, Waheed A, Sly WS, Parkkila
S and Karttunen T: Expression of cancer-related carbonic anhydrases
IX and XII in normal skin and skin neoplasms. APMIS. 122:880–889.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kumar SM, Yu H, Edwards R, Chen L,
Kazianis S, Brafford P, Acs G, Herlyn M and Xu XW: Mutant V600E
BRAF increases hypoxia inducible factor-1alpha expression in
melanoma. Cancer Res. 67:3177–3184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Halaban R, Zhang W, Bacchiocchi A, Cheng
E, Parisi F, Ariyan S, Krauthammer M, McCusker JP, Kluger Y and
Sznol M: PLX4032, a selective BRAF (V600E) kinase inhibitor,
activates the ERK pathway and enhances cell migration and
proliferation of BRAF (WT) melanoma cells. Pigm Cell Melanoma Res.
23:190–200. 2010. View Article : Google Scholar
|
|
10
|
Botton T, Yeh I, Nelson T, Vemula SS,
Sparatta A, Garrido MC, Allegra M, Rocchi S, Bahadoran P, McCalmont
TH, et al: Recurrent BRAF kinase fusions in melanocytic tumors
offer an opportunity for targeted therapy. Pigment Cell Melanoma
Res. 26:845–851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Azijli K, Stelloo E, Peters GJ and VAN DEN
Eertwegh AJ: New developments in the treatment of metastatic
melanoma: Immune checkpoint inhibitors and targeted therapies.
Anticancer Res. 34:1493–1505. 2014.PubMed/NCBI
|
|
12
|
Lee JT, Li L, Brafford PA, van den Eijnden
M, Halloran MB, Sproesser K, Haass NK, Smalley KS, Tsai J, Bollag G
and Herlyn M: PLX4032, a potent inhibitor of the B-Raf V600E
oncogene, selectively inhibits V600E-positive melanomas. Pigment
Cell Melanoma Res. 23:820–827. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chapman PB, Hauschild A, Robert C, Haanen
JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et
al: Improved survival with vemurafenib in melanoma with BRAF V600E
mutation. N Engl J Med. 364:2507–2516. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wagenaar TR, Ma LY, Roscoe B, Park SM,
Bolon DN and Green MR: Resistance to vemurafenib resulting from a
novel mutation in the BRAFV600E kinase domain. Pigm Cell Melanoma
Res. 27:124–133. 2014. View Article : Google Scholar
|
|
15
|
O'Connell MP, Marchbank K, Webster MR,
Valiga AA, Kaur A, Vultur A, Li L, Herlyn M, Villanueva J, Liu Q,
et al: Hypoxia induces phenotypic plasticity and therapy resistance
in melanoma via the tyrosine kinase receptors ROR1 and ROR2. Cancer
Discov. 3:1378–1393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wagner S, Hafner C, Allwardt D, Jasinska
J, Ferrone S, Zielinski CC, Scheiner O, Wiedermann U, Pehamberger H
and Breiteneder H: Vaccination with a human high molecular weight
melanoma-associated antigen mimotope induces a humoral response
inhibiting melanoma cell growth in vitro. J Immunol. 174:976–982.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pastoreková S, Závadová Z, Kostál M,
Babusíková O and Závada J: A novel quasi-viral agent, Ma Tu, is a
2-component system. Virology. 187:620–626. 1992. View Article : Google Scholar
|
|
18
|
Svastova E, Witarski W, Csaderova L, Kosik
I, Skvarkova L, Hulikova A, Zatovicova M, Barathova M, Kopacek J,
Pastorek J and Pastorekova S: Carbonic anhydrase IX interacts with
bicarbonate transporters in lamellipodia and increases cell
migration via its catalytic domain. J Biol Chem. 287:3392–3402.
2012. View Article : Google Scholar :
|
|
19
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–5. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Widmer DS, Hoek KS, Cheng PF, Eichhoff OM,
Biedermann T, Raaijmakers MI, Hemmi S, Dunnmer R and Levesque MP:
Hypoxia contributes to melanoma heterogeneity by triggering
HIF1α-dependent phenotype switching. J Invest Dermatol.
133:2436–2443. 2013. View Article : Google Scholar : PubMed/NCBI
|