Introduction
Glioma is the most common and aggressive type of
primary brain tumor. Despite advances in treatments combining
surgery, radiotherapy and chemotherapy, the survival rate remains
poor (1); therefore, to improve
the prognosis for patients with glioma, novel effective treatments
are required.
Curcumin [1,7-bis (4-hydroxy-3-methoxyphenyl)-1,
6-hepta-diene-3,5-dione] is a lipophilic phenolic compound that is
derived from the rhizome of turmeric (Curcuma longa).
Turmeric is a coloring and flavoring of curry, and is a common
spice, particularly in Asia. In traditional Indian medicine,
turmeric is used in the treatment of several disorders, including
coughs, sinusitis, hepatic disease and anorexia. The potential
effectiveness of turmeric has been associated with the
anti-infectious and anti-inflammatory activities of curcumin
(2–6). It has previously been reported that
curcumin exerts anticancer effects in several types of cancer,
which are attributed to its ability to modulate numerous targets
and kinases associated with survival signaling, cell proliferation
and cell cycle regulation (7–9).
Previous studies have also indicated that curcumin is of promising
research value as a potential anticancer agent in the treatment of
glioma (10,11). However, the effects and underlying
mechanism of curcumin on glioma are currently unclear.
Forkhead box O (FoxO) transcription factors are a
superfamily of proteins, which include FoxO1, FoxO3, FoxO4 and
FoxO6 in humans. It has been reported that FoxO proteins are able
to regulate cell fate by modulating the expression of genes
associated with apoptosis, cell cycle transition, DNA repair,
oxidative stress and longevity, and muscle growth control, as well
as cell differentiation and glucose metabolism (12). Therefore, FoxOs are considered
attractive candidates as tumor suppressors. The hypothesis that
FoxO family members may serve as tumor suppressors has been
confirmed by evidence in human cancer tissue samples. Furthermore,
in nude mice experiments, IkappaB kinase-induced cell proliferation
and tumorigenicity were inhibited by the expression of active FoxO3
(13). The expression of FoxO3 in
breast cancer is negatively correlated with poor patient survival,
and increased FoxO3a expression is common following cytotoxic drug
treatment, and is associated with apoptosis and cell cycle arrest
(14). Similarly, the constitutive
expression of active FoxO4 can reduce tumor onset, as well as tumor
size and progression, in nude mice transplanted with cells
expressing the human epidermal growth factor oncogene (15). Furthermore, the tumorigenesis of
phosphatase and tensin homolog-deficient tumor cells is decreased
by overexpression of a constitutively active form of FoxO1
(16). Therefore, FoxO1, FoxO3 and
FoxO4 may prevent tumor progression.
The present study indicated that curcumin was able
to induce G2/M phase arrest and apoptosis in U87 cells,
and these effects were associated with increased FoxO1 expression
and FoxO1 nuclear localization.
Materials and methods
Curcumin
Curcumin (95%purity) [(E,E)-1,7-bis
(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] was purchased
from Sigma-Aldrich (St. Louis, MO, USA), and was stored as a 100 mM
stock solution in dimethyl sulfoxide (DMSO) at −20°C until use. The
present study was approved by the Ethical Committee of Nanjing
Medical University (Nanjing, China) (no. 2014-109).
Cell culture and treatment
The human U87 glioblastoma cell line was provided by
the China Center for Typical Culture Collection (Shanghai, China).
The U87 cells were cultured in low-glucose (1 g/l) Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.), 1 mM sodium pyruvate,
100 units/ml penicillin and 100 mg/ml streptomycin (all purchased
from Gibco; Thermo Fisher Scientific, Inc.). Cells were incubated
at 37°C under a humidified atmosphere containing 5% CO2.
The cells were incubated for 24 h, after which the medium was
removed and replaced with fresh DMEM containing 20 or 40 µM
curcumin or DMSO (untreated control) for 24 or 48 h. In some
experiments, proliferating cells were transfected with FoxO1 small
interfering (si)RNA (cat. no. sc-35382; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) or cyclin-dependent kinase 1 (CDK1) siRNA
(Suzhou Ribo Life Science Co., Ltd., Suzhou, China), 6 h
post-treatment with Lipofectamine® 2000 in Opti-MEM
serum-free medium (both purchased from Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. Then,
the Opti-MEM medium was changed into DMEM and cells were cultured
for 24 h.
Cell viability assay
Cells were treated with 20 or 40 µM curcumin
for 24 or 48 h. The effects of curcumin on cell viability were
determined using the Cell Counting kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) assay. Briefly, following
incubation with the 20 or 40 µM curcumin for 24 or 48 h, the
cells were incubated with 5 g/l CCK-8 solution for 2 h.
Subsequently, the cells were placed in a 96-well microplate reader
(BioTek, Winooski, VT, USA) for analysis and the optical density
(OD) was detected at 450 nm. Cell viability was evaluated using the
following formula: Cell viability (%) = [1 − (OD of the samples/OD
of the control)] × 100%.
Cell proliferation assay
Cells were treated with curcumin (20 or 40
µM) for 24 h, and the control cells were untreated. The
cells (1×106) were subsequently washed in
phosphate-buffered saline (PBS) and fixed in ice-cold ethanol. Cell
proliferation was determined by 5-ethynyl-2-deoxyuridine (EdU)
incorporation using the Cell-Light EdU Imaging Detecting kit
(Guangzhou RiboBio Co., Ltd., Guangzhou, China), according to the
manufacturer's protocol.
Cell cycle distribution analysis
Cells were cultured in DMEM for 24 h, and were then
treated with 20 or 40 µM curcumin or DMSO for 24 h. To
measure cell cycle distribution, the cells were harvested and fixed
in 70% ethanol overnight at 4°C. Cells were washed twice in PBS,
and re-suspended in 500 µl PBS containing 20 µg/ml
propidium iodide (PI; Sigma-Aldrich) for 30 min in the dark. Cell
cycle distribution was analyzed by flow cytometry using CellQuest™
software (version 3.0; BD Biosciences, Oxford, UK) and a FACScan
flow cytometer (BD Biosciences).
Apoptosis assay
For flow cytometric analysis, Annexin-V-fluorescein
isothiocyanate (FITC) conjugate and binding buffer (Santa Cruz
Biotechnology, Inc.) were used as standard reagents. Cells remained
untreated, or were treated with 20 or 40 µM curcumin for 24
h. The cells (1×106) were then digested with 0.25%
trypsin for 2–3 min, digestion was terminated with FBS
heat-inactivated serum (Biological Industries, Kibbutz Beit Haemek,
Israel), and the cells were re-suspended in PBS. Following fixing
with 70% ethanol and staining with FITC and PI using an Annexin
V-FITC/PI Apoptosis Detection kit (Biobyt, Ltd., Cambridge, UK),
flow cytometry analyses were performed using a FACSCalibur flow
cytometer (Becton-Dickinson, Franklin Lakes, NJ, USA) and
CellQuest™ software (version 3.0). Subsequently, the cells were
analyzed. Fluorescent emission of FITC was measured at 515–545 nm
and that of DNA-PI complexes was measured at 564–606 nm. Cell
debris was excluded from analysis using an appropriate forward
light scatter threshold setting. Compensation was used wherever
necessary.
Subcellular fractionation
The cytoplasmic and nuclear proteins were prepared
using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Treated cells were suspended in buffer A (10 mM HEPES, pH 7.9; 10
mM KCl; 0.1 mM EDTA; 0.1 mM EGTA; 1 mM dithiothreitol; 0.15%
Nonidet P40; 1% protease inhibitor cocktail), were incubated for 10
min on ice, and were centrifuged at 16,000 × g for 5 min. The
supernatant fraction was collected as the cytoplasmic extract.
Subsequently, the pellet was washed, re-suspended in buffer B (20
mM HEPES, pH 7.9; 400 mM NaCl; 1 mM EDTA; 1 mM EGTA; 1 mM
dithiothreitol; 0.5% Nonidet P40; 1% protease inhibitor cocktail)
and was agitated for 15 min at 4°C. The supernatant fraction from
the centrifugation (16,000 × g at 4°C for 10 min) was collected as
the nuclear extract. The nuclear and cytoplasmic expression levels
of FoxO1 were determined by western blot analysis.
Western blot analysis
Using the Compartment Protein Extraction kit (EMD
Millipore, Billerica, MA, USA), cytoplasmic and nuclear proteins
were isolated from the U87 cells. Cellular fractionation was
conducted according to the manufacturer's protocol. Cells were
lysed on ice in radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology, Haimen, China) for 20 min in the
presence of a protease inhibitor (Roche Diagnostics, Basel,
Switzerland). The protein concentration was determined using the
Bio-Rad Protein Assay Reagent (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), according to the manufacturer's protocol. Total
proteins (20 µg) extracted from the untreated and treated
cells were separated by 10% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis and were electophoretically transferred to a
polyvinylidene difluoride membrane (EMD Millipore), according to
standard procedures. Membranes were blocked for 1 h with 5% nonfat
dry milk in TBS-T buffer (20 mM Tris-HCl, pH 7.4; 150 mM NaCl; 0.1%
Tween-20), followed by an overnight incubation at 4°C with the
following primary antibodies: Rabbit anti-FoxO1 (1:1,000 dilution;
cat. no. 2880, Cell Signaling Technology, Inc., Danvers, MA, USA),
rabbit anti-histone h4 (1:3,000 dilution; cat. no. ab61254, Abcam,
Cambridge, UK), anti-phosphorylated (p)-FoxO1 (1:1,000 dilution;
cat. no. sc-16307, Santa Cruz Biotechnology, Inc.), anti-CDK1
(1:1,000 dilution; cat. no. 9116, Cell Signaling Technology, Inc.),
anti-cyclin G2 (1:1,000 dilution; sc-7266, Santa Cruz
Biotechnology, Inc.), anti-cleaved caspase-3 (1:1,000 dilution;
cat. no. 9664, Cell Signaling Technology, Inc.), anti-Fas ligand
(FasL; 1:1,000 dilution; cat. no. 4273S, Cell Signaling Technology,
Inc.) and anti-glyceraldehyde 3-phosphate dehydrogenase (1:1,000
dilution; cat. no. ab9484; Abcam). Blots were subsequently washed
with TBS-T and were then incubated with a horseradish
peroxidase-conjugated anti-rabbit secondary antibody (1:5,000
dilution; cat. no. sc-2004, Santa Cruz Biotechnology, Inc.) for 1 h
at room temperature. Proteins were detected using enhanced
chemiluminescence reagents (EMD Millipore), and were exposed to
X-ray films. Protein expression was semi-quantified using Scion
Image Beta 4.02 (Scion Corporation, Torrance, CA, USA).
Immunofluorescence and confocal
microscopy
Treated cells were fixed with 4% paraformaldehyde
for 30 min and permeabilized with 0.1% Triton X-100. After blocking
with blocking buffer containing 5% nonfat dry milk, the cells were
incubated with rabbit anti-FoxO1 (1:200) overnight at 4°C, followed
by incubation with a goat anti-rabbit immunoglobulin G tagged with
Alexa Fluor 488 (cat. no. 4412, Cell Signaling Technology, Inc.)
for 1 h at room temperature. 4′,6-diamidino-2-phenylindole (DAPI)
was used to stain the nuclei. Morphological alterations in U87
cells were observed and documented under a fluorescence microscope
(Zeiss LSM 510 META; Carl Zeiss AG, Oberkochen, Germany). Fields
were chosen at random from various sections, in order to ensure
objectivity of sampling. Morphometric analyses were performed using
the Zeiss LSM510 v.3.2 analysis software (Carl Zeiss AG).
Statistical analysis
All statistical analyses were performed using SPSS
software (version 19.0; IBM SPSS, Amronk, NY, USA). Quantitative
data is expressed as the mean ± standard deviation and analyzed by
one-way analysis of variance followed by the Tukey's post-hoc test.
Comparison between the groups was made by analyzing data using a
post-hoc method. Representative results from independent
experiments are presented in the present study. Student's t-test or
analysis of variance was used to statistically analyze the results
between the control and treatment groups. P<0.05 is considered
to indicate a statistically significant difference.
Results
Effects of curcumin on cell viability and
proliferation in U87 human glioblastoma cells
The present study observed the effects of curcumin
on the inhibition of U87 cell viability. Curcumin markedly
inhibited the viability of U87 cells in a dose- and time- dependent
manner. The inhibitory effects of curcumin were most obvious when
used at a concentration of 40 µM (Fig. 1A). To detect whether curcumin was
able to affect the proliferation of U87 cells, U87 cell growth was
analyzed using the EdU incorporation assay following treatment with
various concentrations of curcumin for 24 h. Similarly, cell
proliferation was markedly inhibited by curcumin when used at 20 or
40 µM. In addition, the effect of 40 µM curcumin was
more obvious than that of 20 µM curcumin (Fig. 1B).
Effects of curcumin on G2/M
cell cycle arrest and apoptosis in U87 cells
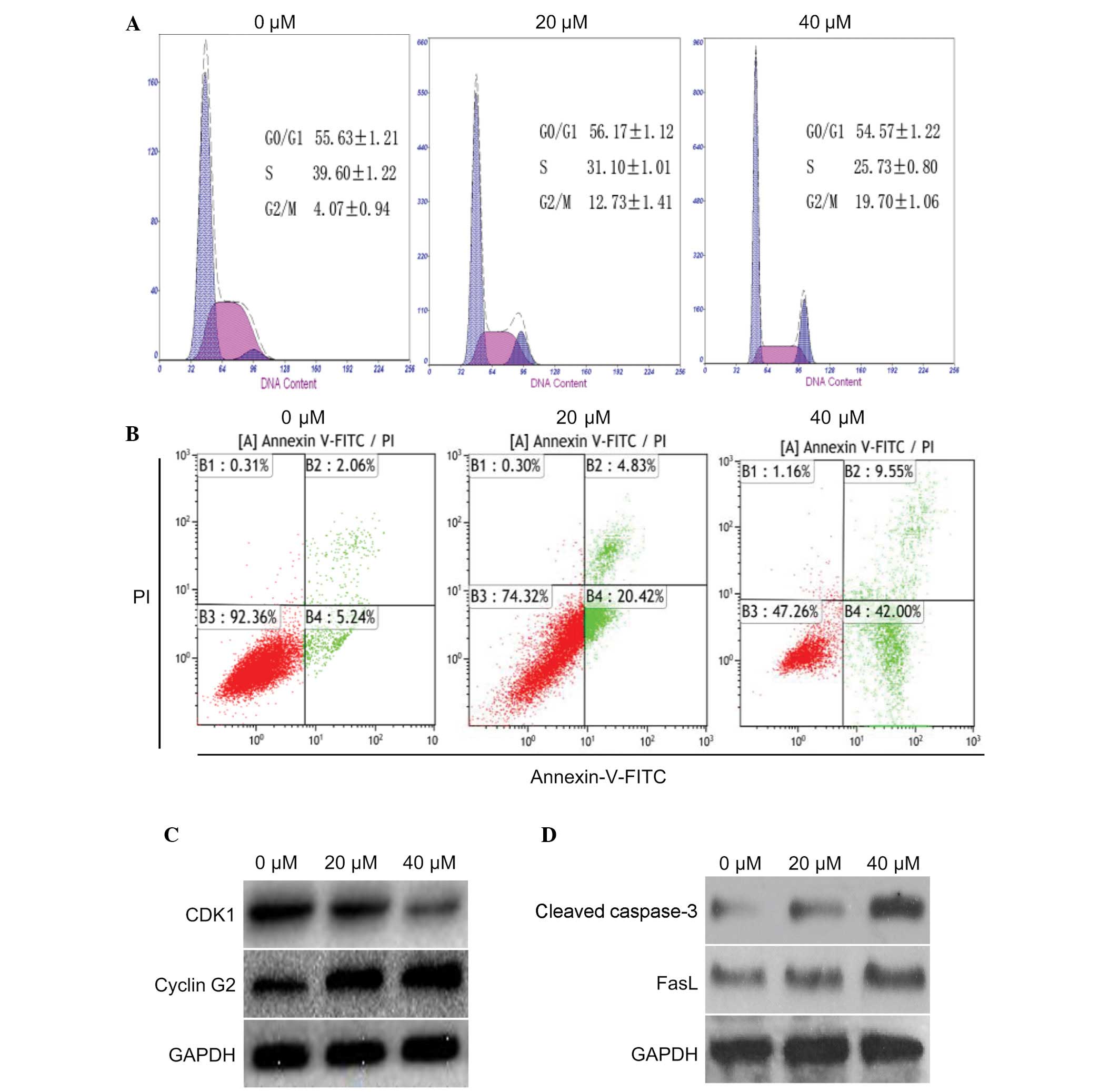
The present study determined the effects of curcumin
on cell cycle progression and apoptosis in U87 cells. To determine
whether curcumin had an effect on cell cycle distribution and
apoptosis, U87 cells were treated with 0, 20 or 40 µM
curcumin for 24 h. According to the results of a flow cytometric
analysis, an increased number of cells were detected in
G2/M phase, and a corresponding reduction in the number
of cells was detected in S phase (Fig.
2A). In addition, the number of apoptotic cells was increased
in a dose-dependent manner. Curcumin (40 µM) was able to
markedly induce apoptosis of U87 cells (Fig. 2B). These results indicate that
curcumin may effectively induce G2/M arrest and
apoptosis in U87 cells.
The expression levels of proteins associated with
the cell cycle and apoptosis were also detected in the cells
following treatment with 20 or 40 µM curcumin for 24 h.
Compared with the control group, cyclin G2 was markedly increased
in the U87 cells; however, the expression levels of CDK1 were
markedly decreased (Fig. 2C).
Furthermore, two apoptotic proteins: Cleaved caspase-3 and FasL,
were markedly upregulated (Fig.
2D). These data indicate that curcumin may induce
G2/M cell cycle arrest and apoptosis in U87 cells by
decreasing CDK1 expression and increasing the expression of cyclin
G2, cleaved caspase-3 and FasL.
Curcumin induces expression and nuclear
localization of FoxO1
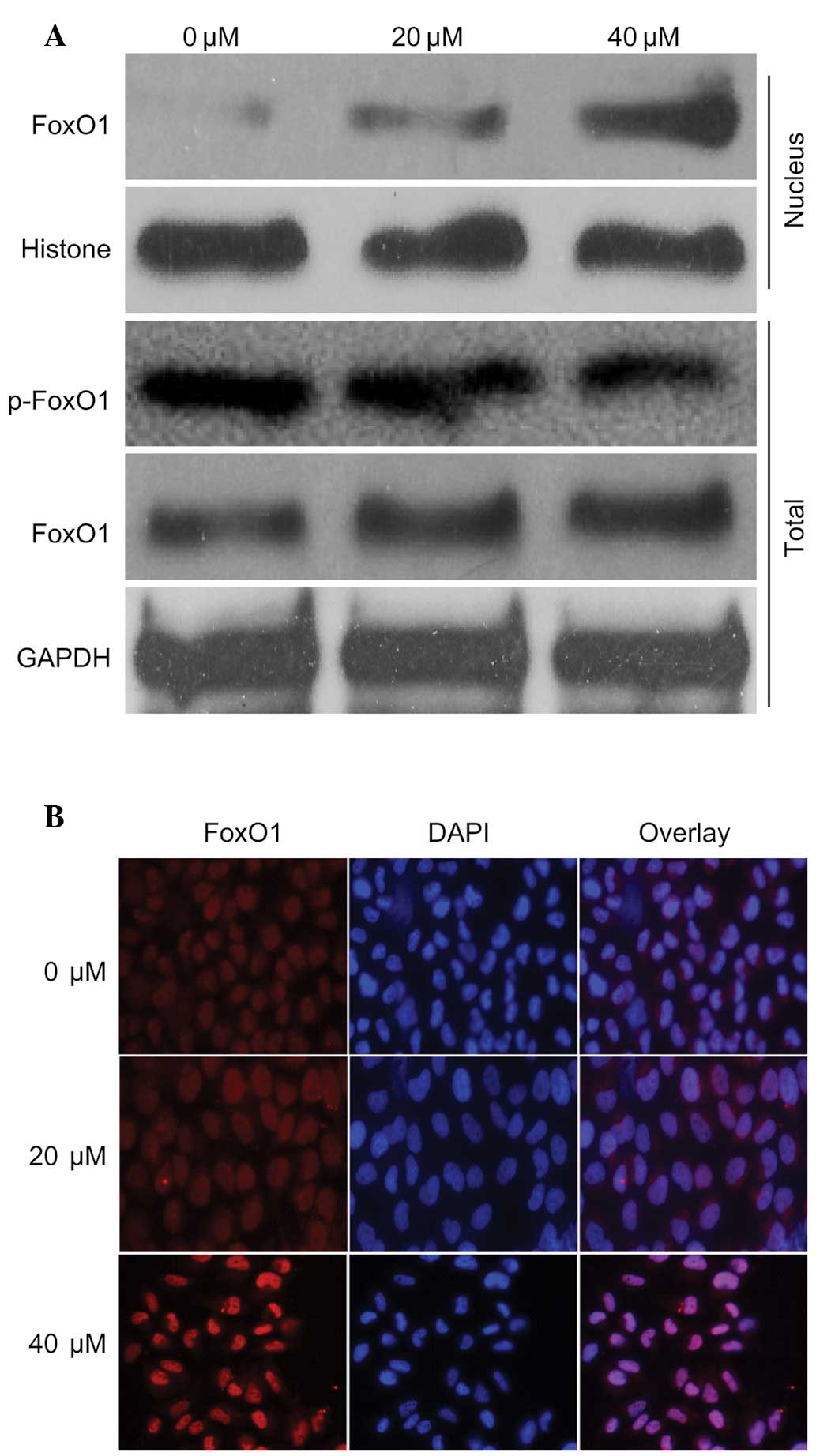
To investigate the mechanism underlying regulation
of the previously mentioned proteins, the present study detected
the expression levels of FoxO1 in the presence of 40 µM
curcumin. The expression levels of FoxO1 in the nucleus and
cytoplasm were increased in response to curcumin in a
dose-dependent manner. Notably, the expression levels of p-FoxO1
were markedly decreased (Fig. 3A).
In addition, the nuclear translocation of FoxO1 was detected
following treatment with curcumin by immunofluorescence, which
demonstrated that the nuclear expression of FoxO1 was markedly
increased (Fig. 3B). These
findings were in accordance with the results presented in Fig. 3A. These results indicate that FoxO1
may have an important role in curcumin-induced regulation of the
cell cycle and apoptosis.
Effects of FoxO1 on the expression of
target proteins associated with the cell cycle and apoptosis
To investigate the role of FoxO1, the present study
determined the effects of FoxO1 on the expression of CDK1, cyclin
G2, cleaved caspase-3 and FasL in the presence of 40 µM
curcumin. Following knockdown of FoxO1 with FoxO1 siRNA, the
expression levels of CDK1 were not affected. Following the knockout
of CDK1 by CDK1-siRNA, the expression of p-FoxO1 decreased;
although the total expression of FoxO1 was not affected, the
expression of FoxO1 in the nucleus decreased. However, the
expression levels of cyclin G2 were inhibited (Fig. 4A), thus indicating that cyclin G2
may be regulated by FoxO1. In addition, the expression levels of
cleaved caspase-3 and FasL were markedly inhibited (Fig. 4B), thus indicating that cleaved
caspase-3 and FasL may be downstream of FoxO1. To determine whether
FoxO1 was regulated by CDK1, CDK1 expression was knocked down using
CDK1 siRNA in untreated cells. In cells transfected with CDK1
siRNA, the expression levels of total FoxO1 were not affected;
however, the expression levels of p-FoxO1 were decreased.
Furthermore, the expression levels of cyclin G2, cleaved caspase-3
and FasL were increased (Fig. 4C),
and the nuclear expression of FoxO1 was increased (Fig. 4D). These results indicate that the
nuclear translocation of FoxO1 may be dependent on the effects of
CDK1 on FoxO1 phosphorylation.
FoxO1 is required for curcumin-induced
G2/M cell cycle arrest and apoptosis in U87 cells
The present study also tested the function of FoxO1
in U87 cells. Following FoxO1 knockdown with FoxO1 siRNA in the
presence of 40 µM curcumin, cell cycle distribution and
apoptosis were detected in U87 cells. Curcumin-induced
G2/M arrest and apoptosis were partly inhibited by FoxO1
knockdown (Fig. 5A and B). These
data suggest that FoxO1 may have an important role in
curcumin-induced G2/M cell cycle arrest and apoptosis in
U87 cells.
Discussion
Curcumin is a botanical polyphenol that is extracted
from the rhizomes of Curcuma longa. Curcumin has garnered
increasing attention due to its low toxic side effects and
promising antitumor effects. Numerous studies have demonstrated
that curcumin has strong inhibitory effects on three phases of
tumor formation (initiation, promotion and progression) (17,18).
In pre-clinical and clinical trials, researchers have reported that
curcumin exerts anticancer effects, particularly in glioma
(19). Although the underlying
mechanism remains to be elucidated, research has focused on several
factors that may affect its anticancer effects, including oxygen
effect, glutathione content, ability to repair radiation damage,
cell cycle phase and cell apoptosis (20).
In the present study, following treatment with
curcumin, compared with the control group, the proliferation of U87
cells was inhibited, and the ratio of cells in G2/M
phase and the rate of apoptosis were significantly increased in a
dose-dependent manner. These findings were concordant with the
results of other studies. Although curcumin has been shown to
induce G2/M arrest and apoptosis (21–23),
the detailed mechanism underlying the effects of curcumin on the
cell cycle and apoptosis have yet to be investigated. Therefore,
the present study also detected the expression levels of proteins
associated with G2/M phase and apoptosis, and
demonstrated that curcumin was able to induce the expression of
cyclin G2 and FasL. Cyclin G2 accumulates in the G2
phase, at the G2/M border, and hinders cell cycle
transition from G2 to M phase (24). In addition, the proapoptotic gene
FasL encodes a protein that activates the death receptor
Fas/CD95/apoptosis antigen 1 and promotes mitochondria-independent
apoptosis (25). These findings
may explain why curcumin induces G2/M arrest and
apoptosis.
FoxO members contain a conserved DNA binding domain
and bind to a consensus DNA binding sequence (TTGTTTAC) in target
genes (26,27). FoxO proteins can be phosphorylated
at their highly conserved sites by the survival kinase Akt (a
downstream target of phosphoinositide 3-kinase signaling) within
and nearby their forkhead domains (28,29).
In addition, FoxO1 can be phosphorylated by CDK1 and CDK2 at serine
249 in vitro and in vivo (30). The exportation of FoxO proteins
from the nucleus to the cytoplasm is controlled by phosphorylation.
Once phosphorylated, FOXO proteins are exported from the nucleus to
the cytoplasm and become inactive (12). The present study demonstrated that
curcumin was able to inhibit the expression of CDK1 and induce the
nuclear expression of FoxO1. These findings may be the result of
decreased FOXO1 phosphorylation, due to the inhibition of CDK1 by
curcumin.
The activity of FoxO transcription factors is
important for cell cycle transition and cell fate. FoxOs are able
to increase the expression of p27, p21 and p130, and inhibit the
expression of cyclin D1 and cyclin D2, which inhibit transition of
the cell cycle from G1 phase to S phase. Growth arrest
and DNA-damage-inducible, alpha and cyclin G2 can also be increased
by active FoxOs, which may result in G2/M arrest
(24). In addition, active FoxO
proteins regulate cell survival by modulating the expression of
death receptor ligands, which function in autocrine and paracrine
pathways (25,31), and are also associated with
transactivation of B-cell lymphoma 1 (Bcl-2) interacting mediator
of cell death, a gene that encodes a member of the proapoptotic
BH3-only subgroup of Bcl-2 family proteins, which functions in the
'intrinsic', mitochondrial apoptotic pathway (32,33).
These results suggested that FoxO transcription factors can induce
cell death through mitochondria-dependent and -independent
mechanisms. However, the role of FoxO1 in cell cycle progression
and apoptosis remains unclear. The results of the present study
demonstrated that following knockdown of FoxO1 by siRNA,
curcumin-induced G2/M arrest and apoptosis were
inhibited, and the expression levels of cyclin G2 and FasL were
decreased; however, the expression of CDK1 was not effected by
FoxO1 knockdown. The subsequent results supported that FoxO1 was a
substrate of CDK1. Once CDK1 was inhibited by curcumin, the
phosphorylation of FoxO1 was decreased; therefore, active FoxO1 was
translocated from the cytoplasm to the nucleus where it triggered
the expression of target genes. However, the present study did not
investigate the mechanism underlying curcumin-induced CDK1
inhibition and FoxO1 upregulation; therefore, further studies are
required.
In conclusion, the present study demonstrated that
curcumin leads to G2/M phase arrest and apoptosis, which
may be associated with the expression of active FoxO1 in the
nucleus, and may mediate the proliferation and apoptosis of U87
cells. The results indicated that this process was associated with
the curcumin-induced inhibition of CDK1. In addition, cyclin G2 and
FasL were identified as potential target genes of FoxO1. The
present study provides evidence for a novel mechanism to explain
the antitumor effects of curcumin, and provides a theoretical basis
for the application of curcumin in glioma.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation China (grant nos. 81272791 and
81502159) and the Social Programs of Wuxi Technology Bureau (grant
no. CSE01N1107).
References
|
1
|
Grossman SA, Ye X, Piantadosi S, Desideri
S, Nabors LB, Rosenfeld M and Fisher J; NABTT CNS Consortium:
Survival of patients with newly diagnosed glioblastoma treated with
radiation and temozolomide in research studies in the United
States. Clin Cancer Res. 16:2443–2449. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garodia P, Ichikawa H, Malani N, Sethi G
and Aggarwal BB: From ancient medicine to modern medicine:
Ayurvedic concepts of health and their role in inflammation and
cancer. J Soc Integr Oncol. 5:25–37. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cole GM, Teter B and Frautschy SA:
Neuroprotective effects of curcumin. Adv Exp Med Biol. 595:197–212.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chuang SE, Kuo ML, Hsu CH, Chen CR, Lin
JK, Lai GM, Hsieh CY and Cheng AL: Curcumin-containing diet
inhibits diethylnitrosamine-induced murine hepatocarcinogenesis.
Carcinogenesis. 21:331–335. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lim GP, Chu T, Yang F, Beech W, Frautschy
SA and Cole GM: The curry spice curcumin reduces oxidative damage
and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci.
21:8370–8377. 2001.PubMed/NCBI
|
|
6
|
Thiyagarajan M and Sharma SS:
Neuroprotective effect of curcumin in middle cerebral artery
occlusion induced focal cerebral ischemia in rats. Life Sci.
74:969–985. 2004. View Article : Google Scholar
|
|
7
|
LoTempio MM, Veena MS, Steele HL,
Ramamurthy B, Ramalingam TS, Cohen AN, Chakrabarti R, Srivatsan ES
and Wang MB: Curcumin suppresses growth of head and neck squamous
cell carcinoma. Clin Cancer Res. 11:6994–7002. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang D, Veena MS, Stevenson K, Tang C, Ho
B, Suh JD, Duarte VM, Faull KF, Mehta K, Srivatsan ES and Wang MB:
Liposome-encapsulated curcumin suppresses growth of head and neck
squamous cell carcinoma in vitro and in xenografts through the
inhibition of nuclear factor kappaB by an AKT-independent pathway.
Clin Cancer Res. 14:6228–6236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin YG, Kunnumakkara AB, Nair A, Merritt
WM, Han LY, Armaiz-Pena GN, Kamat AA, Spannuth WA, Gershenson DM,
Lutgendorf SK, et al: Curcumin inhibits tumor growth and
angiogenesis in ovarian carcinoma by targeting the nuclear
factor-kappaB pathway. Clin Cancer Res. 13:3423–3430. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhuang W, Long L, Zheng B, Ji W, Yang N,
Zhang Q and Liang Z: Curcumin promotes differentiation of
glioma-initiating cells by inducing autophagy. Cancer Sci.
103:684–690. 2012. View Article : Google Scholar
|
|
11
|
Kumar A, Ahuja A, Ali J and Baboota S:
Curcumin-loaded lipid nanocarrier for improving bioavailability,
stability and cytotoxicity against malignant glioma cells. Drug
Deliv. 23:214–229. 2016. View Article : Google Scholar
|
|
12
|
Greer EL and Brunet A: FOXO transcription
factors at the interface between longevity and tumor suppression.
Oncogene. 24:7410–7425. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang
F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, et al: IkappaB kinase
promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell.
117:225–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taylor S, Lam M, Pararasa C, Brown JE,
Carmichael AR and Griffiths HR: Evaluating the evidence for
targeting FOXO3a in breast cancer: A systematic review. Cancer Cell
Int. 15:12015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang H, Zhao R, Yang HY and Lee MH:
Constitutively active FOXO4 inhibits Akt activity, regulates p27
Kip1 stability, and suppresses HER2-mediated tumorigenicity.
Oncogene. 24:1924–1935. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ramaswamy S, Nakamura N, Sansal I,
Bergeron L and Sellers WR: A novel mechanism of gene regulation and
tumor suppression by the transcription factor FKHR. Cancer Cell.
2:81–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Prakobwong S, Gupta SC, Kim JH, Sung B,
Pinlaor P, Hiraku Y, Wongkham S, Sripa B, Pinlaor S and Aggarwal
BB: Curcumin suppresses proliferation and induces apoptosis in
human biliary cancer cells through modulation of multiple cell
signaling pathways. Carcinogenesis. 32:1372–1380. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moragoda L, Jaszewski R and Majumdar AP:
Curcumin induced modulation of cell cycle and apoptosis in gastric
and colon cancer cells. Anticancer Res. 21:873–878. 2001.PubMed/NCBI
|
|
19
|
Shao J, Zheng D, Jiang Z, Xu H, Hu Y, Li X
and Lu X: Curcumin delivery by methoxy polyethylene glycol-poly
(caprolactone) nanoparticles inhibits the growth of C6 glioma
cells. Acta Biochim Biophys Sin (Shanghai). 43:267–274. 2011.
View Article : Google Scholar
|
|
20
|
Vallianou NG, Evangelopoulos A, Schizas N
and Kazazis C: Potential anticancer properties and mechanisms of
action of curcumin. Anticancer Res. 35:645–651. 2015.PubMed/NCBI
|
|
21
|
Weir NM, Selvendiran K, Kutala VK, Tong L,
Vishwanath S, Rajaram M, Tridandapani S, Anant S and Kuppusamy P:
Curcumin induces G2/M arrest and apoptosis in cisplatin-resistant
human ovarian cancer cells by modulating Akt and p38 MAPK. Cancer
Biol Ther. 6:178–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luthra PM, Kumar R and Prakash A:
Demethoxycurcumin induces Bcl-2 mediated G2/M arrest and apoptosis
in human glioma U87 cells. Biochem Biophys Res Commun. 384:420–425.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo H, Xu YM, Ye ZQ, Yu JH and Hu XY:
Curcumin induces cell cycle arrest and apoptosis of prostate cancer
cells by regulating the expression of IkappaBalpha, c-Jun and
androgen receptor. Pharmazie. 68:431–434. 2013.PubMed/NCBI
|
|
24
|
Huang H and Tindall DJ: Dynamic FoxO
transcription factors. J Cell Sci. 120:2479–2487. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo
P, Hu LS, Anderson MJ, Arden KC, Blenis J and Greenberg ME: Akt
promotes cell survival by phosphorylating and inhibiting a Forkhead
transcription factor. Cell. 96:857–868. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Biggs WH III, Cavenee WK and Arden KC:
Identification and characterization of members of the FKHR (FOX O)
subclass of winged-helix transcription factors in the mouse. Mamm
Genome. 12:416–425. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Furuyama T, Nakazawa T, Nakano I and Mori
N: Identification of the differential distribution patterns of
mRNAs and consensus binding sequences for mouse DAF-16 homologues.
Biochem J. 349:629–634. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin K, Dorman JB, Rodan A and Kenyon C:
daf-16: An HNF-3/forkhead family member that can function to double
the life-span of Caenorhabditis elegans. Science. 278:1319–1322.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ogg S, Paradis S, Gottlieb S, Patterson
GI, Lee L, Tissenbaum HA and Ruvkun G: The fork head transcription
factor DAF-16 transduces insulin-like metabolic and longevity
signals in C. elegans. Nature. 389:994–999. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang H, Regan KM, Lou Z, Chen J and
Tindall DJ: CDK2-dependent phosphorylation of FOXO1 as an apoptotic
response to DNA damage. Science. 314:294–297. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Modur V, Nagarajan R, Evers BM and
Milbrandt J: FOXO proteins regulate tumor necrosis factor-related
apoptosis inducing ligand expression. Implications for PTEN
mutation in prostate cancer. J Biol Chem. 277:47928–47937. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dijkers PF, Medema RH, Lammers JW,
Koenderman L and Coffer PJ: Expression of the pro-apoptotic Bcl-2
family member Bim is regulated by the forkhead transcription factor
FKHR-L1. Curr Biol. 10:1201–1204. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stahl M, Dijkers PF, Kops GJ, Lens SM,
Coffer PJ, Burgering BM and Medema RH: The forkhead transcription
factor FoxO regulates transcription of p27Kip1 and Bim in response
to IL-2. J Immunol. 168:5024–5031. 2002. View Article : Google Scholar : PubMed/NCBI
|