Introduction
Leukemia is the most common malignancy and leading
cause of cancer-associated mortality in patients below the age of
20 years (1). Approximately 6,000
new cases of the sub-type acute lymphoblastic leukemia (ALL) are
diagnosed in the US each year (1)
and the curative rate is approaching ≥80% (2). In spite of this high cure rate,
resistant forms of the disease and relapse remain a leading cause
of cancer-associated deaths in children. Furthermore, the cure rate
of ALL in adults is ~50%, which is unsatisfactory (3). Thus, novel treatment strategies,
including the development of novel anti-leukemic drugs and the
optimization of existing chemotherapeutic treatments, are required
to improve current treatments (4).
Due to their low toxicity, naturally occurring
phytochemicals have drawn extensive attention as potential drugs
for cancer prevention (5). A large
number of natural products have previously been discovered for use
as chemotherapeutic agents and natural products offer a pool for
the discovery of novel anti-cancer agents, which is sourced by
current research. Polydatin (PD) is a stilbenoid compound isolated
from the root of Polygonum cuspidatum (6). PD has been shown to have multiple
beneficial therapeutic properties, including cardioprotective
(7–9), hepatoprotective (10), neuroprotective (11) and anti-inflammatory (12) effects. PD has also been reported to
have anti-cancer effects with activity against colon (13) and lung (14) cancer as well as nasopharyngeal
carcinoma (15). However, to the
best of our knowledge, the effects of PD on human leukemic cells
have yet not been studied. In the present study, the effects of PD
on the proliferation, cell cycle distribution and apoptosis of the
MOLT-4 human ALL cell line were investigated. As the JAK2-STAT3
signaling pathway is known to promote cell growth (16) and confer resistance to apoptosis in
leukemic cells (17), it was also
examined whether the JAK2-STAT3 pathway is involved in PD-induced
apoptosis and cell cycle arrest.
Materials and methods
Chemicals
PD was supplied by LKT Laboratories, Inc. (St Paul,
MN, USA; cat. no. P5845) and a 10-mM stock solution in
dimethysulfoxide (DMSO) was prepared, which was diluted with medium
to the appropriate concentrations for use in the assays. A Cell
Counting Kit-8 (CCK-8) was purchased from Signalway Antibody LLC
(College Park, MD, USA) and an Annexin V-conjugated Alexa Fluor 488
apoptosis detection kit was obtained from 7SeaPharmTech (Shanghai,
China). Primary antibodies against B-cell lymphoma 2 (Bcl-2) and
Bcl-2-associated X (Bax) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA), antibodies against cyclin
B1, phosphorylated signal transducer and activator of transcription
(p-STAT3) and STAT3 were from Abcam (Cambridge, UK), and antibodies
against cyclin D1, p-Janus kinase (JAK)2, JAK2 and GAPDH were from
Cell Signaling Technology Inc. (Danvers, MA, USA). Secondary
antibodies were purchased from Beyotime Institute of Biotechnology
(Haimen, China). The Pierce bicinchoninic acid (BCA) Protein Assay
kit was supplied by Thermo Fisher Scientific (Waltham, MA, USA) and
the Immobilon™ Western Chemiluminescent Horseradish Peroxidase
substrate (cat. no. WBKLS0100) was obtained from EMD Millipore
(Billerica, MA, USA). AG490 was obtained from Beyotime Institute of
Biotechnology and dissolved with DMSO to make a stock at 10 mM.
Cells were treated with 10 mM AG490 as indicated.
Cell culture
The MOLT-4 cell line was obtained from the American
Type Culture Collection (Manassas, VA, USA) and maintained in
monolayer culture in RPMI-1640 medium Hyclone (Logan, UT, USA),
supplemented with 10% fetal calf serum, 2 mmol/l glutamine, 100
µg/ml streptomycin and 100 U/ml penicillin (all Gibco;
Thermo Fisher Scientific Inc., Waltham, MA, USA). at 37°C in a
humidified atmosphere containing 5% CO2. Cells in the
logarithmic growth phase were used in all experiments.
Cell proliferation assay
The effects of PD on the proliferation of MOLT-4
cells were determined using the CCK-8 kit according to the
manufacturer's instructions. In brief, the cells were seeded in
96-well plates at a density of 5×103 per well. The cells
were then treated with DMSO (0.1%) or PD (0.5, 1, 2, 4, 10 or 20
µM) at 37°C. Following 0, 6, 12, 24, 48 or 72 h of
incubation, 10 µl CCK-8 was added to each well, followed by
further incubation for 1 h. The optical density was then determined
at 450 nm to calculate the proliferative index.
Assessment of apoptosis
The apoptotic rate of MOLT-4 cells was analyzed
using an Annexin V-propidium iodide (PI)-based immunofluorescence
apoptosis detection kit according to the manufacturer's
instructions. In brief, the cells were incubated in six-well plates
(3×105 cells/well) for 24 h. The cells were then treated
with DMSO or PD (1, 4 or 20 µM) for 24 h. Approximately
1×105 cells were harvested, washed in phosphate-buffered
saline (PBS) and incubated with 5 µl Annexin V stain and PI
at room temperature for 10 min. Fluorescence was measured using a
FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA). Annexin V-positive and PI-negative cells were regarded as
apoptotic.
Cell cycle analysis
Approximately 1×106 cells were harvested
and washed three times with PBS and fixed in 70% cold ethanol for
≥4 h. The cells were then re-suspended in DNA staining solution (50
µg/ml PI, 100 µg/ml RNase A and 0.1% Triton X-100 in
PBS; all from Jrdun Biotech (Shanghai, China), followed by
incubation at room temperature for 30 min. The DNA content was then
determined using a FACSCalibur flow cytometer (BD Biosciences). The
cell cycle distribution was calculated from 10,000 cells using
CellQuest Pro (BD Biosciences) and Modfit software 3.2 (Verity
Software House, Topsham, ME, USA).
Assessment of mitochondrial membrane
potential (MMP)
The MMP was evaluated by assessing the accumulation
of rhodamine 123 (Molecular Probes, Eugene, OR, USA) according to a
method described previously (18).
Briefly, the cells were incubated in six-well plates
(3×105 cells/well) for 24 h and then treated with DMSO
or PD (1, 4 or 20 µM) for 24 h. Cells were harvested, washed
in PBS and incubated with 0.5 µM rhodamine 123 at 37°C for
15 min. Fluorescence was measured using a FACSCalibur flow
cytometer (BD Biosciences) with an excitation wavelength of 485 nm
and an emission wavelength of 520 nm.
Evaluation of reactive oxygen species
(ROS) by flow cytometry using dihydroethidium (DHE)
DHE (Vigorous Biotechnology Beijing Co., Ltd.,
Beijing, China) was used for the flow cytometric assessment of
superoxide production as described previously (19). DHE is rapidly oxidized to ethidium,
a red fluorescent compound, by H2O2 (in the
presence of peroxidase) and superoxide. Cells were harvested,
washed with PBS and incubated with 50 µM DHE at room
temperature for 5 min. Fluorescence was measured using a
FACSCalibur flow cytometer (BD Biosciences). DHE fluorescence in
cells was evaluated using CellQuest software (BD Biosciences).
Western blot analysis
Western blot analyses was performed according to a
previously described method (13).
Cells were treated with DMSO (0.1%) or 20 µM PD and
incubated for 1 or 24 h. Following lysis in RIPA lysis buffer
(Jrdun Biotech), the protein concentration was quantified using the
Pierce BCA Protein Assay kit following the manufacturer's
instructions. Equal amounts (30 µg) of protein were
separated by electrophoresis on 10 or 15% sodium dodecyl
sulfate-polyacrylamide gels (Jrdun Biotech) and transferred onto
nitrocellulose membranes (EMD Millipore). Following blocking with
5% non-fat milk, the membranes were incubated with the desired
primary antibodies overnight at the following dilutions: p-JAK2
(1:1,000; Cell Signaling Technology Inc.; cat. no. 3776); JAK2
(1:1,000; Cell Signaling Technology Inc.; cat. no. 3230); p-STAT3
(1:1,000; Abcam; cat. no. Ab76315); STAT3 (1:1,000; Abcam; cat. no.
Ab119352); Bax, (1:100; Santa Cruz Biotechnology Inc.; cat. no.
Sc-493); Bcl-2 (1:150; Santa Cruz Biotechnology Inc.; cat. no.
Sc-492); cyclin B1 (1:20,000; Abcam; cat. no. Ab32053); cyclin D1
(1:1,000; Cell Signaling Technology Inc.; cat. no. 2922) and GAPDH
(1:1,500 Cell Signaling Technology Inc., cat. no. 5174).
Subsequently, the membranes were incubated with the appropriate
secondary antibodies: Horseradish peroxidase (HRP) conjugated
goat-anti-rabbit antibody (1:1,000; Beyotime Institute of
Biotechnology; cat. no. A0208); HRP-conjugated goat-anti mouse
antibody (1:1,000; Beyotime Institute of Biotechnology; cat. no.
A0216). The membranes were exposed to X-ray film (Kodak XAR-2,
Rochester, NY, USA). The immunoreactive bands were visualized using
the Immobilon™ Western Chemiluminescent Horseradish Peroxidase
substrate (EMD Millipore) according to the manufacturer's
instructions.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical analysis was performed by multifactorial
analysis of variance using SPSS software 17.0 (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
PD inhibits the proliferation of leukemia
cells
The cytotoxicity of PD on MOLT-4 leukemia cells was
determined using the CCK8 assay. In the absence of PD, MOLT-4
proliferated in an exponential manner, while PD inhibited the cell
proliferation in a dose-dependent manner, as well as a
time-dependent manner at 6–12 h (Fig.
1). These results indicated that, due to its marked
anti-proliferative effects, PD may be suitable for the treatment of
leukemia.
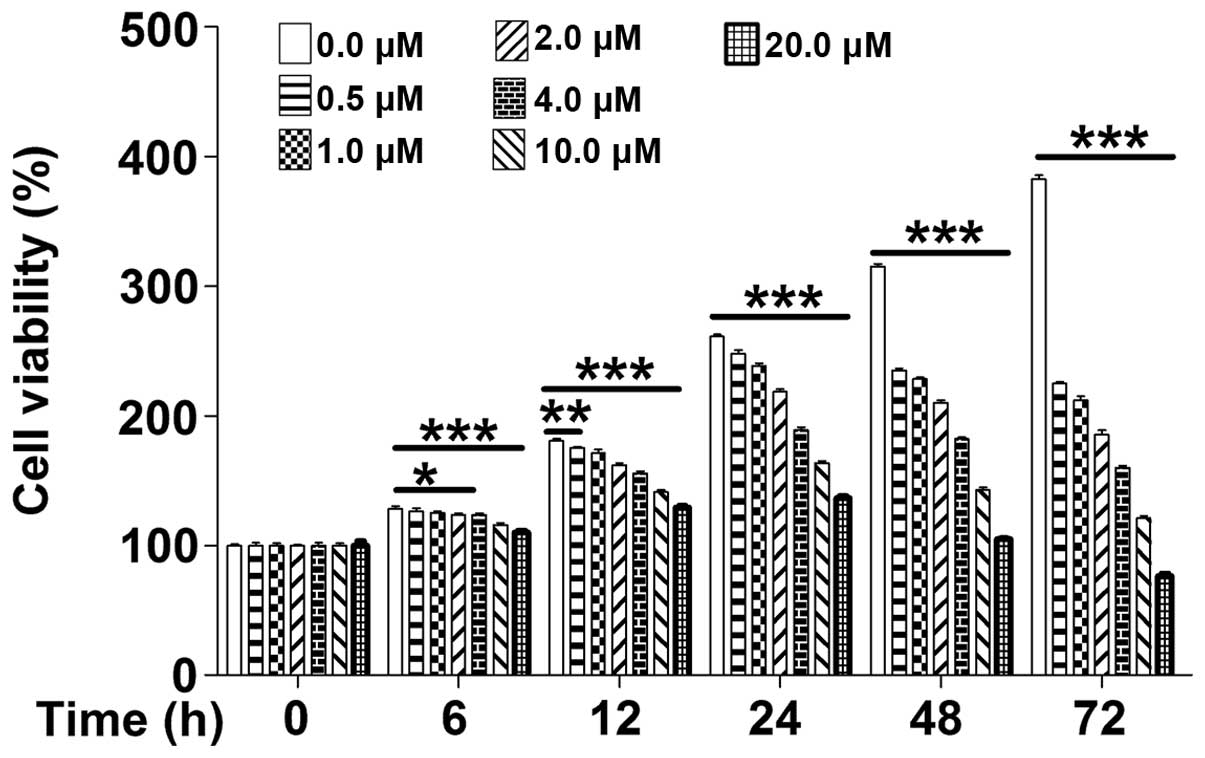 | Figure 1PD dose-and time-dependently reduces
the viability of MOLT-4 cells. Cell viability of MOLT-4 cells was
examined using a Cell Counting Kit 8 assay after treatment with PD
(0, 0.5, 1, 2, 4, 10 or 20 µM) for 0, 6, 12, 24, 48 or 72 h.
Values are expressed as the mean ± standard deviation (n=3). PD,
polydatin. *P<0.05, **P<0.01 and
***P<0.01 compared with 0.0 µM. |
PD induces apoptosis in leukemia
cells
To investigate the underlying mechanism of
PD-induced inhibition of leukemia cell growth, MOLT-4 cells were
incubated with various concentrations of PD for 24 h and their
apoptotic rate was assessed by flow cytometry. PD was shown to
induce apoptosis in leukemia cells in a dose-dependent manner
(P<0.001) (Fig. 2A). The
apoptotic rate increased from 3.63±0.42% in the control group to
9.67±0.42, 26.33±1.00 and 52.13±1.30% following incubation with 1,
4 and 20 µM PD for 24 h, respectively (Fig. 2B). These results suggested that PD
induced apoptotic cell death in leukemia cells.
PD induces cell-cycle arrest in S phase
in leukemia cells
To determine whether interference with cell-cycle
progression is among the underlying mechanisms of PD-induced growth
inhibition of leukemia cells, the effects of PD on cell cycle
progression were examined in exponentially growing MOLT-4 cells.
Treatment of cells with various concentrations of PD for 24 h
resulted in an increased accumulation of cells in S phase and a
corresponding decrease in the G1-phase population (Fig. 3A). In the absence of PD, the
S-phase population was 19.73±2.14% and the G1-phase population was
54.25±1.56%. Following PD treatment of MOLT-4 cells at 1, 4 and 20
µM, the S-phase population was increased to 27.58±2.91,
33.25±3.84 and 45.81±1.86%, whereas the G1-phase population was
decreased to 49.87±2.56, 42.80±2.88 and 27.25±2.25% (Fig. 3B). This result revealed that PD
inhibited cell proliferation by inducing cell-cycle arrest.
PD decreases the MMP in leukemia
cells
Mitochondria have key roles in activating apoptosis
in mammalian cells (20). The MMP
is regarded as a measure for mitochondrial function and it is
decreased during apoptosis (21).
The results of the present study revealed that treatment of MOLT-4
cells with PD for 24 h resulted in a decrease in the MMP (Fig. 4A). The MMP decreased from 1,136±73
in the control group to 859.0±21.1, 625.0±15.7 and 231.9±19.3
following incubation with 1, 4 and 20 µM PD for 24 h
(P<0.001) (Fig. 4B). These
results demonstrated that PD induced apoptosis in leukemia cells
through the mitochondrial pathway.
PD increases ROS in leukemia cells
Mitochondria-dependent apoptosis is often mediated
by ROS (22). To examine the
levels of ROS in MOLT-4 cells, DHE staining was used. Treatment of
cells with PD for 24 h resulted in an increase of ROS in a
dose-dependent manner (P<0.001) (Fig. 5A). ROS levels in leukemia cells
increased from 7,529±5,618 in the control group to 22,448±757,
69,669±2,010 and 154,994±1,587 following incubation with 1, 4 and
20 µM PD for 24 h (Fig.
5B). These results demonstrated that PD treatment led to the
generation of ROS to initiate mitochondria-dependent apoptosis in
leukemia cells.
PD-induced apoptosis of leukemia cells is
potentiated by inhibition of JAK2-STAT3 signaling
Due to the observed effects of PD on apoptosis and
S-phase arrest in leukemia cells, the impact of PD on the
expression of Bcl-2 and Bax, two key regulatory proteins of
apoptosis, as well as cyclin B1 and cyclin D1, two key cell
cycle-regulators, were examined by western blot analysis. The
results showed that the expression of anti-apoptotic protein Bcl-2
as well as cell cycle proteins cyclin B1 and cyclin D1 was
significantly decreased, while the expression of pro-apoptotic
protein Bax was significantly increased following treatment of
MOLT-4 cells with PD for 24 h (Fig.
6A). Inhibition of JAK2 activity by the specific tyrosine
kinase inhibitor AG490 is known to selectively block leukemic cell
growth in vitro and in vivo by inducing programmed
cell death (23). The present
study examined whether the JAK2-STAT3 pathway is involved in
PD-induced apoptosis and cell cycle arrest. Of note, the Bax/Bcl-2
ratio, an indicator of apoptosis (24), was markedly increased in MOLT-4
cells following combined treatment with PD and AG490 as compared
with that following treatment with either drug alone. In parallel,
the expression of cyclin B1 and cyclin D1 was also markedly
decreased following combined treatment with PD and AG490.
Furthermore, combined treatment with PD and AG490 for 1 h potently
reduced the levels of p-STAT3 and p-JAK2, indicating the
de-activation of the JAK2-STAT3 signaling pathway (Fig. 6B). This result suggested that PD
and JAK2 inhibition may have synergistic effects with regard to the
induction of apoptosis in leukemia cells.
 | Figure 6PD potentiates the effects of JAK2
inhibition on cell apoptosis and cell cycle arrest in MOLT-4 cells.
(A) The expression levels of Bcl2, Bax, cyclin B1 and cyclin D1
following PD treatment for 24 h, and (B) the expression levels of
p-JAK2, JAK2, p-STAT3 and STAT3 after PD treatment for 1 h in the
absence or presence of 10 nM JAK2 inhibitor AG490 were examined by
western blotting. GAPDH was used as loading control. p-JAK2,
phosphorylated Janus kinase 2; STAT3, signal transducer and
activator of transcription; Bcl2, B-cell lymphoma 2; Bax,
Bcl2-associated X protein; PD, polydatin. |
Discussion
PD is a natural precursor of resveratrol. Numerous
studies have suggested that resveratrol is a promising candidate
for cancer chemoprevention and therapy (25). However, it is difficult to reach a
therapeutically relevant level in vivo, since resveratrol is
readily metabolized and eliminated from the body (26). As resveratrol derivative with a
glucopyranoside ring substitution of the hydroxyl group in position
three, PD features enhanced stability and water solubility, and is
able to enter cells via glucose transporters (27). Due to these properties, PD is more
easily absorbed in the intestinal tract than resveratrol. PD has
been shown to have cytotoxic effects on various cancer cell lines,
including colon (13), lung
(14) and nasopharyngeal (15) cancer cells; however, the underlying
mechanism of action has largely remained elusive. The present study
showed that PD, exerted anti-proliferative effects via inducing
cell cycle arrest and induced mitochondria-mediated apoptosis in
MOLT-4 leukemia cell lines, which was potentiated by a JAK2
inhibitor.
Apoptosis is a highly organized form of cell death,
via which cells which have suffered significant damage are
eliminated (28). Apoptosis can be
triggered through either a death receptor-mediated extrinsic
pathway or a mitochondria-mediated intrinsic pathway (29). It is desirable to discover drugs
which selectively induce apoptosis in cancer cells through
triggering cancer-specific upstream mechanisms. As these pathways
are frequently altered in tumors, is may be possible to selectively
induce apoptosis in cancer cells or sensitize them to established
cytotoxic agents (30). The
present study demonstrated that PC triggers apoptosis in human
leukemia cells through the mitochondria-mediated intrinsic pathway,
rendering it a promising drug candidate for leukemia therapy.
ROS are mediators of numerous intracellular
signaling cascades; however, upon overproduction, they may induce
the collapse of the mitochondrial membrane potential, which
triggers a series of mitochondria-associated events, including
apoptosis (31). The present study
demonstrated that PD treatment markedly enhanced ROS production in
parallel with mitochondria-mediated leukemia-cell apoptosis;
therefore, it is likely that apoptosis was, at least in part,
triggered by ROS generation.
The proliferation of mammalian cells is driven by
the core cell cycle machinery comprising cyclin and
cyclin-dependent kinase (CDK) complexes (32). D-type cyclins, the ultimate
recipients of numerous oncogenic pathways, are key signaling
molecules and represent targets for cancer treatments (33). Among them, cyclin D1 is dispensable
with regard to normal physiological processes in adults, but is
required for tumor maintenance (34). Cyclin D1 levels must be suppressed
during the S phase for efficient DNA synthesis and must be
re-induced during the G2 phase to support cell proliferation
(35). The present study showed
that PD inhibited the expression of cyclin D1 and cyclin B1 and
caused cell-cycle arrest in S phase, which was the underlying
mechanism of its anti-proliferative effects.
Numerous cancer types, including hematological
malignancies, have been associated with constitutive activation of
members of the STAT family, whereas JAK-mediated tyrosine
phosphorylation is often required for transcriptional activation of
STAT proteins (36). A unique
somatic gain-of-function mutation in JAK2 (JAK2 V617F) was found in
>90% of patients with polycythaemia vera and in 50% of those
with essential thrombocythemia and primary myelofibrosis (37,38).
The combination of JAK2 inhibitors with inhibitors of downstream
effectors, including Bcl-2/Bcl extra large (ABT-737) has been
suggested to enhance the efficacy of polycythaemia vera treatments
(39). This strategy is thought to
be effective for the treatment of subsets of ALL featuring
dysregulation of JAK/STAT signaling. The present study found that a
JAK2 inhibitor indeed potentiated the apoptotic and cell
cycle-inhibitory effects of PD.
In conclusion, the results of the present study
demonstrated that PD-induced apoptosis of human leukemia cells is
mediated by the generation of ROS in mitochondria. Furthermore, the
inhibition of cyclin D1 by PD was shown to be accountable for its
anti-proliferative effects. The present study suggested that
treatment with PD and JAK2 inhibitor holds promise as a novel and
effective combination chemotherapy for leukemia.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81300418) and the
Youth Innovation Fund of the First Affiliated Hospital of Zhengzhou
University.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gregers J, Gréen H, Christensen IJ,
Dalhoff K, Schroeder H, Carlsen N, Rosthoej S, Lausen B,
Schmiegelow K and Peterson C: Polymorphisms in the ABCB1 gene and
effect on outcome and toxicity in childhood acute lymphoblastic
leukemia. Pharmacogenomics J. 15:372–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neumann M, Vosberg S, Schlee C, Heesch S,
Schwartz S, Gökbuget N, Hoelzer D, Graf A, Krebs S, Bartram I, et
al: Mutational spectrum of adult T-ALL. Oncotarget. 6:2754–2766.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pui CH and Evans WE: Acute lymphoblastic
leukemia. N Engl J Med. 339:605–615. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mehta RG and Pezzuto JM: Discovery of
cancer preventive agents from natural products: From plants to
prevention. Curr Oncol Rep. 4:478–486. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Du QH, Peng C and Zhang H: Polydatin: A
review of pharmacology and pharmacokinetics. Pharm Biol.
51:1347–1354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Q, Tan Y, Zhang N and Yao F:
Polydatin prevents angiotensin II-induced cardiac hypertrophy and
myocardial superoxide generation. Exp Biol Med (Maywood). 2014.
View Article : Google Scholar
|
|
8
|
Dong M, Ding W, Liao Y, Liu Y, Yan D,
Zhang Y, Wang R, Zheng N, Liu S and Liu J: Polydatin prevents
hypertrophy in phenylephrine induced neonatal mouse cardiomyocytes
and pressure-overload mouse models. Eur J Pharmacol. 746:186–197.
2015. View Article : Google Scholar
|
|
9
|
Ding W, Dong M, Deng J, Yan D, Liu Y, Xu T
and Liu J: Polydatin attenuates cardiac hypertrophy through
modulation of cardiac Ca2+ handling and calcineurin-NFAT signaling
pathway. Am J Physiol Heart Circ Physiol. 307:H792–H802. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Q, Tan Y, Zhang N and Yao F:
Polydatin supplementation ameliorates diet-induced development of
insulin resistance and hepatic steatosis in rats. Mol Med Rep.
11:603–610. 2015.
|
|
11
|
Sun J, Qu Y, He H, Fan X, Qin Y, Mao W and
Xu L: Protective effect of polydatin on learning and memory
impairments in neonatal rats with hypoxic-ischemic brain injury by
up-regulating brain-derived neurotrophic factor. Mol Med Rep.
10:3047–3051. 2014.PubMed/NCBI
|
|
12
|
Ravagnan G, De Filippis A, Cartenì M, De
Maria S, Cozza V, Petrazzuolo M, Tufano MA and Donnarumma G:
Polydatin, a natural precursor of resveratrol, induces β-defensin
production and reduces inflammatory response. Inflammation.
36:26–34. 2013. View Article : Google Scholar
|
|
13
|
De Maria S, Scognamiglio I, Lombardi A,
Amodio N, Caraglia M, Cartenì M, Ravagnan G and Stiuso P:
Polydatin, a natural precursor of resveratrol, induces cell cycle
arrest and differentiation of human colorectal Caco-2 cell. J
Transl Med. 11:2642013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Zhuang Z, Meng Q, Jiao Y, Xu J
and Fan S: Polydatin inhibits growth of lung cancer cells by
inducing apoptosis and causing cell cycle arrest. Oncol Lett.
7:295–301. 2014.
|
|
15
|
Liu H, Zhao S, Zhang Y, Wu J, Peng H, Fan
J and Liao J: Reactive oxygen species-mediated endoplasmic
reticulum stress and mitochondrial dysfunction contribute to
polydatin-induced apoptosis in human nasopharyngeal carcinoma CNE
cells. J Cell Biochem. 112:3695–3703. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takemoto S, Mulloy JC, Cereseto A, Migone
TS, Patel BK, Matsuoka M, Yamaguchi K, Takatsuki K, Kamihira S,
White JD, et al: Proliferation of adult T cell leukemia/lymphoma
cells is associated with the constitutive activation of JAK/STAT
proteins. Proc Natl Acad Sci USA. 94:13897–13902. 1997. View Article : Google Scholar
|
|
17
|
Alas S and Bonavida B: Rituximab
inactivates signal transducer and activation of transcription 3
(STAT3) activity in B-non-Hodgkin's lymphoma through inhibition of
the interleukin 10 autocrine/paracrine loop and results in
down-regulation of Bcl-2 and sensitization to cytotoxic drugs.
Cancer Res. 61:5137–5144. 2001.PubMed/NCBI
|
|
18
|
Wu EY, Smith MT, Bellomo G and Di Monte D:
Relationships between the mitochondrial transmembrane potential,
ATP concentration and cytotoxicity in isolated rat hepatocytes.
Arch Biochem Biophys. 282:358–362. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Medjkane S, Perichon M, Marsolier J,
Dairou J and Weitzman JB: Theileria induces oxidative stress and
HIF1 α activation that are essential for host leukocyte
transformation. Oncogene. 33:1809–1817. 2014. View Article : Google Scholar
|
|
20
|
Wang C and Youle RJ: The role of
mitochondria in apoptosis. Annu Rev Genet. 43:95–118. 2009.
View Article : Google Scholar
|
|
21
|
Gottlieb E, Armour SM, Harris MH and
Thompson CB: Mitochondrial membrane potential regulates matrix
configuration and cytochrome c release during apoptosis. Cell Death
Differ. 10:709–717. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Herrera B, Alvarez AM, Sánchez A,
Fernández M, Roncero C, Benito M and Fabregat I: Reactive oxygen
species (ROS) mediates the mitochondrial-dependent apoptosis
induced by transforming growth factor (beta) in fetal hepatocytes.
FASEB J. 15:741–751. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meydan N, Grunberger T, Dadi H, Shahar M,
Arpaia E, Lapidot Z, Leeder JS, Freedman M, Cohen A, Gazit A, et
al: Inhibition of acute lymphoblastic leukaemia by a Jak-2
inhibitor. Nature. 379:645–648. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu JY, Meng QH, Chong Y, Jiao Y, Zhao L,
Rosen EM and Fan S: Sanguinarine inhibits growth of human cervical
cancer cells through the induction of apoptosis. Oncol Rep.
28:2264–2270. 2012.PubMed/NCBI
|
|
25
|
Singh CK, Ndiaye MA and Ahmad N:
Resveratrol and cancer: Challenges for clinical translation.
Biochim Biophys Acta. 1852:1178–1185. 2015. View Article : Google Scholar
|
|
26
|
Francioso A, Mastromarino P, Masci A,
d'Erme M and Mosca L: Chemistry, stability and bioavailability of
resveratrol. Med Chem. 10:237–245. 2014. View Article : Google Scholar
|
|
27
|
Krasnow MN and Murphy TM: Polyphenol
glucosylating activity in cell suspensions of grape (Vitis
vinifera). J Agric Food Chem. 52:3467–3472. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Daniel PT, Koert U and Schuppan J:
Apoptolidin: Induction of apoptosis by a natural product. Angew
Chem Int Ed Engl. 45:872–893. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Circu ML and Aw TY: Glutathione and
modulation of cell apoptosis. Biochim Biophys Acta. 1823:1767–1777.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ghobrial IM, Witzig TE and Adjei AA:
Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin.
55:178–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qu K, Shen NY, Xu XS, Su HB, Wei JC, Tai
MH, Meng FD, Zhou L, Zhang YL and Liu C: Emodin induces human T
cell apoptosis in vitro by ROS-mediated endoplasmic reticulum
stress and mitochondrial dysfunction. Acta Pharmacol Sin.
34:1217–1228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: A changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Musgrove EA, Caldon CE, Barraclough J,
Stone A and Sutherland RL: Cyclin D as a therapeutic target in
cancer. Nat Rev Cancer. 11:558–572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi YJ, Li X, Hydbring P, Sanda T,
Stefano J, Christie AL, Signoretti S, Look AT, Kung AL, von Boehmer
H and Sicinski P: The requirement for cyclin D function in tumor
maintenance. Cancer Cell. 22:438–451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang K, Hitomi M and Stacey DW: Variations
in cyclin D1 levels through the cell cycle determine the
proliferative fate of a cell. Cell Div. 1:322006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vainchenker W and Constantinescu SN:
JAK/STAT signaling in hematological malignancies. Oncogene.
32:2601–2613. 2013. View Article : Google Scholar
|
|
37
|
James C, Ugo V, Le Couédic JP, Staerk J,
Delhommeau F, Lacout C, Garçon L, Raslova H, Berger R,
Bennaceur-Griscelli A, et al: A unique clonal JAK2 mutation leading
to constitutive signalling causes polycythaemia vera. Nature.
434:1144–1148. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Levine RL, Wadleigh M, Cools J, Ebert BL,
Wernig G, Huntly BJ, Boggon TJ, Wlodarska I, Clark JJ, Moore S, et
al: Activating mutation in the tyrosine kinase JAK2 in polycythemia
vera, essential thrombocythemia, and myeloid metaplasia with
myelofibrosis. Cancer Cell. 7:387–397. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu M, Wang J, Li Y, Berenzon D, Wang X,
Mascarenhas J, Xu M and Hoffman R: Treatment with the Bcl-xL
inhibitor ABT-737 in combination with interferon α specifically
targets JAK2V617F-positive polycythemia vera hematopoietic
progenitor cells. Blood. 116:4284–4287. 2010. View Article : Google Scholar : PubMed/NCBI
|




















