Introduction
Vascular dementia (VaD) is one of the most common
cognitive disorders of the elderly, after Alzheimer's disease (AD).
VaD is caused by a range of cardiovascular or cerebrovascular
conditions that lead to ischemic, hypoperfusive, or hemorrhagic
brain lesions; which are characterized by a loss of cognitive
function (1–4). Chronic cerebral hypoperfusion (CCH)
is strongly correlated with progressive cognitive decline (5,6).
Since the establishment of the 2VO rat model to reproduce CCH,
which is the permanent bilateral occlusion of the common carotid
artery (7,8), this model has been used widely to
study the molecular mechanisms underlying cognitive impairment,
white matter lesions, and neuronal damage observed in CCH, as well
as in therapeutic intervention studies (9–12).
Among the novel therapeutic strategies that have
been pursued to alleviate cognitive damage, physical activity has
been shown to support brain health and function through the
prevention of oxidative stress (13,14),
with beneficial effects on learning, long-term potentiation and
memory (14–16). Currently, regular physical activity
is consistently indicated as a preventive measure for age-related
neurodegenerative diseases (14,17).
In the mammalian brain, neurogenesis continues
throughout adulthood. Several factors, including exposure to
environmental enrichment, exercise and ischemic insult, have been
demonstrated to affect adult neurogenesis (18–20).
Exercise enhances neurogenesis, expression of growth
factors and synaptic plasticity in the hippocampus of rodents
(21). These changes have been
associated with improved cognitive function, spatial memory and
learning (21–24). In humans, physical activity not
only improves cognitive function, but is also associated with a
reduced risk of dementia, AD, and cognitive impairment in elderly
individuals (25–27).
In the adult brain, brain-derived neurotrophic
factor (BDNF) is known to be important in synaptic plasticity,
learning and neurogenesis, and is considered to be the most
important factor to be upregulated by exercise (14,28).
Mature BDNF activates tyrosine receptor kinase B, which is followed
by the phosphorylation of downstream effectors, including protein
kinase B, extracellular signal-regulated kinase, and
calcium-calmodulin dependent kinase. Ultimately, the transcription
factor cAMP-calcium response element binding protein (CREB), which
mediates the transcription of genes that are essential for the
survival and differentiation of neurons, is phosphorylated and
activated (14,29). Accumulating evidence indicates that
a signaling mechanism is involved in neurogenesis augmentation,
which is associated with the expression of phosphorylated CREB
(p-CREB) and BDNF in the hippocampus (22), and is involved in synaptic
plasticity, learning and memory (30). A previous study reported that
physical activity, such as exercise, enhances ischemic neurogenesis
following stroke (31).
However, the effect of physical activity-induced
hippocampal neurogenesis in VaD has not been investigated.
Therefore, in the present study, the effect of increased
hippocampal neurogenesis induced by exercise on cognitive
impairment was confirmed in rats subjected to 2VO.
Materials and methods
Animals and experimental setup
A total of 52 adult male Wistar rats (age, 8 weeks;
weight, 292±3.05 g; Samtako Bio Korea, Co., Ltd., Seorang-dong,
Korea) were used in this study. The animals were housed in groups
of 3 in smooth-bottomed plastic cages (28×42×20 cm) with beta chip
bedding (Agrolab Group, Bavaria, Germany) in a specific pathogen
free room that was maintained on a 12 h light/dark cycle. Food and
water were available ad libitum. The room temperature was
maintained at 22±1°C and the room was illuminated by incandescent
lamps (luminous flux, 11.77l m). To accustom the animals to the
laboratory environment, an acclimation period of 1 week was allowed
prior to the initiation of the experiment. Animal treatments,
including anesthesia and euthanasia were conducted in accordance
with the Principle of Laboratory Animal Care (NIH Publication no.
85–23, revised 1985). All experimental procedures were approved by
the Animal Experiment Review Board of the Laboratory Animal
Research Center of Konkuk University (Seoul, Korea; KU09059).
The experimental animals were randomly divided into
2 groups: The sham-surgery group [sham control (SC), n=24] and the
2VO group (n=28). A blindness test was performed to exclude blind
rats, as described previously (32), 3 weeks after surgery. The blindness
rates were 10.7% (3 out of 28) among 2VO rats and 0% (0 out of 24)
among the SC rats. Twenty-four rats from the SC group and 25 rats
from the 2VO group were subdivided into treadmill exercise or no
exercise groups. The experimental groups consisted of SC (n=12), SC
with treadmill exercise (SC+EX, n=12), 2VO (n=13), and 2VO with
treadmill exercise (2VO+EX, n=12).
A subset of animals were used for behavioral
(n=12–13/group), morphological (n=8/group), and biochemical
analysis (n=4–5/group). The animals were number coded and
investigators were always blinded to the treatment groups until the
end of the data analysis. Animal allocation and a time line of
experiments are shown in Fig.
1.
Surgical procedures
Chronic cerebral hypoperfusion in the rat was
modeled by 2VO. The rats were anesthetized with 70% nitrogen and
30% isoflurane (Hana Pharm Co., Ltd., Kyonggido, Korea). Both
common carotid arteries were exposed through a midline cervical
incision and were double ligated using silk sutures (n=28; B. Braun
Medical Inc., Bethlehem, PA, USA). The SC rats (n=24) were treated
similarly to the rats in the 2VO group with the exception of the
common carotid artery occlusion. During the surgical procedure, the
rectal temperature was monitored and maintained at 37±0.5°C using a
heating pad (Homeothermic Blanket system; Harvard Apparatus Inc.,
Holliston, MA, USA). Following surgery, rats were kept in an animal
resource facility with access to food and water ad libitum.
Three weeks after the sham or 2VO surgery, the rats underwent a
blindness test as described previously (32), followed by treadmill exercise.
Forced treadmill training
The animals allocated to the exercise groups (SC+EX
and 2VO+EX) ran on a motor-driven treadmill (Columbus Instruments,
Columbus, OH, USA) at a 0° inclination, beginning 3 weeks after the
sham or 2VO operation. The speed of the exercise was 15 m/min, and
the animal performed exercise 30 min daily for 4 weeks (5
days/week). The electrical part of the treadmill delivered light
electric shocks (about 0.3 mA stimulus current) when the rats
entered the rear of the test chamber. The SC and 2VO groups were
placed in neighboring lanes without switching on the treadmill
motor for the exact duration of the experiments (33).
5-Bromo-2-deoxyuridine (BrdU)
administration
Neurogenesis was assessed by analyzing the
incorporation of BrdU (Sigma-Aldrich, St. Louis, MO, USA), which is
a thymidine analog and a marker of proliferating cells. BrdU
solutions were prepared to 5 mg/ml in sterile saline with 0.007 N
NaOH to obtain a 50 mg/kg dose (10 ml/kg). The animals received an
intraperitoneal (IP) injection of 50 mg/kg/day of BrdU
(Sigma-Aldrich) 3 weeks following sham or 2VO surgery, and received
the same dosage every day for 2 weeks (n=12–13/group).
Morris water maze (MWM) test
The MWM test was used to evaluate learning and
memory in rats (11,34). The maze was a round tank that
measured 1.83 m in diameter and was 0.58 m deep. It was filled to a
depth of 35.5 cm with tepid (26±1°C) water that was made opaque by
the addition of a nontoxic white-colored dye. A movable circular
platform with a diameter of 12 cm was placed 2 cm below the surface
of the water. The maze was surrounded by white curtains, on which
black visual stimuli of various shapes and sizes were placed. A
camera (CBC Co., Ltd., Tokyo, Japan) was located above the center
of the maze and relayed images to a videocassette recorder
(AverMedia, New Taipei City, Taiwan) and an HVS Image Analysis
Computer system (HVS, Hampton, United Kingdom). Seven weeks after
sham or 2VO surgery (4 weeks after treadmill exercise), the MWM
test was conducted to evaluate spatial memory (n=12–13/group). Four
consecutive sessions, each consisting of 5 trials over 1 day, were
conducted over 4 consecutive days. The hidden platform was always
placed in the southeast quadrant of the pool. Every trial started
at a different point, which alternated among the 4 quadrants. Rats
were gently handled for 10 min daily for 7 days prior to the test.
In the maze, rats were allowed to swim for a maximum of 90 sec. If
the rat failed to find the platform within 90 sec, it was guided
gently onto the platform and allowed to remain there for 30 sec.
The intertrial interval was 1 min. Performance accuracy was
evaluated by analyzing the search error and time latency data of
all trials. Measurement of the search error was based on a
computation of the average distance from the platform during the
trial. The distance between a rat and the platform was sampled 10
times/sec during each trial, and these distances were averaged in 1
sec bins. The cumulative search error was the sum of these 1 sec
averages of the proximity measure corrected for the specific
platform location and start location, by subtracting the proximity
score that would be produced by a perfect performance in the trial.
A probe trial was conducted 1 min after the 5th training trial at
the 2nd and 4th sessions. The entire training procedure included 2
probe trials for each rat, during which the rats swam with the
platform retracted to the bottom of the pool for 30 sec. After
recording the swimming path, the platform was raised to its normal
position for completion of the trial. Swimming time in the target
quadrant of the retracted platform was used as a parameter of the
retention of spatial memory.
Western blot analysis
Hippocampal tissues were washed with ice-cold
phosphate-buffered saline (PBS) and lysed on ice in
radioimmunoprecipitation assay buffer [50 mM Tris-HCl, pH 7.4; 150
mM NaCl, 1% NP40, 0.25% Na-deoxycholate, and 0.1% sodium dodecyl
sulfate (SDS)] containing a protease inhibitor mixture and
phosphatase inhibitors (Sigma-Aldrich). Soluble proteins (30
µg) were subjected to 12% SDS-polyacrylamide gel (Bio-Rad
Laboratories, Inc., Berkeley, CA, USA) electrophoresis and
electrotransferred onto a polyvinylidene difluoride membrane
(Millipore, Darmstadt, Germany). Membranes were blocked for 1 h in
20 mM Tris-HCl containing 137 mM NaCl and 0.1% Tween 20 (TBST)
containing 5% skim milk at room temperature. They were then
incubated overnight at 4°C with the following primary antibodies:
Rabbit polyclonal anti-rat BDNF antibody (cat. no. sc-546; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA; 1:500), rabbit
polyclonal anti-rat phosphorylated CREB antibody (cat. no. 9191;
Cell Signaling Technology, Inc., Danvers, MA, USA; 1:1,000), rabbit
polyclonal anti-rat-CREB antibody (cat. no. 9197; Cell Signaling
Technology Inc.; 1:1,000), mouse monoclonal anti-rat β-actin
antibody (cat. no. A5441; Sigma-Aldrich; 1:5,000). Membranes were
washed with TBST and then incubated with peroxidase-conjugated
AffiniPure goat anti-mouse IgG (H+L; cat. no. 115-035-003; Jackson
ImmunoResearch, West Grove, PA, USA; 1:3,000 dilution) or
peroxidase-conjugated AffiniPure goat anti-rabbit IgG (H+L; cat.
no. 111-035-003; Jackson ImmunoResearch; 1:3,000) for 1 h at room
temperature. Specific bands were detected using the ECL system
(Amersham, Buckinghamshire, UK) and analyzed using the Bio-Rad
electrophoresis image software (Bio-Rad Laboratories, Inc., Hemel
Hampstead, UK). β-actin was used as a loading control and the band
intensity was normalized to β-actin. Quantitative analysis was
performed using quantity one software (version 4.6.6; Bio-Rad
Laboratories, Inc.).
Immunohistochemistry
Rats were anesthetized with diethyl ether (>4.5%;
Samchun Pure Chemical, Kyonggido, Korea) and sacrificed by
transcardial perfusion following anesthesia with diethyl ether.
Rats were transcardially perfused with saline containing 0.5%
sodium nitrite (Sigma-Aldrich) and 10 U/ml heparin sulfate (Jeil
Pharmaceutical Co., Ltd., Seoul, Korea), followed by 4% cold
formaldehyde (Sigma-Aldrich) generated from paraformaldehyde in 0.1
M PBS (pH 7.2). Following perfusion, rats were decapitated and then
the brains were removed and hippocampus blocks were immersion fixed
in 4% paraformaldehyde and cryoprotected in sucrose. Serial coronal
sections (40 µm) were cut on a cryostat, collected in
cryopreservative (Sigma-Aldrich), and stored at −20°C. Tissue
sections were washed in 0.1 M PBS and endogenous peroxidase was
blocked by immersing sections in 0.3% H2O2 in
0.1 M PBS for 30 min. To denature DNA, brain sections were
incubated in 50% formamide in saline sodium citrate buffer (2X SSC,
0.3 M NaCl buffer, and 0.03 M trisodium citrate) at 60°C for 2 h,
followed by incubation at 37°C in 2 N HCl for 30 min, and rinsing
in 100 mM boric acid (pH 8.5) for 10 min. Sections were washed in
0.1 M PBS, and then incubated in 0.1 M PBS containing 5% normal
goat serum (Jackson ImmunoResearch) and 0.3% Triton X-100
(Sigma-Aldrich) for 1 h, and subsequently incubated overnight with
a rat anti-BrdU antibody (BrdU, 1:400: Abcam, Cambridge, MA, USA)
at 4°C. The sections were then incubated with biotinylated anti-rat
IgG (1:500; cat. no. BA-9400; Vector Laboratories, Burlingame, CA,
USA) for 1 h, followed by avidin/biotin/peroxidase (1:50; Vector
Laboratories) staining for 1 h in a humidified chamber. PBS (0.1 M)
containing 1.5% bovine serum albumin (Sigma-Aldrich) was used to
wash sections on slides between all steps. The antigen-antibody
complexes were visualized by incubation for 5 min in 0.05%
3,3′-diaminobenzidine (Sigma-Aldrich) and 0.003%
H2O2 (Sigma-Aldrich), and sections were then
mounted sequentially on glass slides using permanent mounting
medium (Vector Laboratories). Mounted sections were evaluated using
a light microscope (Eclipse 80i; Nikon, Tokyo, Japan).
Fluorescent immunostaining of
tissues
Brain sections were incubated in antigen retrieval
buffer including 50% formamide in saline sodium citrate buffer (2X
SSC, 0.3 M NaCl buffer, and 0.03 M trisodium citrate) at 60°C for 2
h. To denature DNA, brain sections were incubated at 37°C in 2 N
HCl for 30 min and rinsed in 100 mM boric acid (pH 8.5) for 10 min.
Brain sections (40 µm) were washed in 0.1 M PBS, incubated
in 0.1 M PBS containing 5% normal donkey serum (Jackson
ImmunoResearch) and 0.3% Triton X-100 for 1 h, and subsequently
incubated overnight at 4°C with primary antibodies, including mouse
monoclonal anti-rat NeuN (1:2,000; cat. no. MAB377; Millipore,
Billerica, MA, USA), goat monoclonal anti-rat doublecortin (DCX;
1:500; cat. no. sc-8066; Santa Cruz Biotechnology, Inc.) and rat
monoclonal anti-BrdU (1:400; cat. no. ab6326; Abcam) in 2% normal
donkey serum (Jackson ImmunoResearch) in PBS. Subsequently, they
were incubated in a 1:200 dilution of Alexa Fluor-conjugated 488
donkey anti-mouse IgG (H+L; cat. no. A21202; Invitrogen; Thermo
Fisher Scientific, Inc.), Alexa Fluor-conjugated 488 donkey
anti-goat IgG (H+L; cat. no. A11055; Invitrogen; Thermo Fisher
Scientific, Inc.) or Alexa Fluor-conjugated 647 chicken anti-rat
IgG (H+L; cat. no. A21472; Invitrogen; Thermo Fisher Scientific,
Inc.) for 1 h at room temperature, washed with PBS, and mounted
sequentially on glass slides using Vectashield (Vector
Laboratories). Mounted sections were evaluated for fluorescence
under settings for 488 and 647 emissions on a confocal microscope
(FV-1000 spectral; Olympus, Melville, NY, USA). Quantitative
analysis was performed.
Quantitative analysis
Sections including the hippocampus from 8 rats per
group were subjected to analysis. Five regions of interest (ROI) of
0.25 mm2/section in the subgranular zone (SGZ) of the
hippocampus (bregma −3.30 to −4.30 mm; 5 sections per rat, every
5th section) were selected. The number of BrdU+,
DCX+, NeuN+, BrdU+ and
DCX+, or BrdU+ and NeuN+ cells was
counted in each ROI, and averaged. Data are presented as the
percentage of total cells. All quantitative analyses were conducted
in a blinded manner.
Statistical analysis
Parameters relating to spatial memory, including the
search error, time latency, and swimming speed, were analyzed by
two-way repeated measures analysis of variance (ANOVA), followed by
a post hoc least significant differences multiple-comparison test.
One-way ANOVA was used to compare the results of the probe trials
and the quantitative data of the western blot analysis and
immunohistochemistry (mean ± standard error of the mean).
Statistical analysis was performed using one-way ANOVA followed by
the Newman-Keuls multiple comparison test. P<0.05 was considered
to indicate a statistically significant difference. Data analyses
were performed using the IBM SPSS statistics 22 (IBM Corporation,
Armonk, NY, USA).
Results
Treadmill exercise reduces the memory
impairment induced by CCH
To evaluate the effect of exercise on the recovery
of cognitive function after 2VO, spatial memory was evaluated by
assessing learning and memory retention using the MWM test. In the
acquisition trials, the rats in the SC group gradually learned the
location of the hidden platform, which was demonstrated by the
shorter latencies and fewer search errors recorded throughout the
test period. CCH induced by 2VO resulted in a significant
impairment in spatial learning [time latency, F(3,44)=13.78; and search errors, F(3,44)=11.63] compared with that observed
for the SC group (repeated-measures two-way ANOVA, P<0.01;
Fig. 2A and B). Exercise-treated
2VO animals exhibited shorter mean session latencies and fewer
search errors in locating the platform compared with the 2VO group
(repeated measures two-way ANOVA, P<0.05; Fig. 2A and B). To determine whether the
group differences observed in escape latencies were caused by
differences in swimming, particularly between the SC group and the
2VO group, swimming speeds were calculated for each group. No
differences in swimming speed were observed among the four groups
[repeated measures two-way ANOVA, F(3,44)=0.329; Fig. 2C]. In addition, differences in
performance were observed during the probe trial, as assessed by
the percentage of time spent in the target quadrant during the 30
sec probe trial (Fig. 2D). One-way
ANOVA of the time spent in the target quadrant observed on the
second probe trial showed that the between-group effects were
significant. Post hoc analysis revealed that 2VO rats that were
subjected to treadmill exercise exhibited significant improvements
during the probe trials compared with 2VO rats (percentage of time
spent in the target quadrant, P<0.05, Fig. 2D).
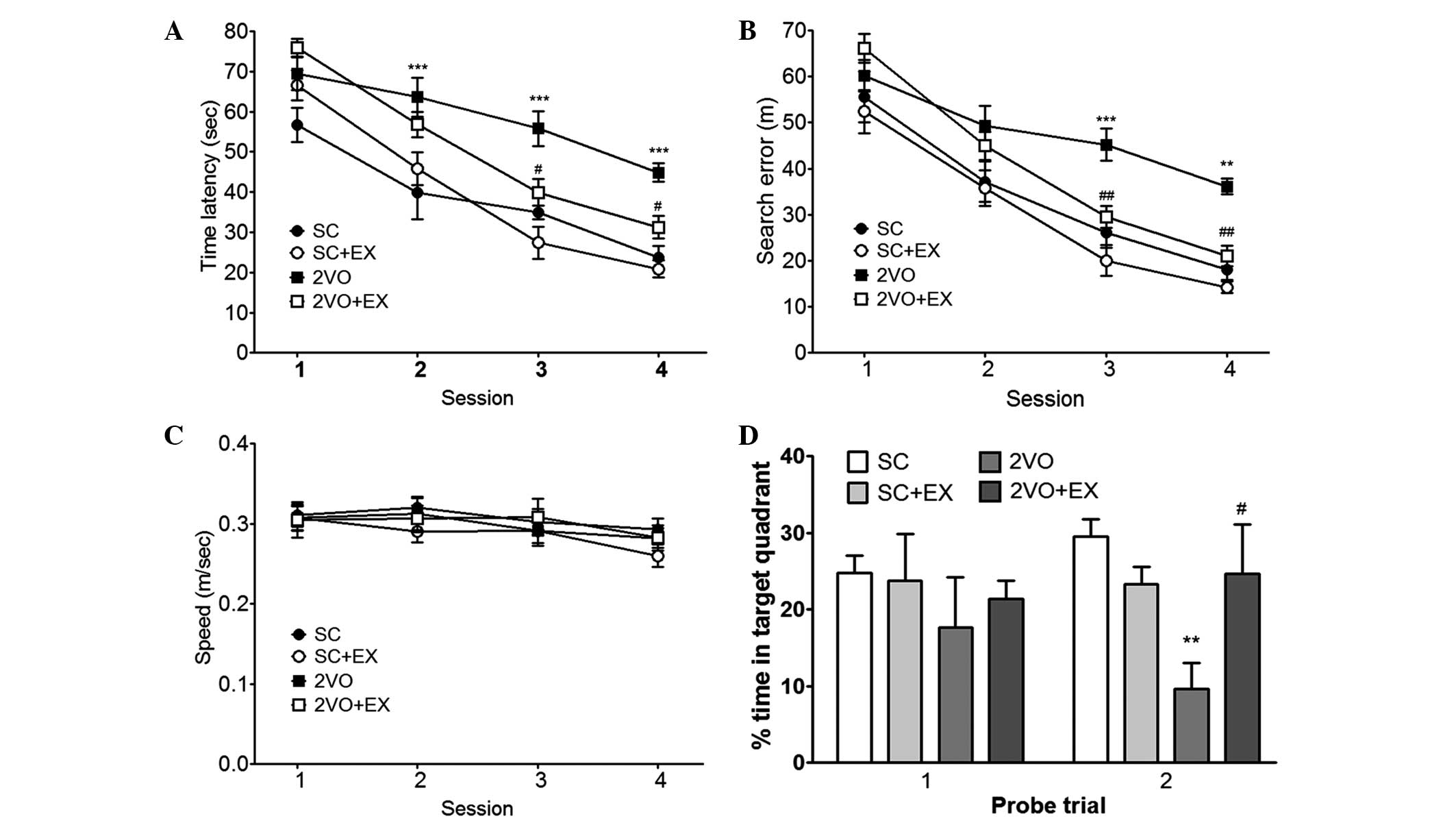 | Figure 2Effect of treadmill exercise on
performance deficits induced by chronic cerebral hypoperfusion in
rats in the Morris water maze test. Spatial memory evaluation using
(A) time latency, (B) search error, (C) swimming speed, and (D)
percentage of time spent in target quadrant. n=12–13/group.
**P<0.01, ***P<0.001 vs. SC;
#P<0.05, ##P<0.01 vs. 2VO. SC,
sham-surgery group; SC+EX, sham-surgery group subjected to
treadmill exercise (15 m/min, 30 min/day for 4 weeks); 2VO, 2VO
surgery group; 2VO+EX, 2VO-surgery group subjected to treadmill
exercise (15 m/min, 30 min/day for 4 weeks). |
Treadmill exercise increases hippocampal
neurogenesis
Rats received an injection of the cell proliferation
marker BrdU (50 mg/kg, IP injection, once a day) 3–5 weeks after
surgery. At 7 weeks after surgery, the majority of BrdU+
cells were localized in the SGZ of the hippocampus (Fig. 3). The number of BrdU+
cells was significantly increased in the SGZ of rats in the SC+EX
and 2VO+EX groups (Fig. 3B).
 | Figure 3Effect of treadmill exercise on the
cell proliferation in the SGZ. (A) Representative photomicrograph
of BrdU immunostaining showing proliferating cells in the SGZ area
7 weeks after SC or 2VO surgery. Scale bars, 100 µm. (B)
BrdU-stained cells were counted in the brain. Results are presented
as the mean ± standard error of the mean, n=8.
***P<0.001 vs. SC; #P<0.05 vs. 2VO. SC,
sham-surgery group; SC+EX, sham-surgery group subjected to
treadmill exercise (15 m/min, 30 min/day for 4 weeks); 2VO, 2VO
surgery group; 2VO+EX, 2VO-surgery group subjected to treadmill
exercise (15 m/min, 30 min/day for 4 weeks); SGZ, subgranular zone;
BrdU, 5-bromo-2-deoxyuridine. |
In order to examine the effect of exercise on
2VO-induced cell proliferation, BrdU and DCX double-labeling
methods were used to detect proliferating cells in the hippocampal
SGZ of rats in the 2VO-EX and SC-EX groups. The number of
BrdU+ cells, DCX+ cells, and BrdU+
and DCX+ cells in the SGZ of the rats in the SC+EX and
2VO+EX groups was increased compared with that observed in the SC
and 2VO groups, respectively (P<0.001 vs. SC; P<0.05 vs. 2VO;
Fig. 4). These results suggest
that exercise increases the proliferation of immature neurons in
the SGZ of the chronically hypoperfused rats. Next, in order to
examine the effect of exercise on the differentiation of progenitor
cells in chronically hypoperfused rats, BrdU- and NeuN-labeling
methods were used to detect differentiated cells in the SGZ of the
SC+EX and 2VO+EX rats. The number of BrdU+ and
BrdU+ and NeuN+ double-labeled cells in the
SGZ of the SC+EX and 2VO+EX rats were increased compared with that
observed for SC and 2VO rats, respectively (P<0.05 vs. SC;
P<0.05 vs. 2VO; Fig. 5). These
results indicate that exercise promotes the differentiation of
progenitor cells into mature neurons in the SGZ of 2VO rats.
 | Figure 4Effect of treadmill exercise on the
proliferation of neural progenitor cells in the SGZ after CCH. (A)
Representative photomicrograph of DCX or BrdU immunostaining
showing proliferating cells in the SGZ area 7 weeks after 2VO
surgery. DCX and BrdU coexpression in the SGZ is depicted as yellow
staining after merging green (DCX) and red (BrdU) images. Arrows
indicate cells showing double-staining for DCX and BrdU. Scale
bars, 50 µm. (B) BrdU+, DCX+, and
DCX+ and BrdU+ cells were counted in the
hippocampal brain sections. Results are presented as the mean ±
standard error of the mean, n=8. *P<0.05,
***P<0.001 vs. SC; #P<0.05,
##P<0.01, ###P<0.001 vs. 2VO. SC,
sham-surgery group; SC+EX, sham-surgery group subjected to
treadmill exercise (15 m/min, 30 min/day for 4 weeks); 2VO, 2VO
surgery group; 2VO+EX, 2VO-surgery group subjected to treadmill
exercise (15 m/min, 30 min/day for 4 weeks); SGZ, subgranular zone;
CCH, chronic cerebral hypoperfusion; BrdU, 5-bromo-2-deoxyuridine;
DCX, doublecortin. |
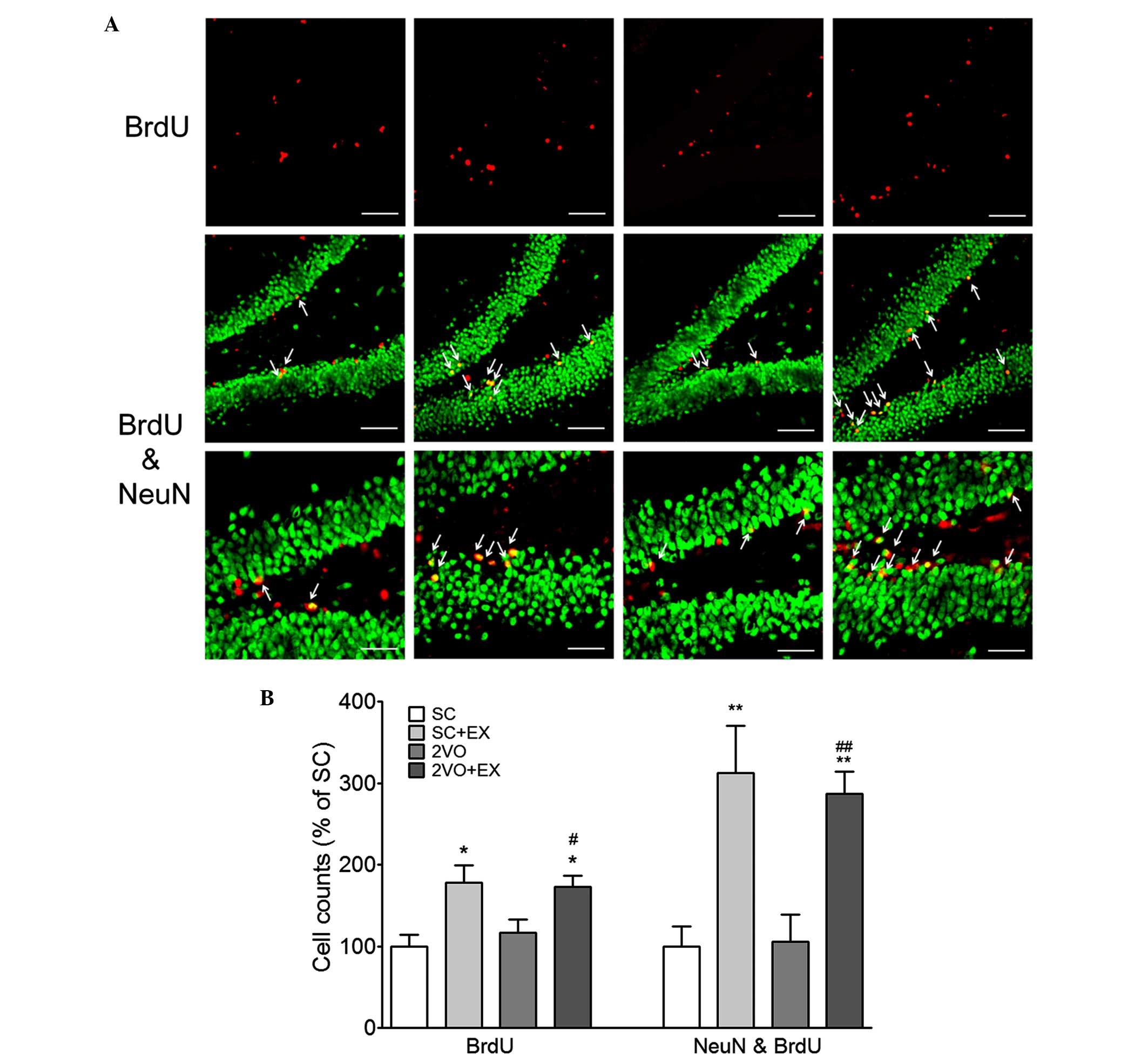 | Figure 5Treadmill exercise increased the
differentiation of progenitor cell in the SGZ after 2VO surgery.
(A) Representative photomicrograph of BrdU (red) and NeuN (green)
double immunostaining showing matured newborn neurons in the SGZ at
4 weeks after 2VO surgery. NeuN and BrdU coexpression in the SGZ is
depicted as yellow staining after merging green (DCX) and red
(BrdU) images. Arrows indicate cells showing double-staining for
NeuN and BrdU. Scale bars, 80 µm (upper and middle panel) or
40 µm (lower panel). (B) DCX+ and
BrdU+ cells were counted in the brain. Results are
presented as the mean ± standard error of the mean, n=8.
*P<0.05, **P<0.01 vs. SC;
#P<0.05, ##P<0.01 vs. 2VO. SC,
sham-surgery group; SC+EX, sham-surgery group subjected to
treadmill exercise (15 m/min, 30 min/day for 4 weeks); 2VO, 2VO
surgery group; 2VO+EX, 2VO-surgery group subjected to treadmill
exercise (15 m/min, 30 min/day for 4 weeks); SGZ, subgranular zone;
BrdU, 5-bromo-2-deoxyuridine. |
Treadmill exercise increases the
expression of BDNF protein in the hippocampus
To determine the effect of exercise on the levels of
expression of mature BDNF in the hippocampus, mature BDNF
expression in the hippocampus was detected by western blot analysis
(Fig. 6A). Mature BDNF expression
in the hippocampus of the SC+EX and 2VO+EX rats was increased
compared with that observed for SC rats (all P<0.01; Fig. 6B). Furthermore, the level of mature
BDNF expression in 2VO rats was enhanced by exercise (P<0.01 vs.
2VO, Fig. 6B).
 | Figure 6Treadmill exercise promotes mature
BDNF expression. (A) Representative photomicrographs of western
blot analysis showing BDNF levels in the hippocampus of rats in the
SC, SC+EX, 2VO and 2VO+EX groups. (B) The intensity of each band
was densitometrically determined and normalized against β-actin.
Results are presented as the mean ± standard error of the mean,
n=4. **P<0.01, ***P<0.001 vs. SC;
#P<0.05 vs. 2VO. SC, sham-surgery group; SC+EX,
sham-surgery group subjected to treadmill exercise (15 m/min, 30
min/day for 4 weeks); 2VO, 2VO surgery group; 2VO+EX, 2VO-surgery
group subjected to treadmill exercise (15 m/min, 30 min/day for 4
weeks); BDNF, brain-derived neurotrophic factor. |
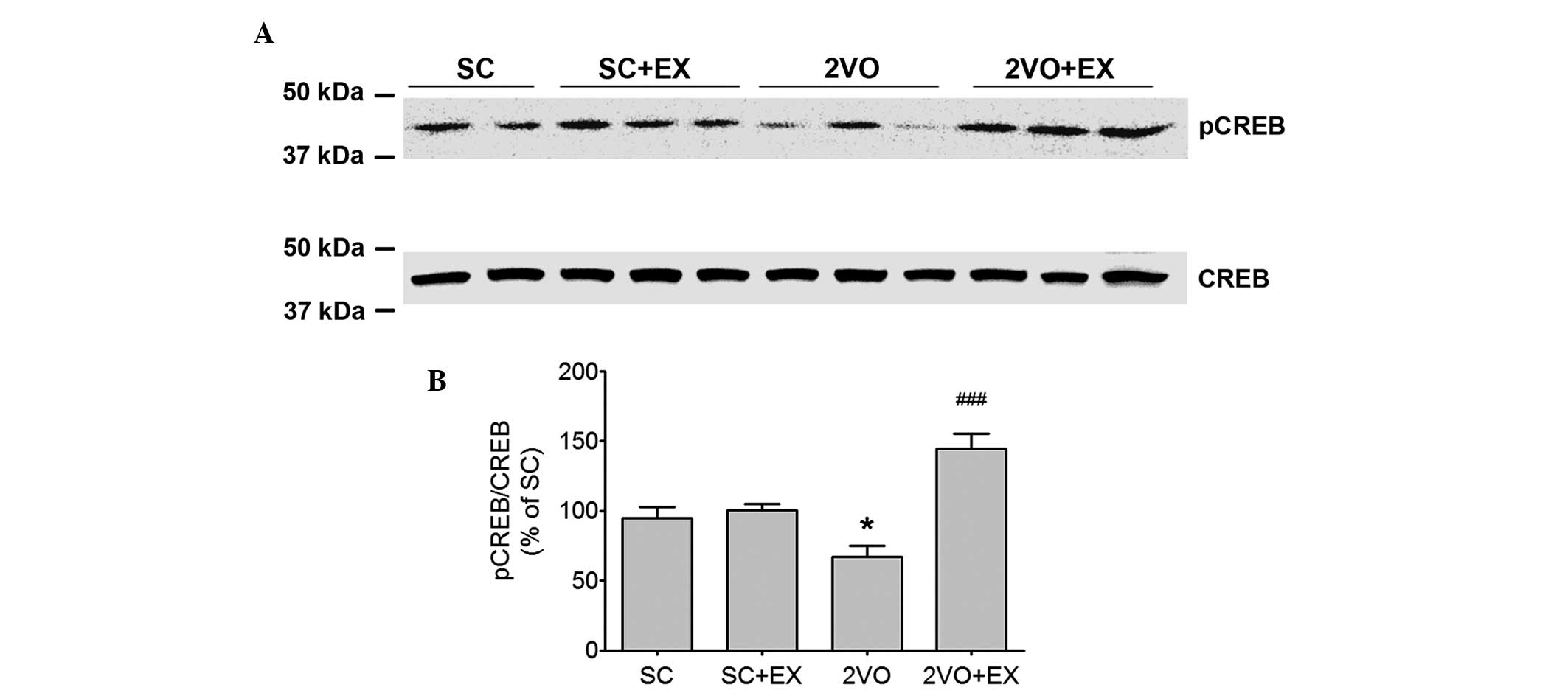
Treadmill exercise increases the
expression of the hippocampal phosphorylated CREB protein
Western blot analysis was used to determine the
effect of exercise on the phosphory-lation of CREB in the
hippocampus (Fig. 7).
Phosphorylated CREB expression was decreased in the hippocampus of
2VO rats (P<0.01, Fig. 7).
Moreover, the decrease in the level of phosphorylated CREB in 2VO
rats was reversed by exercise (P<0.01 vs. 2VO, Fig. 7).
Discussion
To the best of our knowledge, this is the first
study to demonstrate that the performance of forced treadmill
exercise for 4 weeks after CCH increased cognitive function and
hippocampal neurogenesis via the BDNF-pCREB pathway. Enhanced
BDNF-pCREB signaling induced by exercise was beneficial, not only
because it increased the number of new hippocampal neurons, but
also because it reduced functional impairment in the 2VO group.
Notably, treadmill exercise significantly increased cognitive
function and hippocampal neurogenesis in 2VO rats. In addition,
decreased pCREB signaling in the 2VO group was reversed by
treadmill exercise.
CCH is strongly correlated with the severity of
memory dysfunction and the decline of cerebral blood flow in
Alzheimer's disease, VaD and post-stroke hypoperfusion (35). It was previously demonstrated that
CCH induces, oxidative stress in the hippocampus, hippocampal
neurodegeneration, neuroinflammation and a reduction in cerebral
blood flow, which results in memory impairment in the 2VO animal
model (11,30,34).
Exercise enhances cognitive function in a number of
models of brain disease, such as delaying the cognitive decline
associated with aging, preventing cognitive deficits in a variety
of Alzheimer's disease models (36–38),
modifying cognitive function in Parkinson's disease (39,40),
modulating cognitive deficits in Huntington's disease models
(41–43), affording neuroprotective effects in
models of chronic stress (38,44,45),
and improving functional recovery from traumatic brain injury
(38,45,46).
A previous study has shown that forced treadmill exercise prevents
oxidative stress and memory deficits after chronic cerebral
hypoperfusion in the rat (14).
Using exercise training as an upregulator of hippocampal
neurogenesis, an in vivo imaging study performed in humans
indicated a positive association between hippocampal-dependent
cognitive performance and a change in cerebral blood volume
(47). Furthermore, exercise
intervention has been shown to improve performance in a
neurogenesis-dependent cognitive test, the visual pattern
separation task, in human subjects (48). Despite the technical limitations
associated with the direct measurement of neurogenesis in the human
brain, these two studies suggest that new adult-born neurons in the
hippocampus have a functional role in learning and memory in the
human brain (48,49).
Animal studies have suggested that enhanced
hippocampal neurogenesis underlies the reported beneficial effects
of exercise on cognitive function. Studies performed by van Praag
et al showed that physical activity not only increased
hippocampal neurogenesis (16,50),
but also improved MWM performance and selectively increased
long-term potentiation in the dentate gyrus of 3-month-old mice
(50). In addition to upregulating
the neurogenic process, physical activity can also increase the
capacity of neurons in the hippocampus to sustain synaptic
plasticity and facilitate hippocampal-dependent learning in the
same animals (23,49).
In the present study, the significant increase
observed in the total population of neural progenitor cells and
mature neurons identified as BrdU+ and DCX+
or NeuN+ cells in animals that underwent treadmill
exercise suggests increased hippocampal neurogenic activity in rats
from the 2VO group. To the best of our knowledge, this is a novel
finding.
A number of studies have attributed exercise-induced
changes in central nervous system neurogenesis and cognitive
function to BDNF (50,51). However, in addition to being
crucial for controlling several brain functions, BDNF is
particularly important for regulating hippocampal plasticity
(52), which contributes to
learning and memory (40,53,54).
Increased levels of this neurotrophin minimize ischemic neuronal
death (55–60) and enhance hippocampal synaptic
transmission (61–63).
Physical exercise increases the expression of
hippocampal BDNF (64). Several
studies have demonstrated that alterations in BDNF expression
result from the activation/phosphorylation of the transcription
factor, cAMP-response-element binding protein (CREB), following
exercise (65–67). For example, in rats, exercise
activates hippocampal CREB and the mitogen-activated protein kinase
(MAPK) pathway (67). The MAPK
cascade facilitates the phosphorylation of CREB (65,68).
CREB is involved in long-term plasticity (69) and memory (70), and its phosphorylation is involved
in the activation of its target genes, including BDNF (65,71).
In the present study, the expression of BDNF and the
phosphorylation of CREB at the protein level was examined after
exercise in a rat model of VaD. Western blotting results showed
that CCH caused a decrease in the expression of pCREB. Treadmill
exercise increased the expression of hippocampal mature BDNF and
the phosphorylation of the CREB protein. Based on the combined
findings of this study, it was hypothesized that physical exercise
increases post-ischemic neurogenesis via the pCREB-dependent
upregulation of BDNF in 2VO-induced VaD. This evidence implies that
physical exercise provides protection against cognitive impairment
in VaD. Therefore, it was concluded that physical activity may
prevent the development of VaD and could have important clinical
implications for therapeutic intervention of VaD.
Acknowledgments
This study was supported by the Konkuk University
Medical Center Research Grant 2012 (grant no. 201210).
References
|
1
|
Román GC: Vascular dementia:
Distinguishing characteristics, treatment and prevention. J Am
Geriatr Soc. 51(Suppl Dementia 5): S296–S304. 2003. View Article : Google Scholar
|
|
2
|
Aggarwal NT and Decarli C: Vascular
dementia: Emerging trends. Semin Neurol. 27:66–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jellinger KA: Morphologic diagnosis of
'vascular dementia'-a critical update. J Neurol Sci. 270:1–12.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Erkinjuntti T, Román G, Gauthier S,
Feldman H and Rockwood K: Emerging therapies for vascular dementia
and vascular cognitive impairment. Stroke. 35:1010–1017. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de la Torre JC: Vascular risk factor
detection and control may prevent Alzheimer's disease. Ageing Res
Rev. 9:218–225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin MS, Chiu MJ, Wu YW, Huang CC, Chao CC,
Chen YH, Lin HJ, Li HY, Chen YF, Lin LC, et al: Neurocognitive
improvement after carotid artery stenting in patients with chronic
internal carotid artery occlusion and cerebral ischemia. Stroke.
42:2850–2854. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naritomi H: Experimental basis of
multi-infarct dementia: Memory impairments in rodent models of
ischemia. Alzheimer Dis Assoc Disord. 5:103–111. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ni J, Ohta H, Matsumoto K and Watanabe H:
Progressive cognitive impairment following chronic cerebral
hypoperfusion induced by permanent occlusion of bilateral carotid
arteries in rats. Brain Res. 653:231–236. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shibata M, Yamasaki N, Miyakawa T, Kalaria
RN, Fujita Y, Ohtani R, Ihara M, Takahashi R and Tomimoto H:
Selective impairment of working memory in a mouse model of chronic
cerebral hypoperfusion. Stroke. 38:2826–2832. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiwa NS, Garrard P and Hainsworth AH:
Experimental models of vascular dementia and vascular cognitive
impairment: A systematic review. J Neurochem. 115:814–828. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi BR, Lee SR, Han JS, Woo SK, Kim KM,
Choi DH, Kwon KJ, Han SH, Shin CY, Lee J, et al: Synergistic memory
impairment through the interaction of chronic cerebral
hypoperfusion and amlyloid toxicity in a rat model. Stroke.
42:2595–2604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ji HJ, Hu JF, Wang YH, Chen XY, Zhou R and
Chen NH: Osthole improves chronic cerebral hypoperfusion induced
cognitive deficits and neuronal damage in hippocampus. Eur J
Pharmacol. 636:96–101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Radak Z, Toldy A, Szabo Z, Siamilis S,
Nyakas C, Silye G, Jakus J and Goto S: The effects of training and
detraining on memory, neurotrophins and oxidative stress markers in
rat brain. Neurochem Int. 49:387–392. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cechetti F, Worm PV, Elsner VR, Bertoldi
K, Sanches E, Ben J, Siqueira IR and Netto CA: Forced treadmill
exercise prevents oxidative stress and memory deficits following
chronic cerebral hypoperfusion in the rat. Neurobiol Learn Mem.
97:90–96. 2012. View Article : Google Scholar
|
|
15
|
Ogonovszky H, Berkes I, Kumagai S, Kaneko
T, Tahara S, Goto S and Radák Z: The effects of moderate-,
strenuous- and over-training on oxidative stress markers, DNA
repair and memory, in rat brain. Neurochem Int. 46:635–640. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van Praag H, Christie BR, Sejnowski TJ and
Gage FH: Running enhances neurogenesis, learning and long-term
potentiation in mice. Proc Natl Acad Sci USA. 96:13427–13431. 1999.
View Article : Google Scholar
|
|
17
|
Mattson MP: Neuroprotective signaling and
the aging brain: Take away my food and let me run. Brain Res.
886:47–53. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao C, Deng W and Gage FH: Mechanisms and
functional implications of adult neurogenesis. Cell. 132:645–660.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lucassen PJ, Meerlo P, Naylor AS, van Dam
AM, Dayer AG, Fuchs E, Oomen CA and Czéh B: Regulation of adult
neurogenesis by stress, sleep disruption, exercise and
inflammation: Implications for depression and antidepressant
action. Eur Neuropsychopharmacol. 20:1–17. 2010. View Article : Google Scholar
|
|
20
|
Briones TL, Suh E, Hattar H and Wadowska
M: Dentate gyrus neurogenesis after cerebral ischemia and
behavioral training. Biol Res Nurs. 6:167–179. 2005. View Article : Google Scholar
|
|
21
|
Arida RM, Scorza FA, Gomes da Silva S,
Cysneiros RM and Cavalheiro EA: Exercise paradigms to study brain
injury recovery in rodents. Am J Phys Med Rehabil. 90:452–465.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ji JF, Ji SJ, Sun R, Li K, Zhang Y, Zhang
LY and Tian Y: Forced running exercise attenuates hippocampal
neurogenesis impairment and the neurocognitive deficits induced by
whole-brain irradiation via the BDNF-mediated pathway. Biochem
Biophys Res Commun. 443:646–651. 2014. View Article : Google Scholar
|
|
23
|
Vivar C, Potter MC, Choi J, Lee JY,
Stringer TP, Callaway EM, Gage FH, Suh H and van Praag H:
Monosynaptic inputs to new neurons in the dentate gyrus. Nat
Commun. 3:11072012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kobilo T, Yuan C and van Praag H:
Endurance factors improve hippocampal neurogenesis and spatial
memory in mice. Learn Mem. 18:103–107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Verdelho A, Madureira S, Ferro JM, Baezner
H, Blahak C, Poggesi A, Hennerici M, Pantoni L, Fazekas F,
Scheltens P, et al: Physical activity prevents progression for
cognitive impairment and vascular dementia: Results from the LADIS
(Leukoaraiosis and Disability) study. Stroke. 43:3331–3335. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Coelho FM, Pereira DS, Lustosa LP, Silva
JP, Dias JM, Dias RC, Queiroz BZ, Teixeira AL, Teixeira MM and
Pereira LS: Physical therapy intervention (PTI) increases plasma
brain-derived neurotrophic factor (BDNF) levels in non-frail and
pre-frail elderly women. Arch Gerontol Geriatr. 54:415–420. 2012.
View Article : Google Scholar
|
|
27
|
Scarmeas N, Luchsinger JA, Schupf N,
Brickman AM, Cosentino S, Tang MX and Stern Y: Physical activity,
diet and risk of Alzheimer disease. JAMA. 302:627–637. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cotman CW, Berchtold NC and Christie LA:
Exercise builds brain health: Key roles of growth factor cascades
and inflammation. Trends Neurosci. 30:464–472. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cunha C, Brambilla R and Thomas KL: A
simple role for BDNF in learning and memory? Front Mol Neurosci.
3:12010.PubMed/NCBI
|
|
30
|
Kwon KJ, Kim MK, Lee EJ, Kim JN, Choi BR,
Kim SY, Cho KS, Han JS, Kim HY, Shin CY and Han SH: Effects of
donepezil, an acetylcholinesterase inhibitor, on neurogenesis in a
rat model of vascular dementia. J Neurol Sci. 347:66–77. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee SH, Kim YH, Kim YJ and Yoon BW:
Enforced physical training promotes neurogenesis in the subgranular
zone after focal cerebral ischemia. J Neurol Sci. 269:54–61. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sasaki H, Inoue T, Iso H and Fukuda Y:
Recovery of visual behaviors in adult hamsters with the peripheral
nerve graft to the sectioned optic nerve. Exp Neurol. 159:377–390.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alaei H, Borjeian L, Azizi M, Orian S,
Pourshanazari A and Hanninen O: Treadmill running reverses
retention deficit induced by morphine. Eur J Pharmacol.
536:138–141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi DH, Lee KH, Kim JH, Seo JH, Kim HY,
Shin CY, Han JS, Han SH, Kim YS and Lee J: NADPH oxidase 1, a novel
molecular source of ROS in hippocampal neuronal death in vascular
dementia. Antioxid Redox Signal. 21:533–550. 2014. View Article : Google Scholar :
|
|
35
|
Ohta H, Nishikawa H, Kimura H, Anayama H
and Miyamoto M: Chronic cerebral hypoperfusion by permanent
internal carotid ligation produces learning impairment without
brain damage in rats. Neuroscience. 79:1039–1050. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Adlard PA, Perreau VM, Pop V and Cotman
CW: Voluntary exercise decreases amyloid load in a transgenic model
of Alzheimer's disease. J Neurosci. 25:4217–4221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dao AT, Zagaar MA, Levine AT, Salim S,
Eriksen JL and Alkadhi KA: Treadmill exercise prevents learning and
memory impairment in Alzheimer's disease-like pathology. Curr
Alzheimer Res. 10:507–515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pang TY and Hannan AJ: Enhancement of
cognitive function in models of brain disease through environmental
enrichment and physical activity. Neuropharmacology. 64:515–528.
2013. View Article : Google Scholar
|
|
39
|
Ahlskog JE: Does vigorous exercise have a
neuroprotective effect in Parkinson disease? Neurology. 77:288–294.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Alonso M, Vianna MR, Depino AM, Mello e
Souza T, Pereira P, Szapiro G, Viola H, Pitossi F, Izquierdo I and
Medina JH: BDNF-triggered events in the rat hippocampus are
required for both short- and long-term memory formation.
Hippocampus. 12:551–560. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pang TY, Stam NC, Nithianantharajah J,
Howard ML and Hannan AJ: Differential effects of voluntary physical
exercise on behavioral and brain-derived neurotrophic factor
expression deficits in Huntington's disease transgenic mice.
Neuroscience. 141:569–584. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Van Raamsdonk JM, Pearson J, Slow EJ,
Hossain SM, Leavitt BR and Hayden MR: Cognitive dysfunction
precedes neuropathology and motor abnormalities in the YAC128 mouse
model of Huntington's disease. J Neuroscience. 25:4169–4180. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Harrison DJ, Busse M, Openshaw R, Rosser
AE, Dunnett SB and Brooks SP: Exercise attenuates neuropathology
and has greater benefit on cognitive than motor deficits in the
R6/1 Huntington's disease mouse model. Exp Neurol. 248:457–469.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nagata K, Nakashima-Kamimura N, Mikami T,
Ohsawa I and Ohta S: Consumption of molecular hydrogen prevents the
stress-induced impairments in hippocampus-dependent learning tasks
during chronic physical restraint in mice. Neuropsychopharmacology.
34:501–508. 2009. View Article : Google Scholar
|
|
45
|
Kim BS, Kim MY and Leem YH: Hippocampal
neuronal death induced by kainic acid and restraint stress is
suppressed by exercise. Neuroscience. 194:291–301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Griesbach GS, Hovda DA and Gomez-Pinilla
F: Exercise-induced improvement in cognitive performance after
traumatic brain injury in rats is dependent on BDNF activation.
Brain Res. 1288:105–115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pereira AC, Huddleston DE, Brickman AM,
Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR and Small
SA: An in vivo correlate of exercise-induced neurogenesis in the
adult dentate gyrus. Proc Natl Acad Sci USA. 104:5638–5643. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dery N, Pilgrim M, Gibala M, Gillen J,
Wojtowicz JM, Macqueen G and Becker S: Adult hippocampal
neurogenesis reduces memory interference in humans: Opposing
effects of aerobic exercise and depression. Front Neurosci.
7:662013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yau SY, Gil-Mohapel J, Christie BR and So
KF: Physical exercise-induced adult neurogenesis: A good strategy
to prevent cognitive decline in neurodegenerative diseases? Biomed
Res Int. 2014:4031202014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
van Praag H, Shubert T, Zhao C and Gage
FH: Exercise enhances learning and hippocampal neurogenesis in aged
mice. J Neurosci. 25:8680–8685. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Alomari MA, Khabour OF, Alzoubi KH and
Alzubi MA: Forced and voluntary exercises equally improve spatial
learning and memory and hippocampal BDNF levels. Behav Brain Res.
247:34–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Naylor AS, Persson AI, Eriksson PS,
Jonsdottir IH and Thorlin T: Extended voluntary running inhibits
exercise-induced adult hippocampal progenitor proliferation in the
spontaneously hypertensive rat. J Neurophysiol. 93:2406–2414. 2005.
View Article : Google Scholar
|
|
53
|
Alonso M, Vianna MR, Izquierdo I and
Medina JH: Signaling mechanisms mediating BDNF modulation of memory
formation in vivo in the hippocampus. Cell Mol Neurobiol.
22:663–674. 2002. View Article : Google Scholar
|
|
54
|
Mizuno M, Yamada K, Olariu A, Nawa H and
Nabeshima T: Involvement of brain-derived neurotrophic factor in
spatial memory formation and maintenance in a radial arm maze test
in rats. J Neurosci. 20:7116–7121. 2000.PubMed/NCBI
|
|
55
|
Ferrer I, Ballabriga J, Marti E, Pérez E,
Alberch J and Arenas E: BDNF up-regulates TrkB protein and prevents
the death of CA1 neurons following transient forebrain ischemia.
Brain Pathol. 8:253–261. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ferrer I, Krupinski J, Goutan E, Martí E,
Ambrosio S and Arenas E: Brain-derived neurotrophic factor reduces
cortical cell death by ischemia after middle cerebral artery
occlusion in the rat. Acta Neuropathol. 101:229–238.
2001.PubMed/NCBI
|
|
57
|
Kiprianova I, Freiman TM, Desiderato S,
Schwab S, Galmbacher R, Gillardon F and Spranger M: Brain-derived
neurotrophic factor prevents neuronal death and glial activation
after global ischemia in the rat. J Neurosci Res. 56:21–27. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Schäbitz WR, Schwab S, Spranger M and
Hacke W: Intraventricular brain-derived neurotrophic factor reduces
infarct size after focal cerebral ischemia in rats. J Cereb Blood
Flow Metab. 17:500–506. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yamashita K, Wiessner C, Lindholm D,
Thoenen H and Hossmann KA: Post-occlusion treatment with BDNF
reduces infarct size in a model of permanent occlusion of the
middle cerebral artery in rat. Metab Brain Dis. 12:271–280. 1997.
View Article : Google Scholar
|
|
60
|
Zhang Y and Pardridge WM: Neuroprotection
in transient focal brain ischemia after delayed intravenous
administration of brain-derived neurotrophic factor conjugated to a
blood-brain barrier drug targeting system. Stroke. 32:1378–1384.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Vaynman S, Ying Z and Gomez-Pinilla F:
Hippocampal BDNF mediates the efficacy of exercise on synaptic
plasticity and cognition. Eur J Neurosci. 20:2580–2590. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Vaynman S, Ying Z and Gomez-Pinilla F:
Interplay between brain-derived neurotrophic factor and signal
transduction modulators in the regulation of the effects of
exercise on synaptic-plasticity. Neuroscience. 122:647–657. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Vaynman S, Ying Z and Gómez-Pinilla F:
Exercise induces BDNF and synapsin I to specific hippocampal
subfields. J Neurosci Res. 76:356–362. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Molteni R, Ying Z and Gómez-Pinilla F:
Differential effects of acute and chronic exercise on
plasticity-related genes in the rat hippocampus revealed by
microarray. Eur J Neurosci. 16:1107–1116. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Griesbach GS, Hovda DA, Molteni R, Wu A
and Gomez-Pinilla F: Voluntary exercise following traumatic brain
injury: Brain-derived neurotrophic factor upregulation and recovery
of function. Neuroscience. 125:129–139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Vaynman S, Ying Z and Gomez-Pinilla F: The
select action of hippocampal calcium calmodulin protein kinase II
in mediating exercise-enhanced cognitive function. Neuroscience.
144:825–833. 2007. View Article : Google Scholar
|
|
67
|
Shen H, Tong L, Balazs R and Cotman CW:
Physical activity elicits sustained activation of the cyclic AMP
response element-binding protein and mitogen-activated protein
kinase in the rat hippocampus. Neuroscience. 107:219–229. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Finkbeiner S, Tavazoie SF, Maloratsky A,
Jacobs KM, Harris KM and Greenberg ME: CREB: A major mediator of
neuronal neurotrophin responses. Neuron. 19:1031–1047. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Abel T and Kandel E: Positive and negative
regulatory mechanisms that mediate long-term memory storage. Brain
Res Brain Res Rev. 26:360–378. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Silva AJ, Kogan JH, Frankland PW and Kida
S: CREB and memory. Annu Rev Neurosci. 21:127–148. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Finkbeiner S: Calcium regulation of the
brain-derived neurotrophic factor gene. Cell Mol Life Sci.
57:394–401. 2000. View Article : Google Scholar : PubMed/NCBI
|