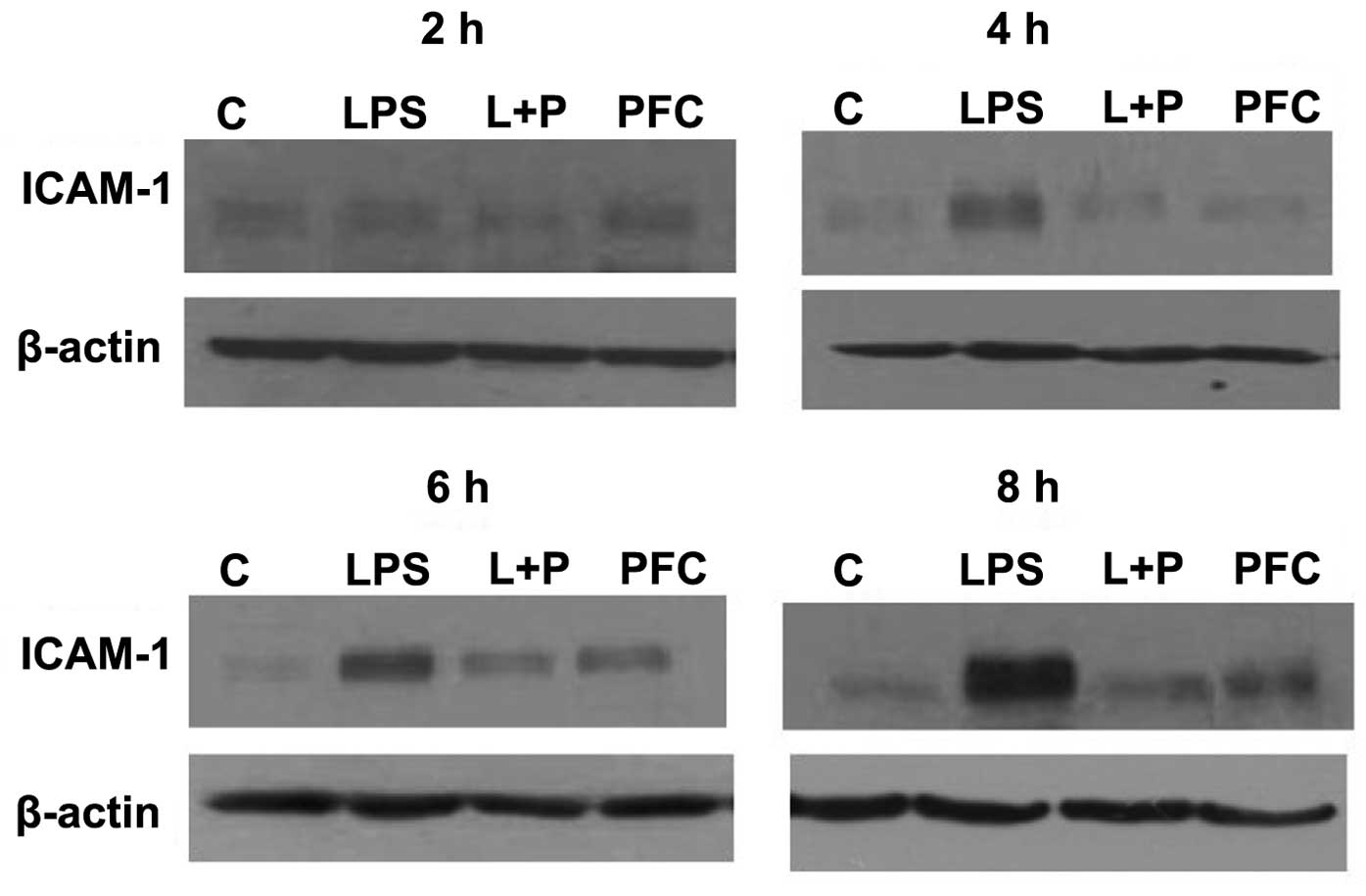
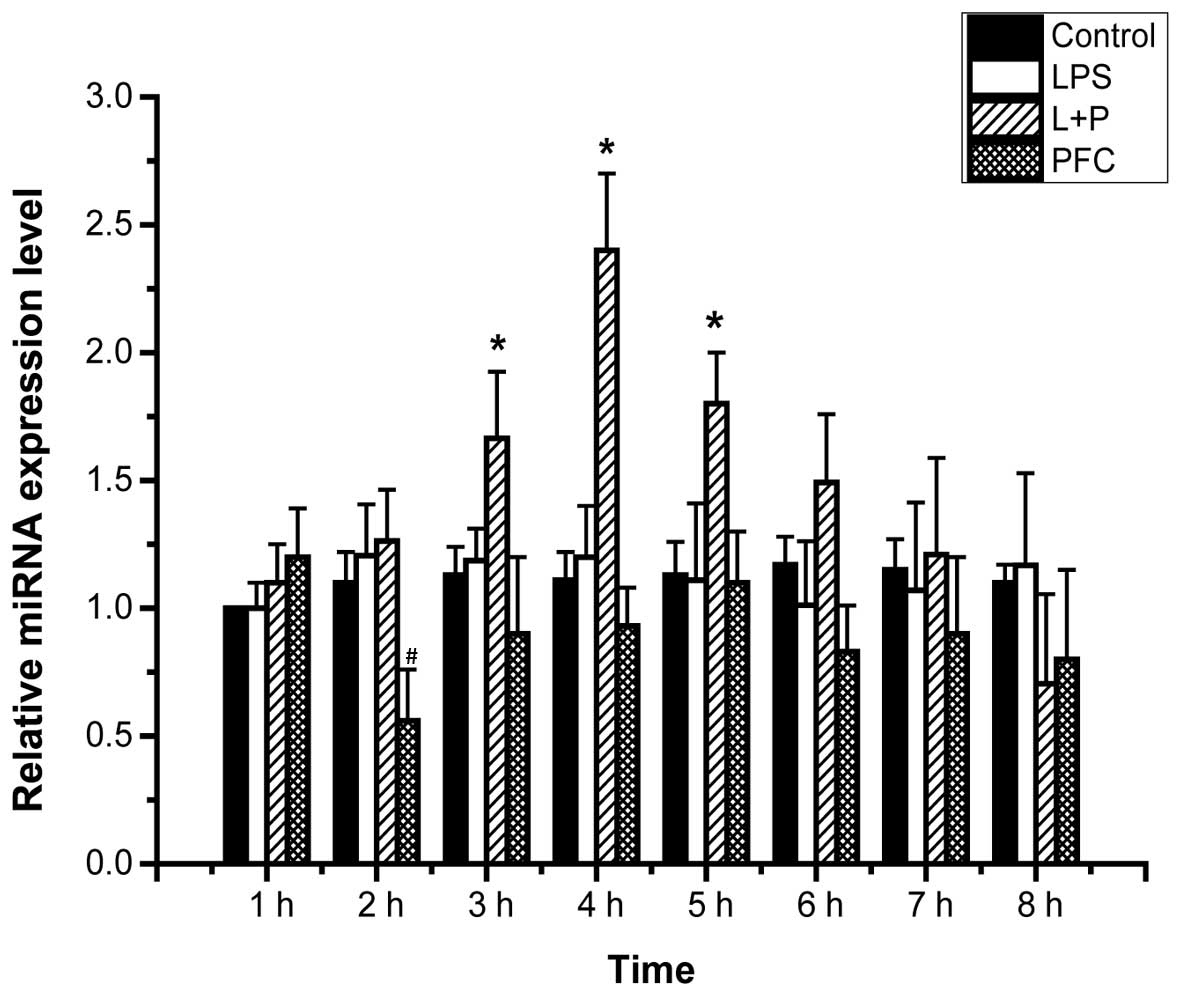
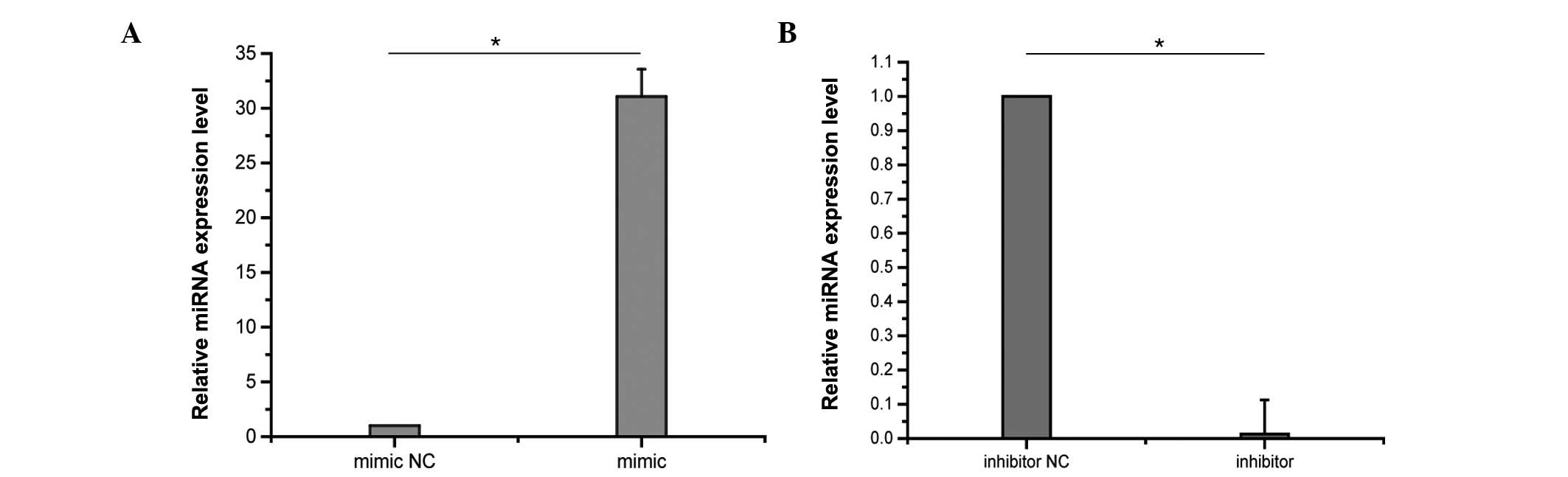
|
1
|
Dushianthan A, Grocott MP, Postle AD and
Cusack R: Acute respiratory distress syndrome and acute lung
injury. Postgrad Med J. 87:612–622. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matthay MA and Zemans RL: The acute
respiratory distress syndrome: Pathogenesis and treatment. Annu Rev
Pathol. 6:147–163. 2011. View Article : Google Scholar :
|
|
3
|
Ashbaugh DG, Bigelow DB, Petty TL and
Levine BE: Acute respiratory distress in adults. Lancet. 2:319–323.
1967. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blank R and Napolitano LM: Epidemiology of
ARDS and ALI. Crit Care Clin. 27:439–458. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rubenfeld GD, Caldwell E, Peabody E,
Weaver J, Martin DP, Neff M, Stern EJ and Hudson LD: Incidence and
outcomes of acute lung injury. N Engl J Med. 353:1685–1693. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wakabayashi T, Tamura M and Nakamura T:
Partial liquid ventilation with low-dose perfluoro chemical and
high-frequency oscillation improves oxygenation and lung compliance
in a rabbit model of surfactant depletion. Biol Neonate.
89:177–182. 2006. View Article : Google Scholar
|
|
7
|
Bleyl JU, Ragaller M, Tschö U, Regner M,
Hübler M, Kanzow M, Vincent O and Albrecht M: Changes in pulmonary
function and oxygenation during application of perfluorocarbon
vapor in healthy and oleic acid-injured animals. Crit Care Med.
30:1340–1347. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
von der Hardt K, Kandler MA, Brenn G,
Scheuerer K, Schoof E, Dötsch J and Rascher W: Comparison of
aerosol therapy with different perfluorocarbons in surfactant
depleted animals. Crit Care Med. 32:1200–1206. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schoof E, von der Hardt K, Kandler MA,
Abendroth F, Papadopoulos T, Rascher W and Dötsch J: Aerosolized
perfluorocarbon reduces adhesion molecule gene expression and
neutrophil sequestration in acute respiratory distress. Eur J
Pharmacol. 457:195–200. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
von der Hardt K, Kandler MA, Fink L,
Schoof E, Dotsch J, Bohle RM and Rascher W: Laser-assisted
microdissection and real-time PCR detect anti-inflammatory effect
of perfluorocarbon. Am J Physiol Lung Cell Mol Physiol.
285:L55–L62. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakstad B, Wolfson MR, Shaffer TH, Kähler
H, Lindemann R, Fugelseth D and Lyberg T: Perfluorochemical liquids
modulate cell-mediated inflammatory responses. Crit Care Med.
29:1731–1737. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakata S, Yasui K, Nakamura T, Kubota N
and Baba A: Perf luorocarbon suppresses lipopolysaccharide- and
alpha-toxin-induced interleukin-8 release from alveolar epithelial
cells. Neonatology. 91:127–133. 2007. View Article : Google Scholar
|
|
13
|
Wissel H, Burkhardt W, Rupp J, Wauer RR
and Rüdiger M: Perfluorocarbons decrease Chlamydophila
pneumoniae-mediated inflammatory responses of rat type II
pneumocytes in vitro. Pediatr Res. 60:264–269. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu SF, Wang P, Liu RJ, Zhao J, Zhang XN,
Fu ZZ, Gao LM, Liang ZX, Sun JP and Chen LA: Perfluorocarbon
attenuates lipopolysaccharide-mediated inflammatory responses of
alveolar epithelial cells in vitro. Chin Med J (Engl).
124:2534–2539. 2011.
|
|
15
|
Haeberle HA, Nesti F, Dieterich HJ,
Gatalica Z and Garofalo RP: Perflubron reduces lung inflammation in
respiratory syncytial virus infection by inhibiting chemokines
expression and nuclear factor-kappa B activation. Am J Respir Crit
Care Med. 165:1433–1438. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fernandez R, Sarma V, Younkin E, Hirschl
RB, Ward PA and Younger JG: Exposure to perflubron is associated
with decreased Syk phosphorylation in human neutrophils. J Appl
Physiol (1985). 91:1941–1947. 2001.
|
|
17
|
Rossman JE, Caty MG, Rich GA,
Karamanoukian HL and Azizkhan RG: Neutrophil activation and
chemotaxis after in vitro treatment with perfluorocarbon. J Pediatr
Surg. 31:1147–1151; discussion 1150–1151. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van de Stolpe A and van der Saag PT:
Intercellular adhesion molecule-1. J Mol Med (Berl). 74:13–33.
1996. View Article : Google Scholar
|
|
19
|
Kang BH, Crapo JD, Wegner CD, Letts LG and
Chang LY: Intercellular adhesion molecule-1 expression on the
alveolar epithelium and its modification by hyperoxia. Am J Respir
Cell Mol Biol. 9:350–355. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ware LB and Matthay MA: The acute
respiratory distress syndrome. N Engl J Med. 342:1334–1349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reutershan J and Ley K: Bench-to-bedside
review: Acute respiratory distress syndrome-how neutrophils migrate
into the lung. Crit Care. 8:453–461. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mendez MP, Morris SB, Wilcoxen S, Greeson
E, Moore B and Paine R III: Shedding of soluble ICAM-1into the
alveolar space in murine models of acute lung injury. Am J Physiol
Lung Cell Mol Physiol. 290:L962–L970. 2006. View Article : Google Scholar
|
|
23
|
Beck-Schimmer B, Schimmer RC, Warner RL,
Schmal H, Nordblom G, Flory CM, Lesch ME, Friedl HP, Schrier DJ and
Ward PA: Expression of lung vascular and airway ICAM-1 after
exposure to bacterial lipopolysaccharide. Am J Respir Cell Mol
Biol. 17:344–352. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Agouridakis P, Kyriakou D, Alexandrakis
MG, Prekates A, Perisinakis K, Karkavitsas N and Bouros D: The
predictive role of serum and bronchoalveolar lavage cytokines and
adhesion molecules for acute respiratory distress syndrome
development and outcome. Respir Res. 3:252002. View Article : Google Scholar
|
|
25
|
Calfee CS, Eisner MD, Parsons PE, Thompson
BT, Conner ER Jr, Matthay MA and Ware LB; NHLBI acute respiratory
distress syndrome clinical trials network: Soluble intercellular
adhesion molecule-1 and clinical outcomes in patients with acute
lung injury. Intensive Care Med. 35:248–257. 2009. View Article : Google Scholar :
|
|
26
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bushati N and Cohen SM: microRNA
functions. Annu Rev Cell Dev Biol. 23:175–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
O'Connell RM, Rao DS and Baltimore D:
microRNA regulation of inflammatory responses. Annu Rev Immunol.
30:295–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Suárez Y, Wang C, Manes TD and Pober JS:
Cutting Edge: TNF-induced micrornas regulate TNF-induced expression
of e-selectin and intercellular adhesion molecule-1 on human
endothelial cells: Feedback control of inflammation. J Immunol.
184:21–25. 2010. View Article : Google Scholar
|
|
31
|
Gabriel JL, Miller TF Jr, Wolfson MR and
Shaffer TH: Quantitative structure-activity relationships of
perfluorinated heterohydrocarbons as potential respiratory media.
Application to oxygen solubility, partition coefficient, viscosity,
vapor pressure and density. ASAIO J. 42:968–973. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wemhöner A, Hackspiel I, Hobi N, Ravasio
A, Haller T and Rüdiger M: Effects of perfluorocarbons on
surfactant exocytosis and membrane properties in isolated alveolar
type II cells. Resp Res. 11:522010. View Article : Google Scholar
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
34
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu M: Alveolar epithelium in host
defense: Cytokine production. Sepsis and Organ Dysfunction, from
Chaos to Rationale. Baue AE, Berlot G, Gullo A and Vincent JL:
Springer-Verlag Mailand; Milan, Italy: pp. 37–50. 2001
|
|
36
|
Simon RH and Paine R III: Participation of
pulmonary alveolar epithelial cells in lung inflammation. J Lab
Clin Med. 126:108–118. 1995.PubMed/NCBI
Zhang X, Wu D and Jiang X: Icam-1 and
acute pancreatitis complicated by acute lung injury. JOP. 10:8–14.
2009.PubMed/NCBI
|
|
37
|
Mendez MP, Morris SB, Wilcoxen S, Greeson
E, Moore B and Paine R III: Shedding of soluble ICAM-1 into the
alveolar space in murine models of acute lung injury. Am J Physiol
Lung Cell Mol Physiol. 290:L962–L970. 2006. View Article : Google Scholar
|
|
38
|
Zhang XD, Hou JF, Qin XJ, Li WL, Chen HL,
Liu R, Liang X and Hai CX: Pentoxifylline inhibits intercellular
adhesion molecule-1 (ICAM-1) and lung injury in experimental
phosgene-exposure rats. Inhal Toxicol. 22:889–895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sumagin R, Lomakina E and Sarelius IH:
Leukocyte endothelial cell interactions are linked to vascular
permeability via ICAM-1-mediated signaling. Am J Physiol Heart Circ
Physiol. 295:H969–H977. 2008. View Article : Google Scholar
|
|
40
|
van de Stolpe A and van der Saag PT:
Intercellular adhesion molecule-1. J Mol Med (Berl). 74:13–33.
1996. View Article : Google Scholar
|
|
41
|
Albelda SM, Smith CW and Ward PA: Adhesion
molecules and inflammatory injury. FASEB J. 8:504–512.
1994.PubMed/NCBI
|
|
42
|
Hopkins AM, Baird AW and Nusrat A: ICAM-1:
Targeted docking for exogenous as well as endogenous ligands. Adv
Drug Deliv Rev. 56:763–778. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ware LB, Koyama T, Billheimer DD, Wu W,
Bernard GR, Thompson BT, Brower RG, Standiford TJ and Martin TR:
Prognostic and pathogenetic value of combining clinical and
biochemical indices in patients with acute lung injury. Chest.
137:288–296. 2010. View Article : Google Scholar :
|
|
44
|
Calfee CS, Eisner MD, Parsons PE, Thompson
BT, Conner ER Jr, Matthay MA and Ware LB; NHLBI acute respiratory
distress syndrome clinical trials network: Soluble intercellular
adhesion molecule-1 and clinical outcomes in patients with acute
lung injury. Intensive Care Med. 35:248–257. 2009. View Article : Google Scholar :
|
|
45
|
Zhang X, Ladd A, Dragoescu E, Budd WT,
Ware JL and Zehner ZE: MicroRNA-17-3p is a prostate tumor
suppressor in vitro and in vivo and is decreased in high grade
prostate tumors analyzed by laser capture microdissection. Clin Exp
Metastasis. 26:965–979. 2009. View Article : Google Scholar
|
|
46
|
Jiang X and Li N: Induction of MiR-17-3p
and MiR-106a by TNFα and LPS. Cell Biochem Funct. 29:164–170. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shan SW, Fang L, Shatseva T, Rutnam ZJ,
Yang X, Du W, Lu WY, Xuan JW, Deng Z and Yang BB: Mature miR-17-5p
and passenger miR-17-3p induce hepatocellular carcinoma by
targeting PTEN, GalNT7 and vimentin in different signal pathways. J
Cell Sci. 126:1517–1530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li H and Yang BB: Stress response of
glioblastoma cells mediated by miR-17-5p targeting PTEN and the
passenger strand miR-17-3p targeting MDM2. Oncotarget. 3:1653–1668.
2012. View Article : Google Scholar
|
|
49
|
Yin R, Wang R, Guo L, Zhang W and Lu Y:
MiR-17-3p inhibits angiogenesis by downregulating Flk-1 in the cell
growth signal pathway. J Vasc Res. 50:157–166. 2013. View Article : Google Scholar
|
|
50
|
Ng EK, Chong WW, Jin H, Lam EK, Shin VY,
Yu J, Poon TC, Ng SS and Sung JJ: Differential expression of
microRNAs in plasma of patients with colorectal cancer: A potential
marker for colorectal cancer screening. Gut. 58:1375–1381. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Faltejskova P, Bocanek O, Sachlova M,
Svoboda M, Kiss I, Vyzula R and Slaby O: Circulating miR-17-3p,
miR-29a, miR-92a and miR-135b in serum: Evidence against their
usage as biomarkers in colorectal cancer. Cancer Biomark.
12:199–204. 2012.PubMed/NCBI
|
|
52
|
Mendell JT: miRiad roles for the miR-17-92
cluster in development and disease. Cell. 133:217–222. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mogilyansky E and Rigoutsos I: The
miR-17/92 cluster: A comprehensive update on its genomics,
genetics, functions and increasingly important and numerous roles
in health and disease. Cell Death Differ. 20:1603–1614. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang ZW, An Y and Teng CB: The roles of
miR-17-92 cluster in mammal development and tumorigenesis. Yi
Chuan. 31:1094–1100. 2009.In Chinese. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Takakura S, Mitsutake N, Nakashima M,
Namba H, Saenko VA, Rogounovitch TI, Nakazawa Y, Hayashi T, Ohtsuru
A and Yamashita S: Oncogenic role of miR-17-92 cluster in
anaplastic thyroid cancer cells. Cancer Sci. 99:1147–1154. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Attar M, Arefian E, Nabiuni M, Adegani FJ,
Bakhtiari SH, Karimi Z, Barzegar M and Soleimani M: MicroRNA 17-92
expressed by a transposone-based vector changes expression level of
cell-cycle-related genes. Cell Biol Int. 36:1005–1012. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hirschl RB, Croce M, Gore D, Wiedemann H,
Davis K, Zwischenberger J and Bartlett RH: Prospective, randomized,
controlled pilot study of partial liquid ventilation in adult acute
respiratory distress syndrome. Am J Respir Crit Care Med.
165:781–787. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kacmarek RM, Wiedemann HP, Lavin PT, Wedel
MK, Tütüncü AS and Slutsky AS: Partial liquid ventilation in adult
patients with acute respiratory distress syndrome. Am J Respir Crit
Care Med. 173:882–889. 2006. View Article : Google Scholar
|
|
59
|
Kaushal A, McDonnell CG and Davies MW:
Partial liquid ventilation for the prevention of mortality and
morbidity in paediatric acute lung injury and acute respiratory
distress syndrome. Cochrane Database Syst Rev.
2:CD0038452013.PubMed/NCBI
|