Introduction
The thecoma-fibroma group, a subgroup of ovarian sex
cord-stromal tumors, encompasses a spectrum of neoplasms ranging
from those entirely composed of lipid-containing cells resembling
theca interna cells to those containing predominantly
spindle-shaped cells with variable inter-cellular collagen.
Although these tumors are benign and relatively rare, accounting
for 3–4% of all ovarian tumors (1), they represent the most common type of
solid primary ovarian tumors. Fibromas are purely composed of
mature fibroblastic cells producing abundant collagen, whereas
thecomas contain numerous cells resembling theca and/or lutein
cells and a number of fibroblasts. However, it is occasionally
difficult to differentiate between fibroma and thecoma, thus
justifying the use of the term thecoma-fibroma tumor. There have
been several studies of thecoma-fibroma tumors with positive F-18
fluorodeoxyglucose (FDG) accumulation mimicking malignant ovarian
tumors (2,3). To the best of our knowledge, none of
those studies described the detailed causes of F-18 FDG
accumulation. Therefore, it was hypothesized that these
false-positive findings may be associated with tumor vascularity
and/or proliferation as a number of thecoma-fibroma tumors appear
as hypovascular tumors with delayed weak enhancement in dynamic
contrast magnetic resonance imaging (MRI) studies (4). Furthermore, cellular fibroma is known
as to possess an uncertain malignant potential (5); in other words, imbalances between the
cell density and blood supply, malignant formation, and/or other
conditions may induce F-18 FDG accumulation by altering the glucose
metabolism. In this study, the cause of F-18 FDG accumulation in
thecoma-fibroma tumors was investigated by addressing passive tumor
ischemia/hypoxia and malignant potential in these tumors and
comparing these tumors with F-18 FDG-negative fibromas and
malignant ovarian tumors.
Materials and methods
Patients
The Research Ethics Committee of the Hirosaki
University Graduate School of Medicine/University Hospital
(Hirosaki, Japan) approved this retrospective study and waived the
requirement for individual patient consent. From 2008 to 2013, 78
female patients underwent preoperative F-18 FDG positron emission
tomography (PET)/computed tomography (CT) scans for various ovarian
tumors at the Hirosaki University Hospital. Of these 78 cases, 46
were pathologically proven to be malignant and 32 were benign. The
sensitivity and specificity of the PET findings were 93.5 and
83.9%, respectively, for distinguishing malignant ovarian tumors
from benign ovarian lesions using a maximum standard uptake value
(SUVmax) cutoff of 2.5. Of the 32 benign cases, six were analyzed
as they showed positive FDG accumulation mimicking malignant
ovarian tumors (four cases of thecoma-fibroma tumors and two cases
of xanthogranulomatous inflammation) with positive F-18 FDG
accumulation. The details of the four FDG-positive thecoma-fibroma
tumor cases are shown in Table I.
One case (fibroma, SUVmax=4.0) exhibited mild cystic degeneration,
whereas the other three cases (cellular fibroma, SUVmax=5.2;
thecoma-fibroma tumor, SUVmax=2.7; and fibroma, SUVmax=4.1)
exhibited severe cystic degeneration. For comparison, two fibroma
cases with negative F-18 FDG accumulation (Table II) were selected and eight
malignant ovarian tumor cases (SUVmax <5.5) without metastasis
or invasion and with undetermined malignant statuses according to
SUV max alone due to the relatively low values (Table III).
 | Table IBenign F-18
fluorodeoxyglucose-positive cases (n=4). |
Table I
Benign F-18
fluorodeoxyglucose-positive cases (n=4).
| Patient | Age (years) | Pathology Tumor
form | | Size (cm) | Ascites | Hyperestrogenism | CA125 |
|---|
| 1 | 67 | Fibroma | SWCC | 15 | Little | None | 186 |
| 2 | 56 | Cellular fibroma | CWSC | 5 | Massive | None | 16 |
| 3 | 69 | Thecoma-fibroma
tumors | CWSC | 20 | Little | None | 144 |
| 4 | 67 | Fibroma | CWSC | 21 | Massive | None | 813 |
 | Table IIBenign F-18
fluorodeoxyglucose-negative cases (n=2). |
Table II
Benign F-18
fluorodeoxyglucose-negative cases (n=2).
| Patient | Age (years) | Pathology | Tumor form | Size (cm) | Ascites | Hyperestrogenism | CA125 |
|---|
| 1 | 31 | Fibroma | Solid | 13 | Little | None | 24 |
| 2 | 51 | Fibroma | Solid | 4 | None | None | 10 |
 | Table IIIMalignant cases (n=8). |
Table III
Malignant cases (n=8).
| Patient | Age (years) | Pathology | Tumor form | Size (cm) | Ascites | SUVmax | CA125 |
|---|
| 1 | 59 | Serous
adenocarcinoma | CWSC | 15 | None | 4.8 | 11 |
| 2 | 46 | Serous
adenocarcinoma | CWSC | 15 | Little | 2.4 | 101 |
| 3 | 29 | Serous
adenocarcinoma | CWSC | 13 | Little | 5.2 | 10 |
| 4 | 36 | Serous
adenocarcinoma | Cystic | 17 | Little | <1.0 | 11 |
| 5 | 37 | Serous
adenocarcinoma | CWSC | 3 | None | 3.9 | 30 |
| 6 | 61 | Clear cell
adenocarcinoma | SWCC | 9 | None | 4.7 | 40 |
| 7 | 59 | Clear cell
adenocarcinoma | CWSC | 20 | Little | 4.5 | 526 |
| 8 | 34 | Mucinous
adenocarcinoma | CWSC | 16 | None | 3.5 | 89 |
F-18 FDG PET/CT and image analysis
In preparation for PET/CT, all patients fasted for
at least 4 h and water intake was encouraged. F-18 FDG (FDG scan
injectable, 185 MBq on the assay date; Nihon Medi-Physics, Tokyo,
Japan) was delivered via intravenous injection ~60 min prior to the
initiation of scanning. During the 60-min uptake period, the
patients were advised to drink a sufficient amount of water and to
remain calm. A PET/CT system (Discovery ST Elite 16; GE Healthcare,
Milwaukee, WI, USA) was used to acquire all data in 7–8 bed
positions with an acquisition time of 2.5–3.0 min per bed position.
CT was performed first (30–80 mA, 120 kV, 3.75–3.27-mm slice
thickness). The CT data were used for attenuation correction of PET
data as well as for co-registration with the attenuation-corrected
PET images. The PET data of the same body regions were acquired
immediately following CT imaging. The PET, CT, and fused PET/CT
images were available for review and were displayed in the axial,
coronal, and sagittal planes on a viewer system (Discovery ST Elite
16; GE Healthcare). SUVmax (g/ml) was evaluated in all
histopathologically proven lesions, as described previously
(4).
MRI and image analysis
MRI examinations were performed on a 3.0-T unit
(Signa HDxt, GE Healthcare) or a 1.5-T unit (Magnetom Vision;
Siemens AG, Erlangen, Germany or Signa HDxt).
The MRI characteristics of each thecoma-fibroma
tumor were recorded separately and included the following items:
Lesion components (solid, cystic, solid with cystic components, or
cystic with solid components), signal intensity on T2-weighted
imaging (WI) (hypointensity, isointensity, or hyperintensity), and
gadopentetate dimeglumine (Gd-DTPA) enhancement (mild, moderate or
severe). The signal intensity of each lesion on T2WI was
quantitatively compared with that of the uterine myometrium and
iliopsoas muscle. On T2WI, the hypointensity and hyperintensity of
the pelvic wall muscle and fat signals were similar. After the
intravenous injection of contrast medium, the degree of lesion
enhancement was graded as follows: Mild enhancement (less than the
myometrium), moderate enhancement (similar to the myometrium), or
severe enhancement (greater than the myometrium).
Histological analysis
For the histological examination, specimens of the
six thecoma-fibroma tumors and eight malignant tumors were
routinely fixed in formalin, embedded in paraffin, sectioned into
thin slices, and stained with hematoxylineosin. Thin (4-μm)
sections were mounted on silane-coated glass slides (Matsunami
Glass Industry, Ltd., Osaka, Japan). A standard automated
immunostainer (Benchmark XT; Ventana Medical Systems, Tucson, AZ,
USA) was used to perform an immunohistochemical examination of the
deparaffinized sections. The following antibodies were used:
Polyclonal rabbit anti-human glucose transporter 1 (GLUT1; 1:200
dilution; cat. no. ab15309; Abcam, Cambridge, UK), monoclonal mouse
anti-human L-type amino acid transporter 1 (LAT-1; 1:50; clone
LAT-1; cat. no. M7279; Dako, Glostrup, Denmark), monoclonal mouse
anti-human hypoxia-inducible factor 1α (HIF-1α; 1:100; clone
HIFa67; cat. no. MAB5382; Millipore, Billerica, MA, USA),
polyclonal rabbit anti-human vascular endothelial growth factor
(VEGF; 1:100; cat. no. A-20; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA), and monoclonal mouse anti-human Ki-67 (1:100; clone
MIB-1; cat. no. M7248; Dako). The GLUT1, LAT1, HIF-1α, and VEGF
immunohistochemical reactions were semiqualitatively scored as
follows: 0, negative; 1, weak; 2, intermediate; and 3, strong.
Images of the immunohistochemical analyses were captured using a
BX50 microscope and DP70 digital camera (Olympus Corporation,
Tokyo, Japan).
Reverse transcription-polymerase chain
reaction (RT-PCR)
For RNA preparation from the formalin-fixed,
paraffin-embedded (FFPE) fibroma-thecoma tumor tissue sections, the
RNeasy FFPE kit (Qiagen GmbH, Hilden, Germany) was used according
to the manufacturer's instructions. RT-PCR of an aliquot of
first-strand cDNA as the template was performed under standard
conditions with Taq DNA polymerase (Qiagen GmbH). The primers were
as follows: HIF-1α-F: 5′-AGCCCTAGATGGCTTTGTGA-3′, HIF-1α-R:
5′-TATCGAGGCTGTGTCGACTG-3′, GAPDH-F:
5′-CCACCCATGGCAAATTCCATGGCA-3′, and GAPDH-R:
5′-AGACCACCTGGTGCTCAGTGTAGC-3′. The amplified products of the
HIF-1α and GAPDH primers were 466 and 696 base pairs in length,
respectively. HIF-1α and GAPDH cDNA were amplified for up to 25
cycles (94°C for 40 sec, 59°C for 40 sec and 72°C for 40 sec) using
a C1000 Touch Thermal Cycler (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The PCR products were separated on 1.5% (w/v) agarose
gels (UltraPure Agarose; Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA).
Results
Imaging and pathological findings of
thecoma-fibroma tumors
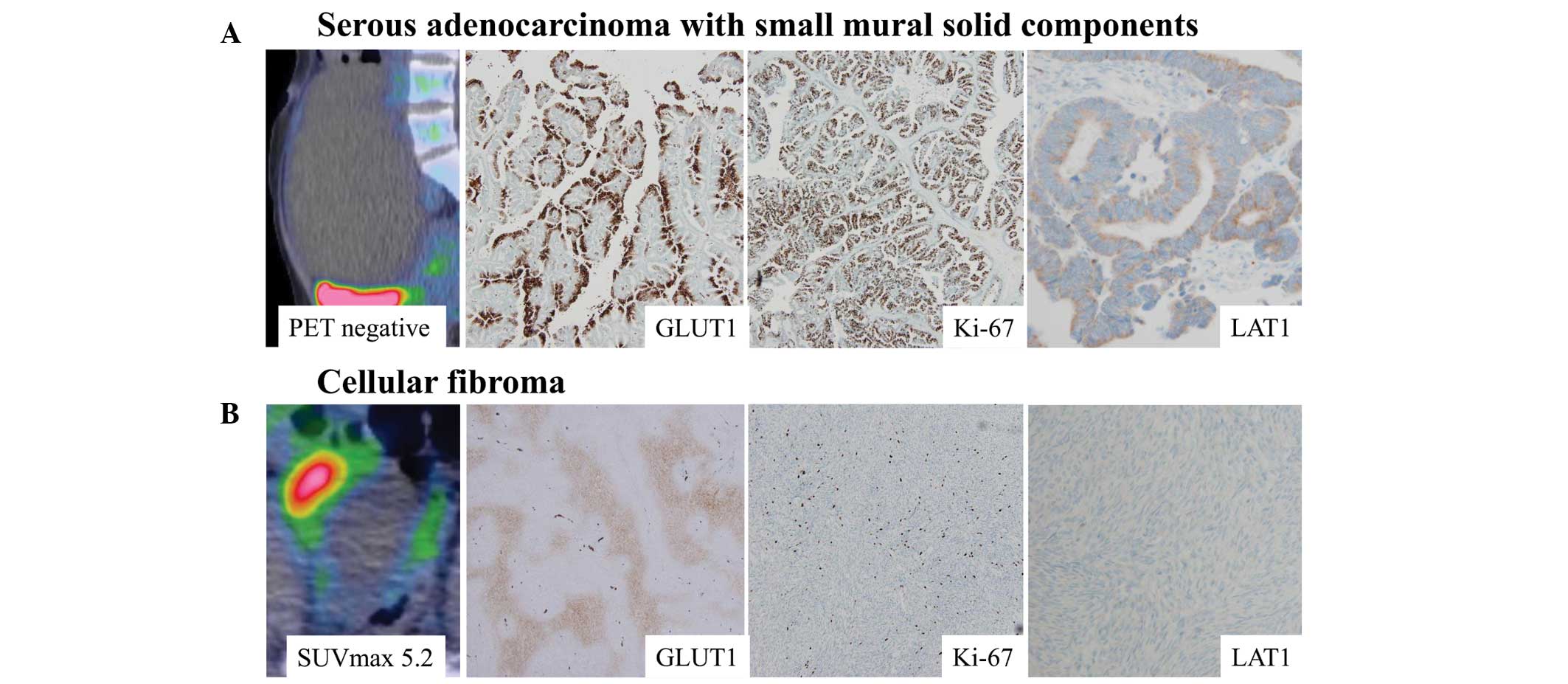
The four false-positive cases exhibited higher
cellularity, SUVmax, signal intensity on T2WI, and Gd-DTPA
enhancement on MRI than the two negative fibroma cases (Table IV). Increases in FDG uptake and
Gd-DTPA enhancement in the tumor tended to occur near the areas of
severe cystic degeneration. Histologically, the four false-positive
tumors comprised an area of high cellularity and an edematous or
degenerated hypocellular area. In the cellular area, spindle-shaped
tumor cells were randomly distributed or arranged in a fascicular
manner. By contrast, the edematous or degenerated area contained
scattered spindle or stellate cells without atypia (Fig. 1).
 | Table IVImaging and pathological findings of
thecoma-fibroma tumors (n=6). |
Table IV
Imaging and pathological findings of
thecoma-fibroma tumors (n=6).
| PET | Pathological
findings | Size (cm) | SUVmax | T2WI | Gd-DTPA
enhancement |
|---|
| Negative | F>HC | 13 | 2.2 | Low intensity | Mild |
| Negative | F>HC | 4 | 2.4 | Low intensity | Mild |
| Positive | HC>F | 15 | 4.0 | High intensity | Moderate |
| Positive | HC>F | 5 | 5.2 | High intensity | Severe |
| Positive | HC>F | 20 | 2.7 | High intensity | Severe |
| Positive | HC>F | 21 | 4.1 | High intensity | Severe |
Immunohistochemical findings and RT-PCR
analysis
SUVmax (Fig. 2A)
and levels of HIF-1α, VEGF, LAT1, Ki-67, and GLUT1 expression in
the malignant ovarian tumors tended to be higher than those in the
false-positive and negative thecoma-fibroma tumors. The
false-positive thecoma-fibroma group exhibited low levels of Ki-67
expression and no LAT1 expression. However, these lesions expressed
considerable quantities of GLUT1, HIF-1α and VEGF. In the two cases
of FDG-negative fibromas, levels of HIF-1α, VEGF, LAT1, Ki-67, and
GLUT1 expression were low or inconclusive. In the malignant ovarian
tumors, the levels of HIF-1α, VEGF, LAT1, Ki-67 and GLUT1
expression varied from high to low (Fig. 2B–F).
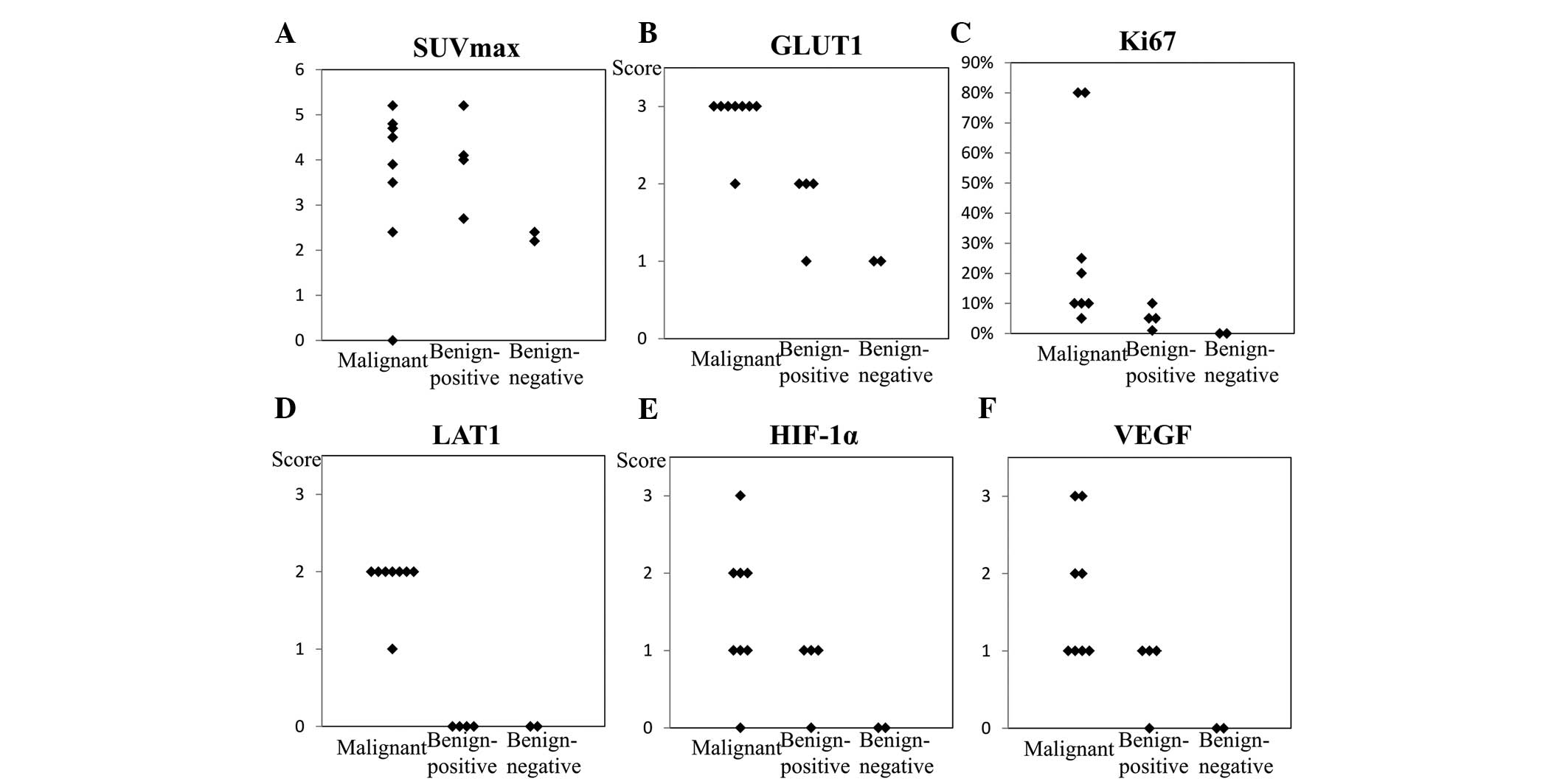 | Figure 2SUVmax (A) and immunohistochemical
analysis findings of (B) GLUT1, (C) Ki-67, (D) LAT1, (E) HIF-1α and
(F) VEGF expression in the imaged lesions. In the malignant ovarian
tumors, HIF-1α, VEGF, LAT1, Ki-67, and GLUT1 expression tended to
be relatively higher than that in the thecoma-fibroma group. In the
F-18 FDG-positive thecoma-fibroma group, Ki-67 expression was low
and LAT1 expression was absent, thereby excluding the possibility
of malignancy in these lesions. However, considerable GLUT1,
HIF-1α, and VEGF expression were observed. In the two cases of F-18
FDG-negative fibroma, HIF-1α, VEGF, LAT1, Ki-67 and GLUT1
expression levels were low or inconclusive. SUV, standard uptake
value; GLUT1, glucose transporter 1; LAT1, L-type amino acid
transporter 1; HIF-1α, hypoxia-inducible factor 1α; FDG,
fluorodeoxyglucose. |
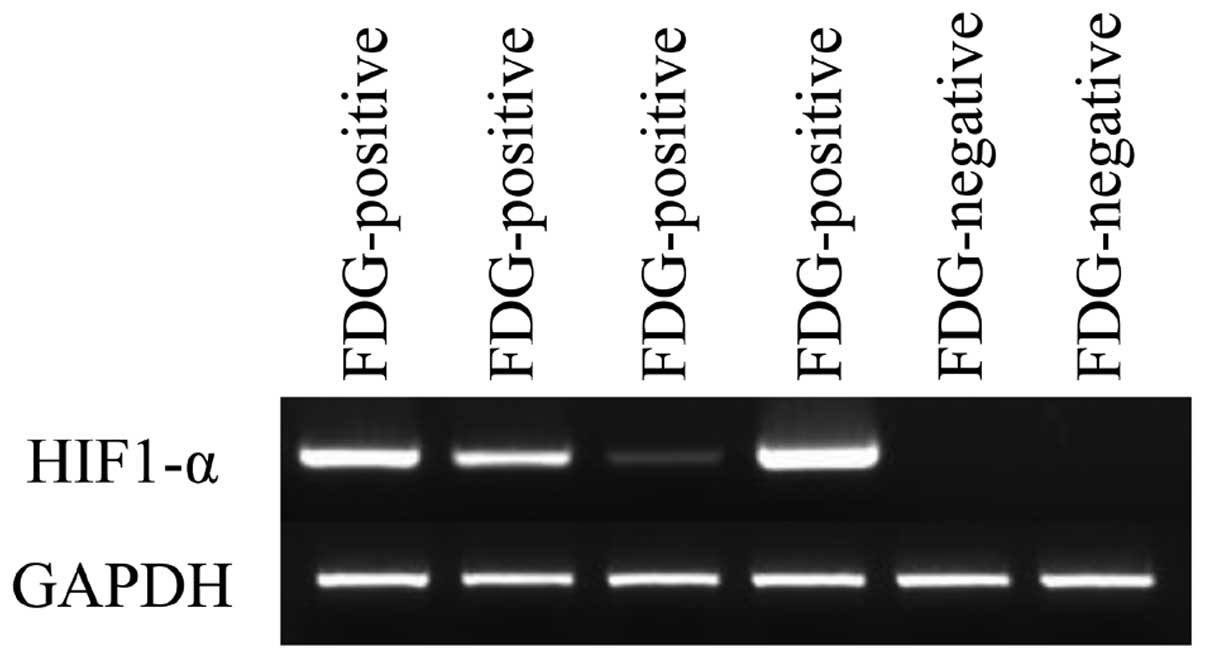
HIF-1α expression was elevated in all F-18
FDG-positive thecoma-fibroma tumors (Fig. 3). By contrast, HIF-1α expression
was not observed in any FDG-negative fibromas. Thecoma-fibroma
tumors with high cellular components exhibited positive F-18 FDG
accumulation and immunohistochemical GLUT1 expression, whereas
negative Ki-67 and LAT1 expression was observed (Fig. 4).
Discussion
In FDG PET, accumulation is determined by the
delivery of FDG to the tissues, as well as the expression and
activity levels of glucose transporter (GLUT) and hexokinase (HK).
HIF-1 activates the transcription of GLUT1 and GLUT3, as well as
HK, the first enzyme in the glycolytic pathway (6). HIF-l, a heterodimer composed of two
subunits termed HIF-lα and HIF-lβ, was identified following the
identification of a hypoxia response element (HRE) (7,8).
HIF-lα expression remains low under physiological oxygen pressure
in normal tissues and increases in response to systemic hypoxia,
whereas HIF-1β is constitutively expressed regardless of the oxygen
availability. Under hypoxic conditions, the HIF-1α protein is
stabilized and initiates a multistep activation pathway that
includes nuclear translocation, dimerization with HIF-1β,
transcriptional coactivator recruitment, and subsequent binding to
HREs of target genes (9). HIF-lα,
which induces the expression of erythropoietin (EPO) (10) and other oxygen-regulated genes,
such as VEGF (11,12) and GLUT1 (13), and several glycolytic enzymes
(14–16), has been shown to be important in
various types of cancer through processes, such as tumor
proliferation, tumor invasion, inflammation and ischemia (17–19).
The results of the present study demonstrated that HIF-1α
expression was increased in all four F-18 FDG-positive
thecoma-fibroma tumors. Accordingly, it was suggested that the
upregulated HIF-1α expression in these F-18 cases was correlated
with increased GLUT1 and VEGF expression, thereby leading to F-18
FDG accumulation and stronger Gd-DTPA enhancement in MRI compared
with the two negative fibroma cases.
In addition, elevated cellularity was also observed
in all four F-18 FDG-positive thecoma-fibroma tumors. The majority
of thecoma-fibroma tumor cases involve hypovascular tumors, as
determined by delayed weak enhancement in dynamic contrast MRI
studies. Therefore, it was hypothesized that this increased
cellularity induces ischemic changes and hypoxia in the tumor,
thereby upregulating HIF-1α expression. The hypoxia consequent to
this increased cellularity stimulated HIF-1α expression, leading to
subsequent responses, such as GLUT1 and VEGF expression and
resulting in F-18 FDG accumulation and strong enhancement. Notably,
within the same tumor, the areas of F-18 FDG accumulation and
stronger Gd-DTPA enhancement were observed near areas of severe
cystic or hyaline degeneration, where hypoxia would be expected. A
recent study revealed novel O2-independent regulatory
mechanisms of HIF transactivation such as mammalian target of
rapamycin (mTOR) activation or altered mitochondrial metabolism,
NAD+ levels, and nitric oxide levels (20); however, no studies have discussed
the correlation between glucose metabolism and
O2-independent HIF regulation in thecoma-fibroma
tumors.
Although thecoma-fibroma tumors are rarely
malignant, certain tumor types included in the thecoma-fibroma
group, such as cellular fibroma, have been recognized as having
uncertain malignant potential (21). Similarly, malignant transformation
induces hypoxia and consequently upregulates HIF-1α expression.
However, LAT-1 and Ki-67 labeling index immunostaining did not
suggest malignant potential in the four false-positive cases. LAT-1
is specifically expressed in malignant tumors (22), and the Ki-67 labeling index is a
classic cellular proliferation marker. Thus, if malignant potential
had been detected in the false-positive cases, levels of LAT-1 or
Ki-67 expression should have been increased. Lee et al
(23) noted that the luteinized
theca-like cells, which may continuously secrete vascular
permeability factors and VEGF, thereby inducing angiogenesis, led
to hypervascularity in ovarian sex cord-stromal tumors. However,
this theory does not explain all four of the false-positive cases
as only one thecoma-fibroma tumor case contained theca cells.
Furthermore, clear differences in VEGF expression between the theca
and fibroma cells were not observed in the thecoma-fibroma
tumors.
One limitation of this study was the relatively
small numbers of cases; this occurred because the incidence of
thecoma-fibroma lesions is low (1), and PET/CT evaluations are not
performed during clinical diagnosis of all thecoma-fibroma lesions.
Thus, further studies are required to validate the present results.
However, to the best of our knowledge, the present study
demonstrated the first evidence of upregulated HIF-1α expression in
a false-positive thecoma-fibroma tumor on FDG/PET from a
clinicopathological viewpoint.
Benign thecoma-fibroma tumors occasionally exhibit
positive F-18 FDG accumulation mimicking malignant ovarian tumors.
Elevated cellularity of the thecoma-fibroma tumor is thought to
induce intratumoral hypoxia, leading to an upregulation of HIF-1α
expression and a subsequent increase in glucose metabolism.
Intratumoral hypoxia may result in F-18 FDG accumulation. Benign
thecoma-fibroma tumors with positive F-18 FDG accumulation should
therefore be listed as one of the potential differential diagnoses
of ovarian carcinoma.
Acknowledgments
This study was supported by Grants-in-Aid for
Science from the Ministry of Education, Culture, Sports, Science,
and Technology of Japan; a grant for Hirosaki University
Institutional Research; and the Fund for the Promotion of
International Scientific Research.
References
|
1
|
Salemis NS, Panagiotopoulos N, Papamichail
V, Kiriakopoulos K and Niakas E: Bilateral ovarian fibrothecoma. An
uncommon cause of a large pelvic mass. Int J Surg Case Rep.
2:29–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fenchel S, Kotzerke J, Stöhr I, Grab D,
Nüssle K, Rieber A, Kreienberg R, Brambs HJ and Reske SN:
Preoperative assessment of asymptomatic adnexal tumors by positron
emission tomography and F 18 fluorodeoxyglucose. Nuklearmedizin.
38:101–107. 1999.In German.
|
|
3
|
Fenchel S, Grab D, Nuessle K, Kotzerke J,
Rieber A, Kreienberg R, Brambs HJ and Reske SN: Asymptomatic
adnexal masses: Correlation of FDG PET and histopathologic
findings. Radiology. 223:780–788. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schwartz RK, Levine D, Hatabu H and
Edelman RR: Ovarian fibroma: Findings by contrast-enhanced MRI.
Abdom Imaging. 22:535–537. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adad SJ, Laterza VL, Dos Santos CD, Ladeia
AA, Saldanha JC, da Silva CS, E Souza LR and Murta EF: Cellular
fibroma of the ovary with multiloculated macroscopic
characteristics: Case report. Case Rep Med.
2012(283948)2012.PubMed/NCBI
|
|
6
|
Iyer NV, Kotch LE, Agani F, Leung SW,
Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY
and Semenza GL: Cellular and developmental control of O2
homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev.
12:149–162. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang GL and Semenza GL: Purification and
characterization of hypoxia-inducible factor 1. J Biol Chem.
270:1230–1237. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl
Acad Sci USA. 92:5510–5514. 1995. View Article : Google Scholar
|
|
9
|
Wenger RH and Gassmann M: Oxygen(es) and
the hypoxia-inducible factor-1. Biol Chem. 378:609–616.
1997.PubMed/NCBI
|
|
10
|
Semenza GL and Wang GL: A nuclear factor
induced by hypoxia via de novo protein synthesis binds to the human
erythropoietin gene enhancer at a site required for transcriptional
activation. Mol Cell Biol. 12:5447–5454. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Levy AP, Levy NS, Wegner S and Goldberg
MA: Transcriptional regulation of the rat vascular endothelial
growth factor gene by hypoxia. J Biol Chem. 270:13333–13340. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Cox SR, Morita T and Kourembanas S:
Hypoxia regulates vascular endothelial growth factor gene
expression in endothelial cells. Identification of a 5′ enhancer.
Circ Res. 77:638–643. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ebert BL, Firth JD and Ratcliffe PJ:
Hypoxia and mitochondrial inhibitors regulate expression of glucose
transporter-1 via distinct Cis-acting sequences. J Biol Chem.
270:29083–29089. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Firth JD, Ebert BL, Pugh CW and Ratcliffe
PJ: Oxygen-regulated control elements in the phosphoglycerate
kinase 1 and lactate dehydrogenase A genes: Similarities with the
erythropoietin 3′ enhancer. Proc Natl Acad Sci USA. 91:6496–6500.
1994. View Article : Google Scholar
|
|
15
|
Semenza GL, Roth PH, Fang HM and Wang GL:
Transcriptional regulation of genes encoding glycolytic enzymes by
hypoxia-inducible factor 1. J Biol Chem. 269:23757–23763.
1994.PubMed/NCBI
|
|
16
|
Firth JD, Ebert BL and Ratcliffe PJ:
Hypoxic regulation of lactate dehydrogenase A. Interaction between
hypoxia-inducible factor 1 and cAMP response elements. J Biol Chem.
270:21021–21027. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marti HJ, Bernaudin M, Bellail A, Schoch
H, Euler M, Petit E and Risau W: Hypoxia-induced vascular
endothelial growth factor expression precedes neovascularization
after cerebral ischemia. Am J Pathol. 156:965–976. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin KL, Mao XO, Nagayama T, Goldsmith PC
and Greenberg DA: Induction of vascular endothelial growth factor
and hypoxia-inducible factor-1alpha by global ischemia in rat
brain. Neuroscience. 99:577–585. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Talks KL, Turley H, Gatter KC, Maxwell PH,
Pugh CW, Ratcliffe PJ and Harris AL: The expression and
distribution of the hypoxia-inducible factors HIF-1alpha and
HIF-2alpha in normal human tissues, cancers and tumor-associated
macrophages. Am J Pathol. 157:411–421. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Semenza GL: HIF-1: Upstream and downstream
of cancer metabolism. Curr Opin Genet Dev. 20:51–56. 2010.
View Article : Google Scholar :
|
|
21
|
McCluggage WG, Staats PN, Kiyokawa T and
Young RH: Sex cord-stromal tumours - pure stromal tumours. WHO
Classification of Tumours of Female Reproductive Organs. 4th
edition. Kurman RJ, Carcangiu MS, Herrington CS and Young RH:
International Agency for Research of Cancer; Lyon: 2014
|
|
22
|
Kanai Y, Segawa H, Miyamoto Ki, Uchino H,
Takeda E and Endou H: Expression cloning and characterization of a
transporter for large neutral amino acids activated by the heavy
chain of 4F2 antigen (CD98). J Biol Chem. 273:23629–23632. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee MS, Cho HC, Lee YH and Hong SR:
Ovarian sclerosing stromal tumors: Gray scale and color Doppler
sonographic findings. J Ultrasound Med. 20:413–417. 2001.PubMed/NCBI
|