Introduction
The prolyl hydroxylase inhibitor dimethyloxallyl
glycine (DMOG) is a type of hypoxia-mimetic agent, which has been
increasingly studied with regards to stem cell therapy. DMOG, which
is a small molecular drug, is a cell-permeable prolyl-4-hydroxylase
inhibitor. At normal oxygen tension, DMOG is able to inhibit the
effects of hypoxia-inducible factor prolyl hydroxylase, and
stabilize the expression of hypoxia-inducible factor-1α (HIF-1α) in
cells (1), thus mediating the
function of signaling pathways associated with cellular or tissue
alterations and repair.
A previous study regarding DMOG application reported
that DMOG was able to induce cardioprotective effects via HIF-1α
stabilization in rabbits (2). In
the past decade, studies on DMOG have gradually increased with
regards to its role in various cells and tissues. In recent
studies, DMOG has been successfully used to increase bone healing
capacity (3), attenuate renal
injury in the remnant kidney model (4), induce angiogenesis in ischemic
skeletal muscle (5), promote
vascularization in the arterovenous loop via HIF-1α upregulation
(6), and provide neuroprotection
in a middle cerebral artery occlusion model (7). Furthermore, it has been reported that
DMOG may have an important role in mesenchymal stem cell (MSC)
transplantation therapy for the functional recovery of ischemic
heart disease (8).
MSCs are plastic-adherent, fibroblast-like adult
stem cells, which possess the capacity to self-renew and
differentiate into numerous types of cells, including adipocytes,
osteoblasts, chondroblasts, myoblasts and neuron-like cells
(9–11). In addition, MSCs can be obtained
from various connective tissue sources, including bone marrow
(12), adipose (13), dermal tissue (14), synovial fluid (15), deciduous teeth (16,17)
and umbilical cord blood (18).
Following adequate stimulation, stem and progenitor cells may leave
the cell niche and enter the peripheral blood, which is termed stem
cell mobilization (19). Although
it has been confirmed that allograft MSCs, following in
vitro amplification, can repair numerous types of tissue
damage, it has been suggested that in vitro-amplified MSCs
may induce the development of tumors (20), thus arousing doubts regarding the
safety of allograft MSCs. Therefore, increasing attention has been
paid with regards to the repairing effect of autologous MSCs.
Unfortunately, the strategy by which endogenous MSCs are used to
treat tissue damage is limited by the paucity of circulating MSCs
in peripheral blood. Recently, pharmacological preconditioning has
been proposed hypothetically and experimentally as an efficient
approach for modification and improvement in the function of
various organs, tissues and cells. Granulocyte colony-stimulating
factor (G-CSF) is a potent stimulator of hematopoietic stem cells
mobilization. Numerous studies have attempted to use G-CSF to
induce MSC mobilization into the circulation, however,
disappointing results have been obtained (21–23).
Recent studies have reported that recombinant wingless-related
integration site (Wnt)3a (24),
insulin-like growth factor-1 + AMD3100 (25) and LiCl (26) mobilize MSCs into the circulation
and promote the proliferation and differentiation ability of
PB-MSCs. Our previous study confirmed that DMOG was able to
mobilize MSCs into peripheral blood circulation (27). Peripheral blood-MSCs were
demonstrated to have weaker proliferation and migration ability and
similar multilineage differentiation potential when compared with
bone marrow-MSCs and the mechanism underlying MSCs mobilization was
investigated (28).
MSC mobilization is commonly measured using the
fibroblast-colony forming unit assay and flow cytometry. Our
previous study demonstrated that compared with normal
saline-treated mice, the number of colony forming units and
percentage of cluster of differentiation
(CD)90+/CD45− cells in the peripheral blood
was significantly increased in mice treated with DMOG (27).
Whether DMOG may be feasible as a novel mobilization
agent remains to be elucidated. Since bone marrow is one of the
most important sources of mobilized peripheral blood MSCs, the
present study conducted preliminary studies to characterize BM-MSCs
collected from mice following DMOG intraperitoneal injection. The
biological properties of BM-MSCs from DMOG preconditioned-mice
(DBM-MSCs), including cell morphology, immune phenotype,
multilineage differentiation, proliferation, migration and
paracrine capacity, were investigated and compared with the
properties of BM-MSCs from normal saline-treated mice (NBM-MSCs).
The results may provide an experimental basis for the application
of DMOG as a novel mobilization agent in future clinical
trials.
Materials and methods
Animals and DMOG preconditioning
The present study was approved by the ethics
committee of Zhejiang Chinese Medical University (Hangzhou, China).
Male Institute of Cancer Research (ICR) mice (age, 8–10 weeks) were
purchased from the Zhejiang Chinese Medical University Animal
Center (Hangzhou, China; Laboratory Animal Certificate: SCXK
2008–0115). All procedures related to the care of animals were
performed according to the National Institutes of Health Guide for
the Care and Use of Laboratory Animals (29).
ICR mice were randomly assigned into the normal
saline control group (NS group) or the DMOG preconditioning group
(DMOG group; n=5/group). Mice of the DMOG group received an
intraperitoneal injection of DMOG (40 mg/kg in 0.5 ml normal
saline; Cayman Chemical Company, Ann Arbor, MI, USA) for 7
consecutive days, whereas mice in the NS group received 0.5 ml
normal saline. The DMOG dose was chosen according to our previous
study (27).
Isolation and culture of BM-MSCs
Following DMOG or normal saline preconditioning,
mice were sacrificed by cervical dislocation and BM-MSCs were
obtained from the mice femur and tibia, as previously described
(30). Briefly, muscles and
adherent tissue were detached, and the epiphyses were removed. The
whole bone marrow plugs were flushed using a 25-gauge needle and a
1.0 ml syringe loaded with Dulbecco's modified Eagle's medium/Ham's
F-12 (DMEM/F-12; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin
(HyClone; Thermo Fisher Scientific, Inc., Logan, UT, USA).
Harvested marrow cells were centrifuged, resuspended, counted, and
cultured as described previously (30). All non-adherent cells were removed
by media replacement after 24 h. Subsequently, the medium was
replaced every 3–4 days. Upon reaching 80–90% confluence, the
primary cells were trypsinized (0.25% trypsin-EDTA; Gibco; Thermo
Fisher Scientific, Inc.), resuspended in complete culture medium,
and subcultured at a 1:2 ratio. BM-MSCs from passages 3–4 were used
in the subsequent experiments.
For identification of BM-MSCs, a colony-forming unit
fibroblast assay was conducted, and cell surface markers CD90 and
CD45 were detected by flow cytometry. The detailed characteristics
were confirmed in our previous study (27).
CD44+/CD90+/CD45−cells were
detected and selected in the present study.
Flow cytometry assay
To detect the effects of DMOG on the immune
phenotype of BM-MSCs, flow cytometry was conducted. The fourth
generation NBM-MSCs and DBM-MSCs were suspended in
phosphate-buffered saline (PBS) at a concentration of
1×106 cells/ml in five tubes. Fluorescein isothiocyanate
(FITC) and phycoerythrin (PE) isotype controls were added into the
two tubes as controls, whereas 2 μl CD44-FITC, 5 μl
CD90-PE and 2 μl CD45-FITC were added into the three
remaining tubes, respectively (all obtained from Abcam (Hong Kong)
Ltd., Hong Kong, China). Following a 30 min incubation at room
temperature the cells were washed twice with PBS. Subsequently, a
FACSCalibur™ flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA) and Cell Quest software (version 7.5.3; BD Biosciences) were
used to test and analyze the results.
Multilineage differentiation assay
The multilineage differentiation potential of the
NBM-MSCs and DBM-MSCs was evaluated in detail, as described in the
following subsections.
Adipogenic differentiation
NBM-MSCs and DBM-MSCs were seeded in 6-well plates
at a density of 2×104 cells/cm2. Once the
cells reached 90–100% confluence, ICR mouse MSC adipogenic
differentiation medium [Cyagen Biosciences (Guangzhou), Inc.,
Guangzhou, China] was added, and the induction process was
conducted according to the manufacturer's protocol. Cells were then
stained with Oil Red O solution [Cyagen Biosciences (Guangzhou),
Inc.) and were observed under an inverted fluorescence microscope
(ECLIPSE TE2000-S; Nikon Corporation, Tokyo, Japan).
Osteogenic differentiation
NBM-MSCs and DBM-MSCs (1×104
cells/cm2) were seeded in 6-well plates in DMEM-low
glucose (DMEM-LG; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS, 0.1 μM dexamethasone, 10 mM
β-glycerol phosphate and 50 μM ascorbic acid (all
Sigma-Aldrich, St. Louis, MO, USA), and the medium was replaced
every 3 days. Cell morphology was observed under an inverted
fluorescence microscope (ECLIPSE TE2000-S). Von Kossa
(Sigma-Aldrich) staining was used to reveal mineralized areas.
Chondrogenic differentiation
NBM-MSCs and DBM-MSCs were resuspended in serum-free
DMEM-high glucose (Gibco; Thermo Fisher Scientific, Inc.)
containing insulin-transferrin-selenium-A (Gibco; Thermo Fisher
Scientific, Inc.), 0.1 μM dexamethasone and 200 μM
ascorbic acid, and were plated in 6-well plates at a density of
5×104 cells/ml. Subsequently, 10 ng/ml transforming
growth factor (TGF)-β1 and TGF-β3 (PeproTech, Rocky Hill, NJ, USA)
were added to the cells. The medium was changed every 3 days, and
cell factors were renewed each time. Cells were stained with
Toluidine Blue (Sigma-Aldrich) after 21 days and visualized under
an ECLIPSE TE2000-S microscope.
Neuronal differentiation
The following neuronal differentiation induction
media were used in the present study: Pre-induction medium (DMEM-LG
containing 1 mM β-mercaptoethanol and 20% FBS), and induction
medium (serum-free DMEM-LG containing 5 mM β-mercaptoethanol).
NBM-MSCs and DBM-MSCs were plated at a density of 1×105
cells/well on 18×18 mm slides on 6-well plates. Once the cells
reached ~70% confluence, differentiation was successively induced
by incubation with pre-induction medium for 24 h, followed by
incubation with induction medium for 3–6 h.
Immunofluorescence
Immunofluorescence assay was used to identify the
expression of the nerve cell-specific marker Nestin, and the
astrocyte-specific marker glial fibrillary acidic protein (GFAP).
Briefly, slides were gently washed twice with PBS, and were fixed
with immune dyeing fixative (Hangzhou Dawen Biotec Co., Ltd.,
Hangzhou, China) for 10 min. Subsequently, immune dyeing wash
buffer (Hangzhou Dawen Biotec Co., Ltd.) was used to wash the
slides, and the slides were blocked with immune dyeing block
buffers (Hangzhou Dawen Biotec Co., Ltd.) for 60 min. The remaining
liquid was aspirated, and the slides were then incubated with
polyclonal rabbit anti-mouse Nestin (1:50; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA; cat. no. sc-20978) and GFAP
(1:100; Santa Cruz Biotechnology, Inc.; cat. no. sc-9065) primary
antibodies for 90 min at room temperature. Following three washes,
FITC-conjugated goat polyclonal anti-rabbit immunoglobulin
G-H&L secondary antibody [1:100; Abcam (Hong Kong) Ltd.; cat.
no. ab97050] was added to the slides and incubated for 30 min. The
slides were rinsed three times, and were incubated with
4′,6-diamidino-2-phenylindole for 5 min. Following a further three
washes with PBS, the specimens were sealed with glycerin and
observed under an inverted fluorescence microscope (ECLIPSE
TE2000-S).
Cell Counting kit (CCK)-8 cell
proliferation assay
In order to determine the influence of DMOG on
BM-MSC proliferation, NBM-MSCs and DBM-MSCs were trypsinized,
neutralized, and centrifuged at 100 × g for 6 min. Resuspended in
fresh complete medium, a 200 μl cell suspension was added to
96-well plates at a density of 2×103 cells/well. After
24 h, 20 μl CCK-8 reagent (CCK-8; Dojindo Laboratories,
Inc., Kumamoto, Japan) was added to the five experimental wells for
2 h. Subsequently, a microplate reader (SpectraMax Plus384;
Molecular Devices, LLC, Sunnyvale, CA, USA) was used to detect
optical density (OD) values at 450 nm wavelength. In addition, a
blank control well was set containing only 200 μl culture
medium. OD values were determined for 7 consecutive days at the
same time-point.
Cell migration assay
In order to determine the effects of DMOG on BM-MSCs
migration capacity, a Transwell assay was conducted. NBM-MSCs and
DBM-MSCs were resuspended in DMEM containing 2% FBS. Briefly, 600
μl culture medium containing 150 ng/ml stromal cell-derived
factor-1α (SDF-1α; PeproTech) was placed into the lower chamber of
the 24-well plate, and 150 μl cell suspension
(1×104 cells/ml) was plated into the upper chamber of
the 24-well plate that contained Transwell inserts (diameter, 6.5
mm; pore size, 8 μm; Corning, Inc., Corning, NY, USA).
Following a 15 h incubation, the inside compartments were removed
and a cotton swab was used to remove the cells. Following fixation
with 4% paraformaldehyde, crystal violet staining and flushing with
double distilled water, the cells that had migrated to the lower
chamber were observed under a microscope. The migrated cells were
counted in five random microscope fields (ECLIPSE TE2000-S).
Enzyme-linked immunosorbent assay
(ELISA)
An ELISA was performed to analyze the paracrine
capacity of the cells. NBM-MSCs and DBM-MSCs were plated in 12-well
plates at a density of 5×104 cells/well. The medium was
discarded after 24 h and the cells were washed twice with PBS.
Subsequently, 500 μl serum-free DMEM-LG was added and the
supernatants were collected after 48 h. TGF and platelet-derived
growth factor (PDGF) concentration was detected using corresponding
ELISA kits. Mouse TGF (β IG-H3) and PDGF ELISA kits (Wuhan Boster
Biological Co., Ltd., Wuhan, China) were used, according to the
manufacturer's protocol.
Statistical analysis
All statistical analyses were performed using SPSS
19.0 statistical software (SPSS IBM, Armonk, NY, USA). Experiments
were repeated at least three times and data are presented as the
mean ± standard deviation. Student's two-tailed t-test was used to
compare the two independent experimental groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Cell morphology, immune phenotype and
multilineage differentiation ability is similar in DBM-MSCs and
NBM-MSCs
To detect the immune phenotype and differentiation
potential of the cells, NBM-MSCs and DBM-MSCs were cultured in
parallel. The homogeneous layer of fibroblast-like BM-MSCs obtained
from DMOG-preconditioned mice was similar to that obtained from
NS-treated mice (Fig. 1A).
The cell surface antigen expression of NBM-MSCs and
DBM-MSCs was analyzed by flow cytometry for three samples. The two
types of cells were positive for CD44 (homing-associated cell
adhesion molecule; 93.8 and 97.9%, in NBM-MSCs and DBM-MSCs,
respectively) and CD90 (Thy-1; 93.5 and 96.6% in NBM-MSCs and
DBM-MSCs, respectively), but were negative for hematopoietic stem
cell marker CD45 (leukocyte common antigen; 2.59 and 1.70% in
NBM-MSCs and DBM-MSCs, respectively; Fig. 1B). These results indicate that
DBM-MSCs possess a similar immune phenotype to NBM-MSCs. In
addition, no significant differences were detected between them
(P>0.05).
NBM-MSCs and DBM-MSCs were separately cultured in
adipogenic, osteogenic or chondrogenic induction medium for 14–21
days. Subsequently, the presence of cytoplasmic olesomes, the
formation of calcium precipitation, or the secretion of acid
glycosaminoglycan was examined by specific staining. NBM-MSCs and
DBM-MSCs were able to be differentiated into adipocytes that
contained secreting orange-red lipid droplets, osteoblasts that are
surrounded by black calcium precipitation and chondrocytes that
have cell matrix that dyes purple and nuclei that stain blue
(Fig. 2A). In addition, DBM-MSCs
induced by β-mercaptoethanol for 5 h were immunocytochemically
shown to display typical neuron-like characteristics, and express
nerve cell-specific marker Nestin, but not astrocyte-specific
marker GFAP (Fig. 2B; data not
shown). These results suggest that the multi-lineage
differentiation capacity of DBM-MSCs was similar to that of
NBM-MSCs.
 | Figure 2Multilineage differentiation capacity
of NBM-MSCs and DBM-MSCs. (A) NBM-MSCs and DBM-MSCs were cultured
in Ad., Os., or Ch. differentiation medium. Adipogenic
differentiation was stained with Oil Red O. Osteogenic
differentiation was stained with Von Kossa. Chondrogenic
differentiation was stained with Toluidine Blue. (Magnification,
x100). (B) NBM-MSCs and DBM-MSCs were induced into neuron-like
cells. Immunofluorescence staining: Nuclei were dyed blue with DAPI
and Nestin expression was dyed green. (Magnification, ×200).
NBM-MSCs, bone marrow-derived mesenchymal stem cells from normal
saline-treated mice; DBM-MSCs, bone marrow-derived mesenchymal stem
cells from dimethyloxallyl glycine-preconditioned mice; Ad.,
adipogenic; Os., osteogenic; Ch., chondrogenic; DAPI,
4′,6-diamidino-2-phenylindole. |
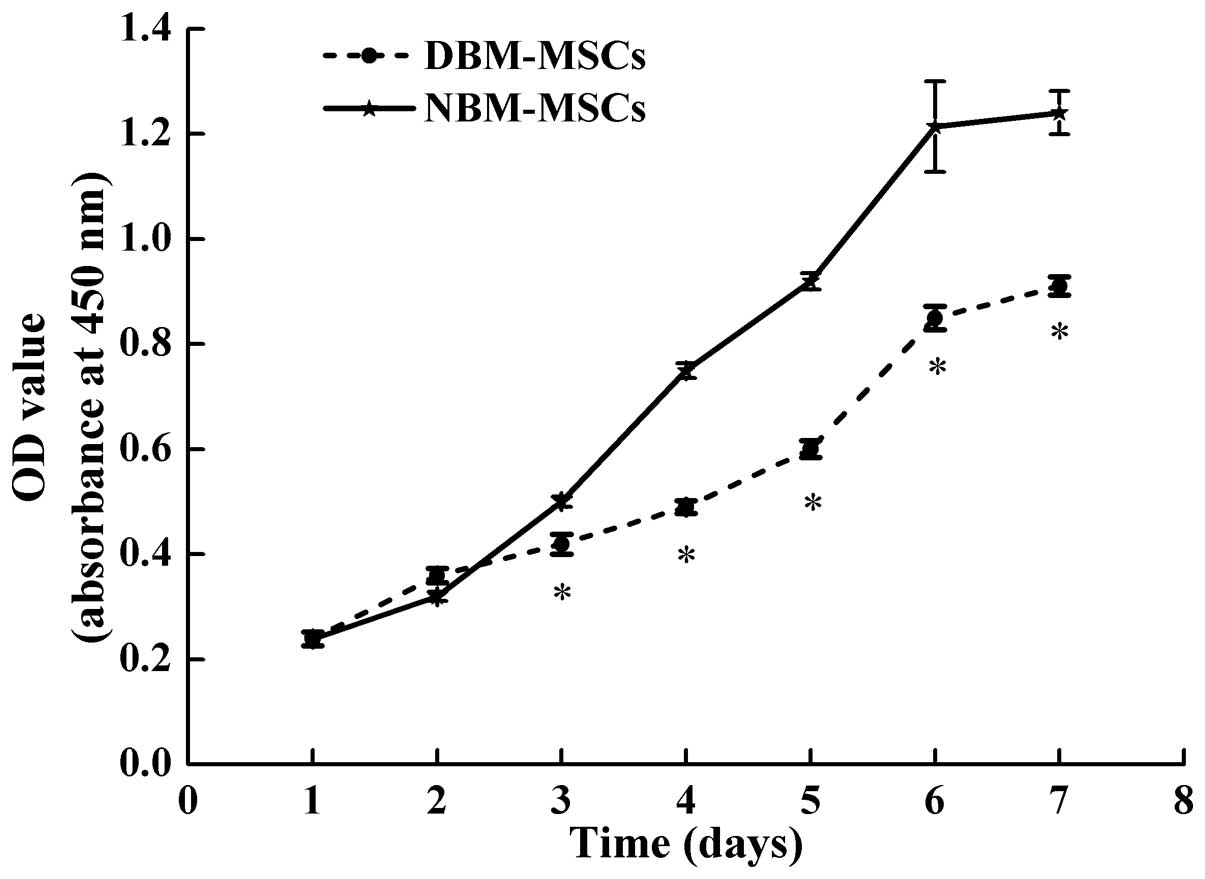
Cell proliferation is slightly reduced in
DBM-MSCs compared with NBM-MSCs
To determine the influence of DMOG on MSCs
proliferation, the number of MSCs derived from the two groups was
measured using the CCK-8 test. DBM-MSCs were in a latent period of
growth between days 1 and 3, exhibited logarithmic growth between
days 4 and 6, and reached a plateau after day 6. Compared with
NBM-MSCs, the proliferation rate of DBM-MSCs was reduced, and
starting from day 3 the total number of cells was significantly
decreased compared with the number of NBM-MSCs at the same time
(P=0.003, P=0.001, P=0.004, P=0.007 and P=0.003 for day 3 to day 7,
respectively). The results also indicated that DBM-MSCs had a
similar latent period, logarithmic phase and plateau to the
NBM-MSCs (Fig. 3). Both cell
growth curves were "S"-shaped; however, the proliferative ability
of DBM-MSCs was slightly reduced compared with NBM-MSCs.
Migratory ability is slightly reduced in
DBM-MSCs compared with NBM-MSCs
The Transwell assay was used to compare differences
in migratory ability between DBM-MSCs and NBM-MSCs. As shown in
Fig. 4A, the migrated cells from
the two groups were detected (Fig.
4A). Results indicated that following 15 h of SDF-1α as a
chemoattractant, the number of DBM-MSCs that had migrated to the
lower compartment was reduced compared with the number of NBM-MSCs
(92.00±4.85 vs. 101.40±5.18; n=5; P=0.018; Fig. 4B). These results suggest that the
migratory ability of DBM-MSCs was inferior to that of NBM-MSCs.
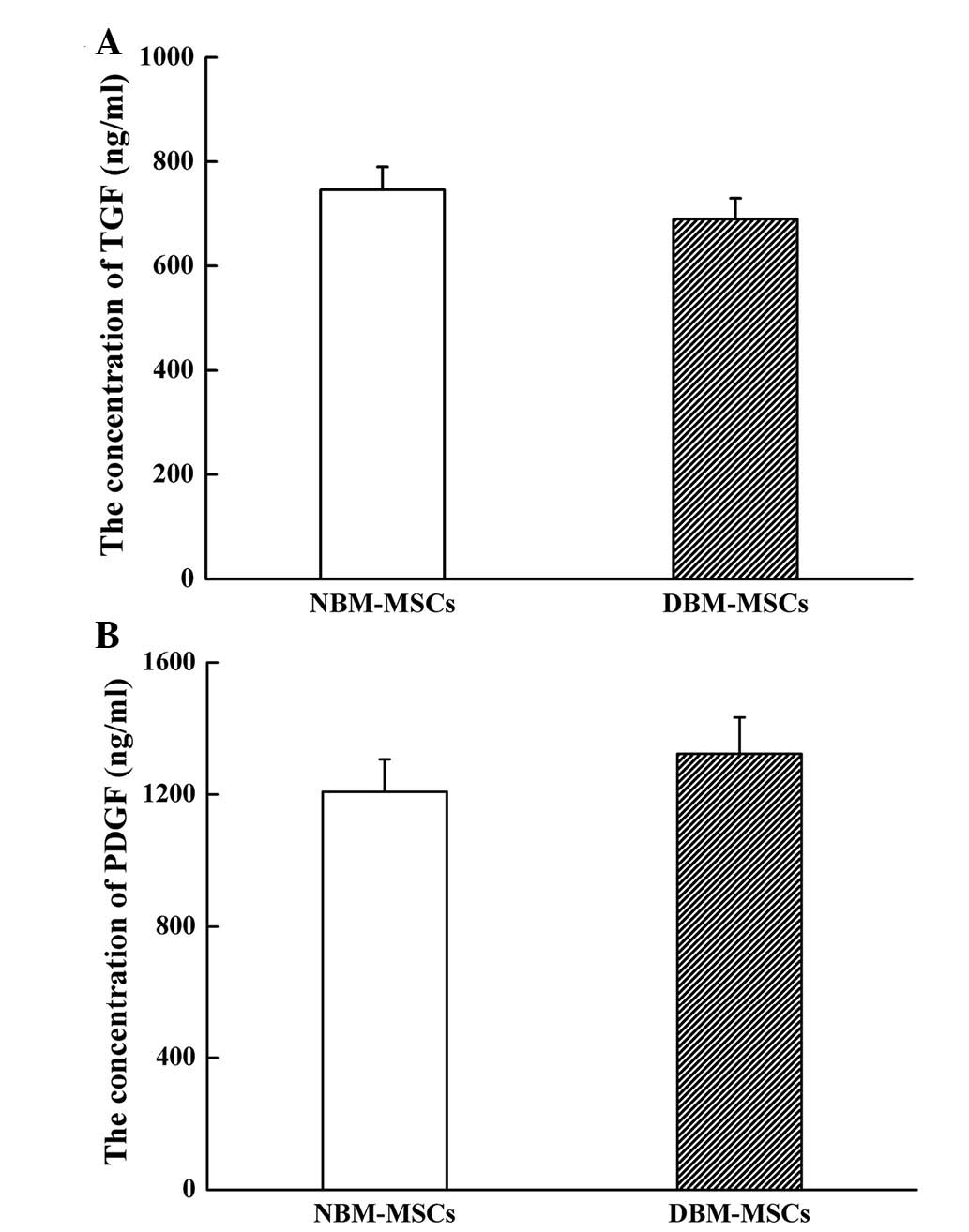
DBM-MSCs exhibit a similar TGF and PDGF
secretory capacity compared with NBM-MSCs
In order to determine whether cytokine production
was affected by DMOG, the TGF and PDGF cytokine concentrations were
detected in DBM-MSCs and NBM-MSCs by ELISA. Compared with NBM-MSCs,
TGF secretion of DBM-MSCs was decreased; however, there was no
significant difference between the groups (689.9±40.2 vs.
746.4±43.8 ng/ml; P=0.066; Fig.
5A). Conversely, PDGF secretion of DBM-MSCs was increased;
however, this difference was also not significant (1323.5±110.3 vs.
1207.9±98.7 ng/ml; P=0.119; Fig.
5B). These results indicate that DBM-MSCs have a similar
secretory capacity with regards to TGF and PDGF, as compared with
NBM-MSCs.
Discussion
Our previous study revealed that MSCs could be
mobilized into peripheral blood circulation by hypoxia induction,
and the transcription factor HIF-1α had a pivotal role in
hypoxia-induced MSCs mobilization (31). Considering clinical and ethical
safety, hypoxia mobilization is not feasible in clinical therapy.
However, it has been reported that similar effects may be obtained
using the prolyl hydroxylase inhibitor DMOG (32), which is a type of hypoxia-mimetic
agent that stabilizes and upregulates HIF-1 signaling under
normoxic conditions.
The present study investigated the biological
properties of BM-MSCs obtained from ICR mice preconditioned with
DMOG. Previous studies have mainly investigated the effects of DMOG
by directly preconditioning stem cells in vitro, in order to
assess the benefits (3,8). However, in the present study, a DMOG
preconditioning strategy was used, which differs from the methods
used in previous studies. The present study detected the effects of
DMOG on BM-MSCs by collecting cells from mice that were
intraperitoneally injected with DMOG.
At present, no specific surface markers of MSCs have
been identified. Previous studies have indicated that the surface
antigen phenotype of MSCs is not singular, but possesses the
characteristics of mesenchymal cells, epithelial cells and muscle
cells at the same time (33,34).
MSCs are negative for hematopoietic cell surface antigens,
including CD34, CD45, CD11, CD14 and CD235a, and adhesion
molecules, such as CD31, CD18 and CD56. Conversely, MSCs are
positive for CD105, CD73, CD90, CD71, CD29, CD44, CD106, CD166,
etc. (35). The results of the
present study revealed that DBM-MSCs and NBM-MSCs were negative for
CD45, and positive for CD44 and CD90, thus suggesting that DBM-MSCs
exhibit a similar immune phenotype to NBM-MSCs, as expected.
MSCs can be expanded in vitro and maintain
multilineage differentiation potential (36). Detection of adipogenic, osteogenic,
chondrogenic and neuronal differentiation potential is the most
common method used to identify whether analyzed cell populations
are capable of multilineage differentiation. Wnt and Rho are the
main signaling pathways associated with regulation of adipogenic
differentiation of MSCs (37).
Adipogenic stimuli induce terminal differentiation of committed
preadipocytes via the epigenomic activation of peroxisome
proliferator-activated receptor-γ (PPARγ). The coordination of
PPARγ with CCAAT/enhancer-binding protein transcription factors is
able to maintain adipocyte gene expression (38). In addition, Wnt, Notch and bone
morphogenetic protein signaling has an important role in the
regulation of MSCs osteogenic differentiation (39). DMOG has been reported to increase
the bone healing capacity of adipose-derived MSCs by promoting
osteogenic differentiation and angiogenic potential in rat
critical-sized calvarial defects (3). In addition, a previous study
suggested that TGF-β signals have a pivotal role in chondrogenic
differentiation (40).
Hypoxia-enhanced chondrogenesis of BM-MSCs has also been reported
to occur via activation of the mitogen-activated protein kinase P38
pathway (41). Furthermore,
sirtuin 1 activation may be essential for the induction of neuronal
differentiation, due to its effects on mammalian target of
rapamycin downregulation and neurite outgrowth stimulation
(42). In the present study,
compared with NBM-MSCs, DBM-MSCs exhibited similar adipogenic,
osteogenic, chondrogenic and neuronal differentiation abilities,
thus suggesting that DMOG had no obvious stimulatory or inhibitory
effects on BM-MSCs multilineage differentiation. While previous
studies have predominantly investigated the effects of DMOG by
directly preconditioning stem cells in vitro (3,8), the
present study investigated the biological properties of BM-MSCs
obtained from ICR mice preconditioned with DMOG. By contrast to
in vitro treatment, the current study hypothesizes the in
vivo treatment would be influenced by complex metabolic
reactions in the animal body. No notable change in the
differentiation ability of BM-MSCs was observed in the present
in vitro culture and our previous study also demonstrated
DMOG could mobilize MSCs to the peripheral blood with no effect on
differentiation in pretreated mice (28). Thus, DMOG appears to be feasible as
a stem cell mobilization agent.
A cell growth curve was generated using the CCK-8
assay, and the proliferative ability of DBM-MSCs was slightly
reduced, compared with that of NBM-MSCs. These results suggested
that DMOG slightly inhibited BM-MSCs proliferation. However, DMOG
treatment maintained a normal growth curve, thus suggesting that
DMOG had no obvious cytotoxic effects on BM-MSCs as only a slightly
reduced proliferation was observed. This result is similar to the
findings of a previous study on the effects of DMOG on
adipose-derived MSCs (3). In the
in vivo microenvironment, MSCs constantly update themselves,
with the majority of cells maintained in the latent period of
growth. There are few studies that have reported the effects of
DMOG on MSCs proliferation. A previous study demonstrated that DMOG
was able to inhibit the proliferation of vascular smooth muscle
cells in vitro (43).
Furthermore, it has been reported that DMOG may significantly
reduce the apoptosis of MSCs, stabilize the expression of HIF-1α to
induce glucose transport protein synthesis, and reduce the release
of mitochondrial cytochrome c, thus promoting protein kinase
phosphorylation (44). Therefore,
the reduction in the proliferative ability of DBM-MSCs may be
associated with the varying expression levels of proteins involved
in cell cycle regulation.
The present study detected the migratory capacity of
DBM-MSCs, and SDF-1α was used as a chemotaxin. The results
demonstrated that DBM-MSCs possessed weaker migratory ability
compared with NBM-MSCs. Our research group and others have revealed
that the SDF-1α/CXC chemokine receptor (CXCR) axis has an important
role in mediating MSCs migration (45,46).
In addition, Wang et al (47) indicated that CXCR-4 and CXCR-7
receptors were co-expressed in BM-MSCs and synergistically promoted
BM-MSC migration. However, the in vitro migration assay
employed in the present study may not directly mimic the in
vivo conditions necessary for BM-MSCs migration. Hu et
al (48) demonstrated that
pretreatment of the BM-MSCs with the CXCR4 antagonist AMD3100
significantly inhibited the mobilization of BM-MSCs in vitro
and in vivo. Therefore, the decreased migratory capacity of
DBM-MSCs may be associated with reduced CXCR expression.
Paracrine capacity is one of the main mechanisms by
which MSCs exert their functions on damage repair, blood vessel
formation and blood supply (49).
In the present study, TGF and PDGF concentrations were detected in
cell culture supernatants. DBM-MSCs exhibited reduced TGF secretion
and increased PDGF secretion compared with NBM-MSCs. Ng et
al (50) reported that
paracrine TGF-β and PDGF signaling was essential for MSCs
differentiation and proliferation. TGF-β is significantly
associated with chondrogenesis, whereas PDGF is significantly
associated with adipogenesis and chondrogenesis. Although there
were differences in cytokine secretion between DBM-MSCs and
NBM-MSCs, no statistical significance was detected.
In conclusion, the results of the present study
provide a novel insight into the biological changes of BM-MSCs
obtained from mice preconditioned with DMOG. DBM-MSCs were similar
in aspects of cell morphology, immune phenotype, multilineage
differentiation, and TGF and PDGF secretion, but were slightly
distinct with regards to proliferative and migratory capacity
compared with NBM-MSCs; however, they may have therapeutic
potential for future stem cell therapy. In addition, the present
study suggested that DMOG may be used as a novel mobilization agent
in future clinical trials as no adverse effects were observed with
the mobilization of MSCs to the peripheral blood.
Acknowledgments
The present study was financially supported by
grants from the National Natural Science Foundation of China (grant
no. 81270566) and the Medicine and Technology Program of Zhejiang
Province (grant no. 2014KYA069).
Abbreviations:
|
DMOG
|
dimethyloxallyl glycine
|
|
MSCs
|
mesenchymal stem cells
|
|
BM-MSCs
|
bone marrow-derived mesenchymal stem
cells
|
|
DBM-MSCs
|
bone marrow-derived mesenchymal stem
cells from dimethyloxallyl glycine-preconditioned mice
|
|
NBM-MSCs
|
bone marrow-derived mesenchymal stem
cells from normal saline-treated mice
|
|
CCK-8
|
Cell Counting kit-8
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
TGF
|
transforming growth factor
|
|
PDGF
|
platelet-derived growth factor
|
|
HIF-1α
|
hypoxia-inducible factor-1α
|
|
ICR
|
Male Institute of Cancer Research
|
|
NS
|
normal saline
|
|
DMEM/F-12
|
Dulbecco's modified Eagle's
medium/Ham's F-12
|
|
FBS
|
fetal bovine serum
|
|
PBS
|
phosphate-buffered saline
|
|
DMEM-LG
|
Dulbecco's modified Eagle's medium-low
glucose
|
|
SDF-1α
|
stromal cell-derived factor-1α
|
|
PPARγ
|
peroxisome proliferator-activated
receptor-γ
|
References
|
1
|
Jaakkola P, Mole DR, Tian YM, Wilson MI,
Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji
M, Schofield CJ, et al: Targeting of HIF-alpha to the von
Hippel-Lindau ubiquitylation complex by O2-regulated prolyl
hydroxylation. Science. 292:468–472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ockaili R, Salloum F, Natarajan R, Jones
DG, Fisher BJ, Ghosh S, Fowler AA and Kukreja RC: Dimethyloxallyl
glycine-A competitive inhibitor of prolyl hydroxylases induces
cardioprotective effect via hypoxia inducible factor-1 alpha
stabilization in rabbits. Circulation. 108:219. 2003.
|
|
3
|
Ding H, Gao YS, Wang Y, Hu C, Sun Y and
Zhang CQ: Dimethyloxaloylglycine increases the bone healing
capacity of adipose-derived stem cells by promoting osteogenic
differentiation and angiogenic potential. Stem Cells Dev.
23:990–1000. 2014. View Article : Google Scholar :
|
|
4
|
Song YR, You SJ, Lee YM, Chin HJ, Chae DW,
Oh YK, Joo KW, Han JS and Na KY: Activation of hypoxia-inducible
factor attenuates renal injury in rat remnant kidney. Nephrol Dial
Transplant. 25:77–85. 2010. View Article : Google Scholar
|
|
5
|
Milkiewicz M, Pugh CW and Egginton S:
Inhibition of endogenous HIF inactivation induces angiogenesis in
ischaemic skeletal muscles of mice. J Physiol. 560:21–26. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuan Q, Bleiziffer O, Boos AM, Sun J,
Brandl A, Beier JP, Arkudas A, Schmitz M, Kneser U and Horch RE:
PHDs inhibitor DMOG promotes the vascularization process in the AV
loop by HIF-1a up-regulation and the preliminary discussion on its
kinetics in rat. BMC Biotechnol. 14:1122014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagel S, Papadakis M, Chen R, Hoyte LC,
Brooks KJ, Gallichan D, Sibson NR, Pugh C and Buchan AM:
Neuroprotection by dimethyloxalylglycine following permanent and
transient focal cerebral ischemia in rats. J Cereb Blood Flow
Metab. 31:132–143. 2011. View Article : Google Scholar :
|
|
8
|
Liu XB, Wang JA, Ji XY, Yu SP and Wei L:
Preconditioning of bone marrow mesenchymal stem cells by prolyl
hydroxylase inhibition enhances cell survival and angiogenesis in
vitro and after transplantation into the ischemic heart of rats.
Stem Cell Res Ther. 5:1112014. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beyer Nardi N and da Silva Meirelles L:
Mesenchymal stem cells: Isolation, in vitro expansion and
characterization. Handb Exp Pharmacol. 174:249–282. 2006.
View Article : Google Scholar
|
|
10
|
Deans RJ and Moseley AB: Mesenchymal stem
cells: Biology and potential clinical uses. Exp Hematol.
28:875–884. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sanchez-Ramos J, Song S, Cardozo-Pelaez F,
Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W,
Patel N, et al: Adult bone marrow stromal cells differentiate into
neural cells in vitro. Exp Neurol. 164:247–256. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bianco P, Riminucci M, Gronthos S and
Robey PG: Bone marrow stromal stem cells: Nature, biology, and
potential applications. Stem Cells. 19:180–192. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakagami H, Morishita R, Maeda K, Kikuchi
Y, Ogihara T and Kaneda Y: Adipose tissue-derived stromal cells as
a novel option for regenerative cell therapy. J Atheroscler Thromb.
13:77–81. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chunmeng S and Tianmin C: Skin: A
promising reservoir for adult stem cell populations. Med
Hypotheses. 62:683–688. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Bari C, Dell'Accio F, Vandenabeele F,
Vermeesch JR, Raymackers JM and Luyten FP: Skeletal muscle repair
by adult human mesenchymal stem cells from synovial membrane. J.
Cell Biol. 160:909–918. 2003. View Article : Google Scholar
|
|
16
|
Pierdomenico L, Bonsi L, Calvitti M,
Rondelli D, Arpinati M, Chirumbolo G, Becchetti E, Marchionni C,
Alviano F, Fossati V, et al: Multipotent mesenchymal stem cells
with immunosuppressive activity can be easily isolated from dental
pulp. Transplantation. 80:836–842. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamada Y, Fujimoto A, Ito A, Yoshimi R and
Ueda M: Cluster analysis and gene expression profiles: A cDNA
microarray system-based comparison between human dental pulp stem
cells (hDPSCs) and human mesenchymal stem cells (hMSCs) for tissue
engineering cell therapy. Biomaterials. 27:3766–3781. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL
and Chen TH: Isolation of multipotent mesenchymal stem cells from
umbilical cord blood. Blood. 103:1669–1675. 2004. View Article : Google Scholar
|
|
19
|
Pitchford SC, Hahnel MJ, Jones CP and
Rankin SM: Troubleshooting: Quantification of mobilization of
progenitor cell subsets from bone marrow in vivo. J Pharmacol
Toxicol Methods. 61:113–121. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alhadlaq A and Mao JJ: Mesenchymal stem
cells: Isolation and therapeutics. Stem Cells Dev. 13:436–448.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lazarus HM, Haynesworth SE, Gerson SL and
Caplan AI: Human bone marrow-derived mesenchymal (stromal)
progenitor cells (MPCs) cannot be recovered from peripheral blood
progenitor cell collections. J Hematother. 6:447–455.
1997.PubMed/NCBI
|
|
22
|
Wexler SA, Donaldson C, Denning-Kendall P,
Rice C, Bradley B and Hows JM: Adult bone marrow is a rich source
of human mesenchymal 'stem' cells but umbilical cord and mobilized
adult blood are not. Br J Haematol. 121:368–374. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roufosse CA, Direkze NC, Otto WR and
Wright NA: Circulating mesenchymal stem cells. Int J Biochem Cell
Biol. 36:585–597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Neth P, Ciccarella M, Egea V, Hoelters J,
Jochum M and Ries C: Wnt signaling regulates the invasion capacity
of human mesenchymal stem cells. Stem Cells. 24:1892–1903. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kumar S and Ponnazhagan S: Mobilization of
bone marrow mesenchymal stem cells in vivo augments bone healing in
a mouse model of segmental bone defect. Bone. 50:1012–1018. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deng J, Zou Z, Zhou T, Ai G, Wang J, Dong
S and Su S: The mobilization of rat mesenchymal stem cells into
peripheral blood by LiCL and its potency differentiation. Chinese
Science Bulletin. 53:2632–2638. 2008.
|
|
27
|
Liu W, Yu Q, Liu L, Zhou L and Hu S:
Effect of prolylhydroxylase inhibitor on mobilization of
mesenchymal stem cells in mice. Zhejiang Zhongyiyaodaxue Xuebao.
37:1371–1376. 2013.In Chinese.
|
|
28
|
Hu S, Yu Q, Liu L and Ge T: Mechanism of
HIF-1 signaling pathway in mediating MSCs mobilization with DMOG.
Zhongguo Bijiaoyixue Zazhi. 25:9–14. 2014.In Chinese.
|
|
29
|
National Research Council: Guide for the
care and use of laboratory animals. 7th edition. National Academies
Press; Washington, DC: 1996
|
|
30
|
Campagnoli C, Roberts IA, Kumar S, Bennett
PR, Bellantuono I and Fisk NM: Identification of mesenchymal
stem/progenitor cells in human first-trimester fetal blood, liver,
and bone marrow. Blood. 98:2396–2402. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu L, Yu Q, Lin J, Lai X, Cao W, Du K,
Wang Y, Wu K, Hu Y, Zhang L, et al: Hypoxia-inducible factor-1α is
essential for hypoxia-induced mesenchymal stem cell mobilization
into the peripheral blood. Stem Cells Dev. 20:1961–1971. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ivan M, Kondo K, Yang H, Kim W, Valiando
J, Ohh M, Salic A, Asara JM, Lane WS and Kaelin WG Jr: HIFalpha
targeted for VHL-mediated destruction by proline hydroxylation:
Implications for O2 sensing. Science. 292:464–468. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Noort WA, Oerlemans MIFJ, Rozemuller H,
Feyen D, Jaksani S, Stecher D, Naaijikens B, Martens AC, Bühring
HJ, Doevendans PA and Sluijter JPG: Human versus porcine
mesenchymal stromal cells: Phenotype, differentiation potential,
immunomodulation and cardiac improvement after transplantation. J
Cell Mol Med. 16:1827–1839. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lv FJ, Tuan RS, Cheung KM and Leung VY:
Concise review: The surface markers and identity of human
mesenchymal stem cells. Stem Cells. 32:1408–1419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sordi V, Malosio ML, Marchesi F, Mercalli
A, Melzi R, Giordano T, Belmonte N, Ferrari G, Leone BE, Bertuzzi
F, et al: Bone marrow mesenchymal stem cells express a restricted
set of functionally active chemokine receptors capable of promoting
migration to pancreatic islets. Blood. 106:419–427. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Laudes M: Role of WNT signalling in the
determination of human mesenchymal stem cells into preadipocytes. J
Mol Endocrinol. 46:R65–R72. 2011.PubMed/NCBI
|
|
38
|
Cristancho AG and Lazar MA: Forming
functional fat: A growing understanding of adipocyte
differentiation. Nat Rev Mol. Cell Biol. 12:722–734. 2011.
|
|
39
|
Lin GL and Hankenson KD: Integration of
BMP, Wnt, and notch signaling pathways in osteoblast
differentiation. J Cell Biochem. 112:3491–3501. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang W, Li B, Yang J, Xin L, Li Y, Yin H,
Qi Y, Jiang Y, Ouyang H and Gao C: The restoration of
full-thickness cartilage defects with BMSCs and TGF-beta 1 loaded
PLGA/fibrin gel constructs. Biomaterials. 31:8964–8973. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hirao M, Tamai N, Tsumaki N, Yoshikawa H
and Myoui A: Oxygen tension regulates chondrocyte differentiation
and function during endochondral ossification. J Biol Chem.
281:31079–31092. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guo W, Qian L, Zhang J, Zhang W, Morrison
A, Hayes P, Wilson S, Chen T and Zhao J: Sirt1 overexpression in
neurons promotes neurite outgrowth and cell survival through
inhibition of the mTOR signaling. J Neurosci Res. 89:1723–1736.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schultz K, Murthy V, Tatro JB and Beasley
D: Prolyl hydroxylase 2 deficiency limits proliferation of vascular
smooth muscle cells by hypoxia-inducible factor-1{alpha}-dependent
mechanisms. Am. J Physiol Lung Cell Mol Physiol. 296:L921–L927.
2009. View Article : Google Scholar
|
|
44
|
Liu XB, Wang JA, Ogle ME and Wei L: Prolyl
hydroxylase inhibitor dimethyloxalylglycine enhances mesenchymal
stem cell survival. J Cell Biochem. 106:903–911. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yu Q, Liu L, Lin J, Wang Y, Xuan X, Guo Y
and Hu S: SDF-1α/CXCR4 axis mediates the migration of mesenchymal
stem cells to the hypoxic-ischemic brain lesion in a rat model.
Cell J. 16:440–447. 2015.PubMed/NCBI
|
|
46
|
Peled A, Petit I, Kollet O, Magid M,
Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, et al:
Dependence of human stem cell engraftment and repopulation of
NOD/SCID mice on CXCR4. Science. 283:845–848. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang Y, Fu W, Zhang S, He X, Liu Z, Gao D
and Xu T: CXCR-7 receptor promotes SDF-1α-induced migration of bone
marrow mesenchymal stem cells in the transient cerebral
ischemia/reper-fusion rat hippocampus. Brain Res. 1575:78–86. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hu C, Yong X, Li C, Lü M, Liu D, Chen L,
Hu L, Teng M, Zhang D, Fan Y and Liang G: CXCL12/CXCR4 axis
promotes mesenchymal stem cell mobilization to burn wounds and
contributes to wound repair. J Surg Res. 183:427–434. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Doorn J, Moll G, Le Blanc K, van
Blitterswijk C and de Boer J: Therapeutic applications of
mesenchymal stromal cells: Paracrine effects and potential
improvements. Tissue Eng Part B Rev. 18:101–115. 2012. View Article : Google Scholar
|
|
50
|
Ng F, Boucher S, Koh S, Sastry KS, Chase
L, Lakshmipathy U, Choong C, Yang Z, Vemuri MC, Rao MS and Tanavde
V: PDGF, TGF-beta, and FGF signaling is important for
differentiation and growth of mesenchymal stem cells (MSCs):
Transcriptional profiling can identify markers and signaling
pathways important in differentiation of MSCs into adipogenic,
chondrogenic, and osteogenic lineages. Blood. 112:295–307. 2008.
View Article : Google Scholar : PubMed/NCBI
|