Introduction
Obstructive sleep apnea (OSA) is a breathing
disorder that is characterized by repetitive episodes of complete
or partial upper airway obstruction during sleep, which leads to
intermittent reduction or complete blockage of airflow (1). The prevalence of OSA is 3–7% in men
and 2–5% in women (1). Repetitive
OSA results in chronic intermittent hypoxia (IH), which is followed
by reoxygenation (ROX), and is characterized by frequent decreases
in blood O2 saturation. The clinical symptoms of sleep
apnea were reported as early as the 19th century
(2); however, it was not until the
1980s that researchers began to investigate and understand OSA
(3).
OSA has been associated with numerous comorbidities,
including cardiovascular alterations, diabetes and depression
(4). Although efforts have been
made to comprehend the consequences of OSA and the underlying state
of IH, there may be more problems or comorbidities associated with
OSA than originally expected. The gastrointestinal system is likely
to be affected by OSA, since the gastrointestinal epithelium is
particularly sensitive to tissue hypoxia and reduced perfusion.
Furthermore, a clinical study involving 35,480 patients indicated
sleep apnea as an independent risk factor for gastric and duodenal
ulcer bleeding (5), thus
suggesting that OSA may compromise the gastrointestinal system;
however, the underlying mechanism is not well understood.
Therefore, the present study hypothesized that IH, a characteristic
of OSA, may induce intestinal injury.
Integrity of the intestinal epithelium is essential
for normal physiological function and the prevention of disease,
since it restricts the free passage of toxic and infectious
molecules from the gut lumen whilst allowing selective paracellular
absorption of nutritive material. The major determinants of
intestinal barrier function are the intercellular tight junctions
(TJs), which are located in the uppermost region of the lateral
membranes of epithelial and endothelial cells (6). Several TJ compounds have been
identified, including the transmembrane proteins occludin and
claudins, and the peripheral membrane proteins zonula occludens
(ZOs) (7). Claudins are considered
integral proteins of TJs that regulate size selectivity of the TJ
barrier. Occludin is thought to be the primary sealing protein of
the epithelial intercellular space, whereas ZOs are the critical
scaffold proteins that link transmembrane TJ components to the
intracellular actin cytoskeleton (8).
Growing evidence from cellular and animal models,
and population surveys of OSA, has demonstrated that exposure to IH
is associated with the activation of oxidative stress and
inflammatory processes (9). IH
induces the accumulation of reactive oxygen species (ROS), which
initiates oxidative stress-sensitive signaling pathways and
inflammatory processes. Various transcription factors and
inflammatory mediators implicated in this process have previously
been identified (10). Among
these, much attention has been focused on hypoxia-inducible
factor-1 (HIF-1), nuclear factor-κB (NF-κB) and activator protein-1
(AP-1). The transcription factor HIF-1 is the main regulator of
oxygen homeostasis and serves a key role in the response to hypoxia
in most tissues (11). NF-κB and
AP-1 are transcription factors implicated in inflammatory
processes. Once they are activated, several target genes are
transcribed, triggering an inflammatory cascade. A previous study
suggested that the expression and function of TJs are affected by
proinflammatory cytokines and intracellular signaling molecules
(12). Therefore, it is essential
to identify whether IH has an effect on the intestinal gene
expression of transcription factors and inflammatory mediators, and
whether it induces TJ disruption via activation of oxidative stress
and inflammatory processes.
In the present study, a rat model was developed to
mimic the recurrent IH and subsequent ROX experienced by patients
with OSA. It was hypothesized that this pathological environment
may result in activation of oxidative stress and inflammatory
processes in the duodenum, subsequently compromising intestinal
barrier function by disrupting TJs.
Materials and methods
Ethics statement
Rats were used in strict accordance with the
protocol approved by the Animal Care Committee of Tianjin Medical
University General Hospital (Tianjin, China).
Animals and treatments
Male Wistar rats (180±20 g; n=30; 6-weeks-old) were
purchased from the Model Animal Center of Radiological Medicine
Research Institute, China Academy of Medical Science (Tianjin,
China). Rats were housed in standard laboratory cages (n=5/cage) at
22°C with a 12 h light/dark cycle and free access to food and
water. The rats were randomly divided into two groups (n=15/group)
matched for body weight: The IH-exposed group and the control
group. Rats in the IH-exposed group were exposed to IH for 8 h/day
during the rodent diurnal sleep period, between 9 AM and 5 PM,
repeatedly for 7 days/week for 8 consecutive weeks, in a
specialized plexiglas chamber (dimensions 30×20×20 cm), as
previously described (13). Pure
nitrogen and compressed air were flushed into the chamber in turn
to maintain an IH cycle. Each cycle of IH lasted 120 sec, the first
30 sec being the hypoxic phase and the following 90 sec the ROX
phase (during which the nitrogen was replaced with clean air). Gas
flow was regulated by timer-controlled solenoid valves and an
O2 flow meter. The O2 and CO2
concentrations were continuously monitored by an O2 and
CO2 concentration monitor (Hamilton Medical AG, Bonaduz,
Switzerland). The control rats underwent an identical protocol;
however, the nitrogen source was replaced with a clean air
source.
Histological analysis
Following treatment, rats were anesthetized by
intraperitoneal injection of 30 mg/kg pentobarbital sodium
(Sigma-Aldrich, St. Louis, MO, USA), and the duodenum was excised
and rinsed in ice-cold phosphate-buffered saline (pH 7.4). The
duodenal tissues were subsequently fixed in 10% neutral buffered
formalin for 24 h, were paraffin-embedded, cut into 5 µm
sections, and were processed for hematoxylin and eosin (H&E)
staining (Solarbio Science & Technology Co., Ltd., Beijing,
China). The stained sections were analyzed, and images of the
representative fields were captured using an Olympus BX53
microscope (Olympus Corporation, Tokyo, Japan). Morphological
injury of the duodenal mucosa was assessed using the Chiu
histological injury scoring system for intestinal villi. The
numerical scores were as follows: 0, normal mucosa; 1, development
of subepithelial Gruenhagen's space and vacuolization at the apex
of the villi; 2, extension of the subepithelial space with moderate
lifting of the epithelial layer from the lamina propria; 3, massive
subepithelial lifting down the sides of villi; 4, epithelial
lifting and vacuolization from the tip to the lower portion of
villi; and 5, mucosal hemorrhage, ulceration and disintegration of
the lamina propria (14). Two
independent and blinded researchers performed the histological
scoring.
Total RNA isolation
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) was used to extract RNA
from homogenized duodenal tissues, according to the manufacturer's
protocol. Extract yield and quality were determined by measuring
the absorbance at 260 and 280 nm using a MaestroNano Micro-volume
Spectrophotometer (Maestrogen, Inc., Las Vegas, NV, USA). The
absorbance ratio of 260:280 nm was between 1.8 and 2.0.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
mRNA (3 µg) was reverse transcribed into cDNA
with an oligo (dT) primer for 1 h at 50°C using the TIANScript RT
kit (Tiangen Biotech Co., Ltd., Beijing, China), according to the
manufacturer's protocol. RT-qPCR was performed using iQ SYBR Green
Supermix (#1708880; Bio-Rad Laboratories, Inc., Hercules, CA, USA)
with a reaction volume of 20 µl, according to the
manufacturer's protocol. Gene-specific primers were designed using
the Primer-Quest SM software (sg.idtdna.com/Primerquest/Home/Index; Integrated DNA
Technologies, Inc., Coralville, IA, USA), and were commercially
produced by BGI Tech (BGI Tech Solutions Co., Ltd., Shenzhen,
China). Primer sequences are listed in Table I. DNA amplification was carried out
using a CFX96 Touch Real-Time PCR Detection system (Bio-Rad
Laboratories, Inc.) with the following reaction conditions: Initial
heating cycle at 95°C for 2 min; followed by 40 cycles alternating
between denaturation at 95°C for 25 sec, primer annealing at 60°C
for 25 sec, and extension at 72°C for 20 sec. A final extension
step at 72°C for 10 min was conducted. The housekeeping gene,
glyceraldehyde 3-phosphate dehydrogenase (GAPDH), was used as an
internal control. Melting curves were used to identity the
amplicons. Relative mRNA expression levels of the target genes were
calculated using the 2−ΔΔCq method, and were normalized
to the levels of GAPDH in the same sample (15).
 | Table IDNA primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I
DNA primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer | Reverse primer |
|---|
| GAPDH |
5′-TGGAGTCTACTGGCGTCTTC-3′ |
5′-TTCACACCCATCACAAACATG-3′ |
| Nox2 |
5′-GGCTGTGAATGAGGGACTC-3′ |
5′-CCAGTGCTGACCCAAGAAG-3′ |
| p22phox |
5′-AAGTACCTGACCGCTGTGG-3′ |
5′-AGGTAGATCACACTGGCAATG-3′ |
| HIF-1α |
5′-AAGAAACCGCCTATGACGTG-3′ |
5′-CCACCTCTTTTTGCAAGCAT-3′ |
| NF-κB |
5′-AGCCCTATGCCTTTTCAACAT-3′ |
5′-CACTCCTGGGTCTGTGTTGTT-3′ |
| c-fos |
5′-CGAAGGGAAAGGAATAAGA-3′ |
5′-GTCCAGGGAGGTCACAGA-3′ |
| Claudin-1 |
5′-TGTCCACCATTGGCATGAAG-3′ |
5′-GCCACTAATGTCGCCAGACC-3′ |
| Claudin-2 |
5′-ACAGCACTGGCATCACCCA-3′ |
5′-GCGAGGACATTGCACTGGAT-3′ |
| Claudin-4 |
5′-AAGGCCAAGGTCATGATCACAG-3′ |
5′-GAAGTCGCGGATGACGTTGT-3′ |
| Occludin |
5′-CTACTCCTCCAACGGCAAAG-3′ |
5′-AGTCATCCACGGACAAGGTC-3′ |
| ZO-1 |
5′-ATTCAGTTCGCTCCCATGAC-3′ |
5′-GCTGTGGAGACTGTGTGGAA-3′ |
Statistical analysis
Results are presented as the mean ± standard error
of the mean and experiments were repeated three times. The data
were analyzed using SPSS software, version 13.0 (SPSS, Inc.,
Chicago, IL, USA) and differences between paired groups were
analyzed using Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
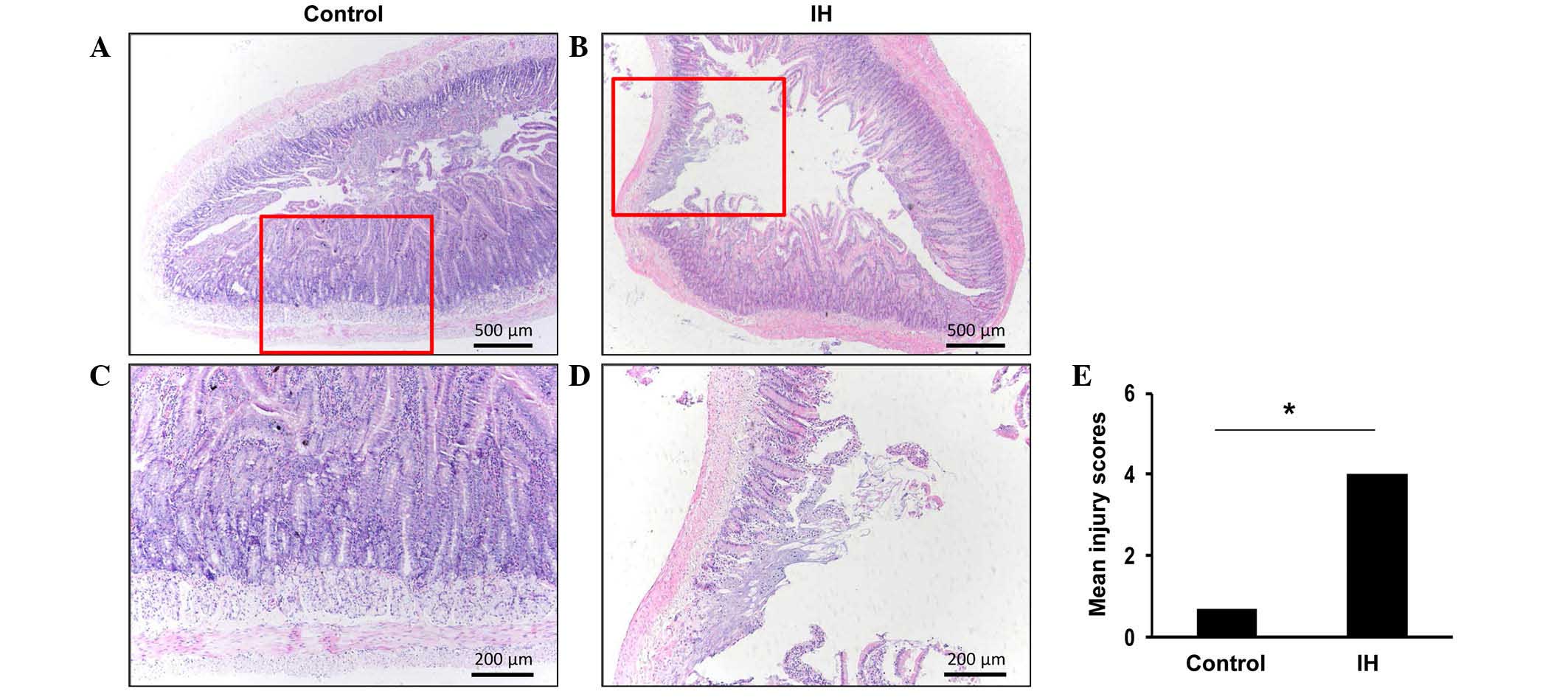
Exposure to IH results in damage to the
duodenal epithelium
Hypoxia is known to lead to inflammation; in order
to assess whether IH contributes toward injury to the duodenal
epithelium, duodenal morphology was examined. Evaluation of the
H&E-stained sections revealed morphological alterations to the
duodenal mucosa in response to IH exposure (Fig. 1A and B). High-power images of the
general epithelial structures of the duodenum from the control or
IH-exposed rats were captured (Fig. 1C
and D). The histological images of the duodenal specimens from
the control rats (Fig. 1A)
exhibited normal-appearing mucosal villi with consistent mucosa, as
compared with the IH-exposed rats (Fig. 1B). IH-exposed rats exhibited
disintegration of the mucosal villi and infiltration of
inflammatory cells (Fig. 1B).
Furthermore, necrosis and superficial ulceration were detected in
the mucosa of certain IH-exposed rats (data not shown). The villous
injury score of the IH-exposed rats (mean injury score, 4.00±0.63)
was markedly higher compared with the control rats (mean injury
score, 0.67±0.58; Fig. 1E). These
findings suggest that exposure to IH may result in marked
pathophysiological alterations in duodenal tissue.
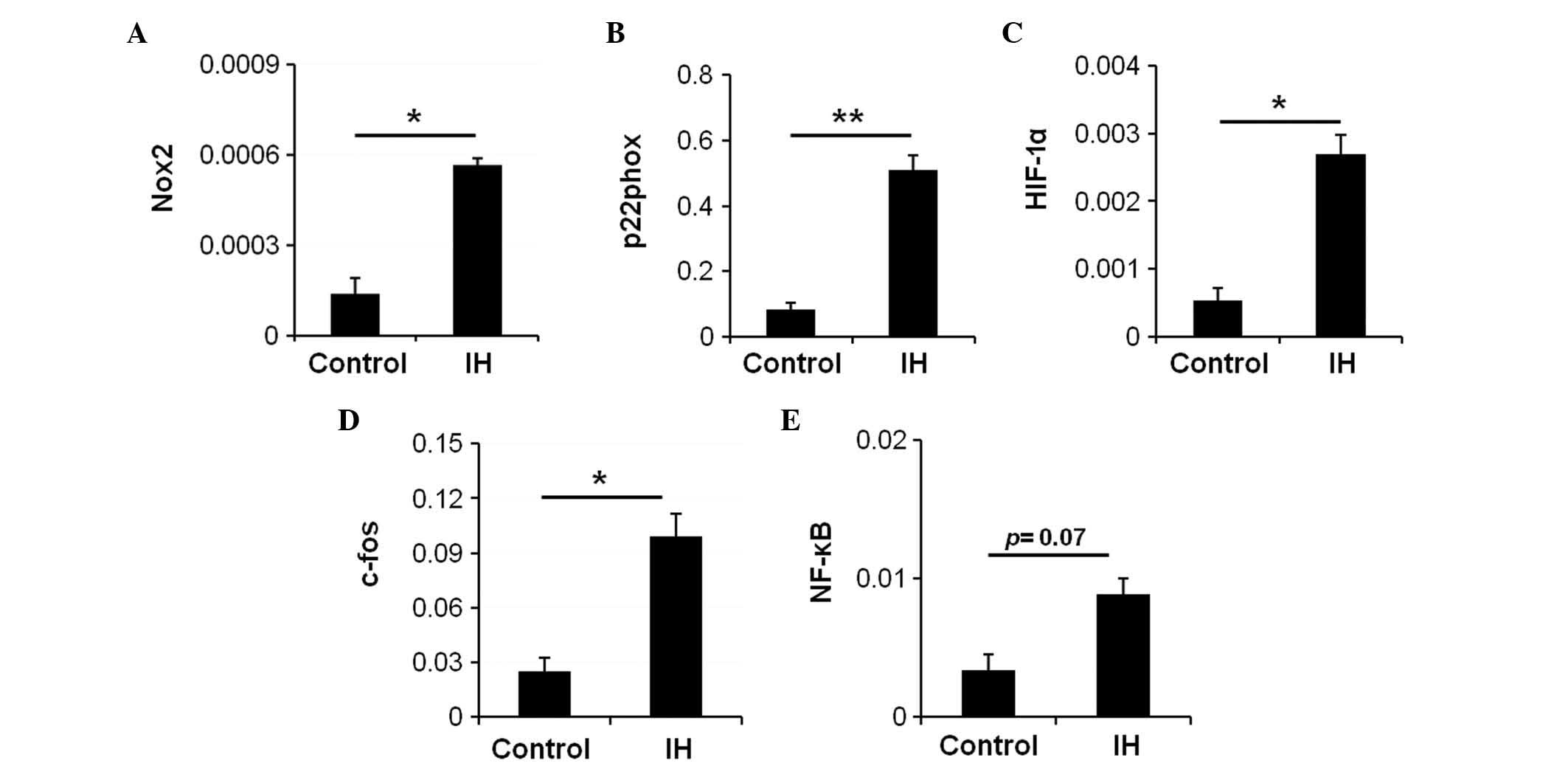
IH exposure induces activation of
oxidative stress and transcription factor expression
A previous study indicated that recurrent hypoxia
and ROX cycles increase the production of ROS in OSA (16). Nicotinamide adenine dinucleotide
phosphate (NADPH) oxidase serves a key role in oxidative stress and
is an enzyme involved in the production of ROS (9). To examine whether IH affects NADPH
oxidase activity in the intestine, and if so, whether NADPH oxidase
activation contributes to the expression of IH-induced
transcription factors, the expression levels of NADPH oxidase
subunit genes were measured in the IH-exposed and control rats.
There was a significant increase in the mRNA expression levels of
the NADPH oxidase subunits NADPH oxidase 2 (Nox2) (Fig. 2A; P=0.003) and p22phox (Fig. 2B; P=0.005) in the IH-exposed rats.
These data suggest an overexpression of NADPH oxidase in the
IH-exposed rats. Therefore, it may be hypothesized that NADPH
oxidase is a major source of ROS in the IH-exposed duodenum, and
that upregulation of NADPH oxidase results in increased ROS,
thereby mediating the onset of oxidative stress.
HIF-1 is a heterodimeric protein that is composed of
an O2-regulated HIF-1α subunit and a constitutively
expressed HIF-1β subunit. Hypoxia induces upregulation of HIF-1,
and the activity of HIF-1 is primarily determined by the HIF-1α
subunit. To examine whether IH activated HIF-1, the mRNA expression
levels of HIF-1α were assessed. Compared with the control group, a
significant increase in the mRNA expression levels of HIF-1α was
detected in the IH group (Fig. 2C;
P=0.014).
AP-1 is a protein complex formed by the protein
products of immediate early genes, including c-fos and c-jun.
Activation of AP-1 is usually indirect and represented by c-fos
mRNA expression levels. The mRNA expression levels of c-fos
(Fig. 2D; P=0.033) were
significantly increased in the IH-exposed rats. In addition, an
increase in the mRNA expression levels of NF-κB was detected in the
duodenum of the IH-exposed rats (Fig.
2E; P=0.07). These data indicate that IH may activate
transcription factors in the duodenum.
IH exposure selectively regulates the
mRNA expression levels of TJ proteins
Due to the key function of TJ proteins in the
integrity of intestinal mucosa, the present study examined whether
IH exposure regulated TJ components in the duodenum, including
claudin-1, -2, -4, occludin and ZO-1. RT-qPCR demonstrated that the
mRNA expression levels of claudin-1 (Fig. 3A) and claudin-4 (Fig. 3B) were significantly reduced by IH
exposure compared with the control group (P<0.01 and P<0.05,
respectively). However, no significant alterations were detected in
claudin-2, occludin or ZO-1 mRNA expression (Fig. 3C–E; P>0.05). These data suggest
that IH exposure selectively loosens TJ proteins of the intestinal
luminal cells to increase intestinal permeability, which
subsequently leads to a breach in the mucosal barrier during
IH.
Discussion
The present study used a rat model to provide
evidence that IH exposure, the hallmark feature of OSA, may lead to
disruption in the duodenum. In addition, increased mRNA expression
levels of oxidative stress-related genes and transcription factors
were detected in the duodenum following exposure to IH.
IH and subsequent ROX are characteristics of OSA,
which is similar to ischemia/reperfusion (I/R) injury. Although no
direct study has observed intestinal injury in OSA, it has
previously been reported that intestinal damage occurs following
I/R injury (17). Intestinal
morphological injury alongside a raised Chiu score has been
observed in response to I/R injury (17). In addition, functional studies of
intestinal barrier function have demonstrated that intestinal
permeability increases following I/R injury (18,19).
Conversely, no previous studies have reported a direct link between
IH and intestinal injury. The present study demonstrated that the
intestinal mucosa was significantly compromised following IH
exposure, as evidenced by morphological alterations to intestinal
structures and elevated Chiu scores. It may therefore be
hypothesized that these changes increase mucosal permeability,
leading to intestinal barrier dysfunction.
IH-induced oxidative stress represents a
pathological link between OSA and resultant multiple organ injury.
A previous study demonstrated that IH induces severe oxidative
stress in the myocardium, brain, carotid body, adrenal gland and
liver in animal models (20).
Excess ROS may lead to radical-induced oxidation and damage,
serving as key activator for transcription factors and inflammatory
pathways (11). Cell culture and
animal model studies have demonstrated that HIF-1 is activated by
IH exposure (21,22), due to the increased generation of
ROS via activated NADPH oxidase and the resultant changes in
intracellular Ca2+ (23). The present study demonstrated that
HIF-1α mRNA expression was upregulated in the duodenum following IH
exposure. A previous study demonstrated the feed-forward
interactions between HIF-1 and ROS under IH conditions (24). IH may activate HIF-1 via a
ROS-dependent manner, whereas antioxidants prevent HIF-1 activation
(25). Conversely, HIF-1 is
required for IH-induced ROS generation, that is, IH elevates ROS
levels in wild-type mice, but not in HIF-1α-deficient mice
(26). These results suggested
that IH may initially induce an increase in ROS levels by
activating NADPH oxidase, which upregulates HIF-1α, and once HIF-1
is activated, it may further promote increases in ROS.
NF-κB and AP-1 are transcription factors, which have
been investigated in IH. The classical NF-κB pathway is thought to
be activated by ROS. Previous studies have reported that IH induces
activation of NF-κB and upregulation of NF-κB-dependent genes
(26), which is mediated via
activation of p38 mitogen-activated protein (MAP) kinase (27). In addition, increased protein and
mRNA expression levels of c-fos have been detected in animal and
cell models following exposure to IH (28), thus suggesting that AP-1 serves an
important role in IH.
TJs are important for maintaining integrity of the
intestinal barrier (29).
Disruption of TJs and increased paracellular permeability serve a
role in the pathogenesis of several intestinal diseases (30). Furthermore, TJ proteins may be
influenced by numerous transcription factors, including HIF-1. A
previous study on HIF-1β knockdown cells detected significantly
reduced levels of claudin-1, which subsequently led to increased
intestinal permeability (31).
However, the roles of HIF-1α in the regulation of barrier integrity
seem controversial. In addition, HIF-1 has been identified as a
factor associated with barrier protection under hypoxic conditions
(32). The present study
demonstrated that HIF-1 may serve a gut-injurious role in
IH-induced intestinal injury, since the expression of TJ-related
proteins was upregulated.
The NF-κB signaling pathway has a role in intestinal
epithelial homeostasis and repair (29), and disruption or anomalous
activation of NF-κB may exaggerate the inflammatory response
(33). A previous cell culture
study demonstrated that TNF-α induced downregulation of claudin-1,
-2, -4, and occludin, which could be partially alleviated via
pharmacological inhibition of NF-κB (34). Furthermore, the NF-κB signaling
pathway has been reported to mediate increased expression of myosin
light chain kinase, which induces opening of intestinal TJ
proteins, thus resulting in TJ barrier breakdown (35). Activation of NF-κB may also mediate
claudin-1 internalization and increase paracellular permeability
(36). Furthermore, NF-κB
associates with AP-1 to induce redistribution of intestinal TJ
permeability via increased MAP kinase phosphorylation (37) and interleukin-6 secretion (38). Taken together, these data
demonstrate that NF-κB and AP-1 may disrupt intestinal epithelium
by regulating TJ components.
Increasing evidence has illustrated the association
between hypoxia and gastrointestinal disease (39,40).
The absorptive and barrier functions of the intestinal epithelium
may be physiologically regulated by the availability of oxygen
(39). It is well known that
hypoxia may induce inflammation, and conversely, inflamed lesions
often become severely hypoxic (41). In addition, hypoxia influences
innate and adaptive immunity via activation of HIF-1α (42). Therefore, it may be suggested that
hypoxia is a significant component of the inflammatory
microenvironment within the intestinal mucosa (40).
The present study has certain limitations.
Constrained to the experimental technique, the present study failed
to detect ROS accumulation directly. In addition, future
experiments that analyze the expression levels of proteins
associated with intestinal TJs and transcription factors by western
blotting or immunohistochemistry are required.
In conclusion, the major observation of the present
study is that OSA, characterized by IH and subsequent ROX, may
cause disruption of the duodenum. The mechanism underlying the
effects of OSA on duodenal morphology is associated with increased
oxidative stress and activation of transcription factors, which may
subsequently induce intestinal TJ disruption and intestinal injury.
These data may provide a novel insight into the clinical treatment
of patients with OSA, but intestinal complications should be kept
in mind and caution taken to avoid these.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 31471121 and
81270144) and the Natural Science Foundation of Tianjin City (grant
nos. 13JCYBJC22400, 13JCYBJC40000 and 14JCYBJC25700).
References
|
1
|
Lurie A: Obstructive sleep apnea in
adults: Epidemiology, clinical presentation, and treatment options.
Adv Cardiol. 46:1–42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dempsey JA, Veasey SC, Morgan BJ and
O'Donnell CP: Pathophysiology of sleep apnea. Physiol Rev.
90:47–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Punjabi NM: The epidemiology of adult
obstructive sleep apnea. Proc Am Thorac Soc. 5:136–143. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kendzerska T, Mollayeva T, Gershon AS,
Leung RS, Hawker G and Tomlinson G: Untreated obstructive sleep
apnea and the risk for serious long-term adverse outcomes: A
systematic review. Sleep Med Rev. 18:49–59. 2014. View Article : Google Scholar
|
|
5
|
Shiao TH, Liu CJ, Luo JC, Su KC, Chen YM,
Chen TJ, Chou KT, Shiao GM and Lee YC: Sleep apnea and risk of
peptic ulcer bleeding: A nationwide population-based study. Am J
Med. 126:249–255.e1. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsukita S, Furuse M and Itoh M:
Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol.
2:285–293. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zahraoui A, Louvard D and Galli T: Tight
junction, a platform for trafficking and signaling protein
complexes. J Cell Biol. 151:F31–F36. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mitic LL, Van Itallie CM and Anderson JM:
Molecular physiology and pathophysiology of tight junctions I.
Tight junction structure and function: Lessons from mutant animals
and proteins. Am J Physiol Gastrointest Liver Physiol.
279:G250–G254. 2000.PubMed/NCBI
|
|
9
|
Lurie A: Inflammation, oxidative stress,
and procoagulant and thrombotic activity in adults with obstructive
sleep apnea. Adv Cardiol. 46:43–66. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prabhakar NR: Oxygen sensing during
intermittent hypoxia: cellular and molecular mechanisms. J Appl
Physiol (1985). 90:1986–1994. 2001.
|
|
11
|
Bonsignore MR and Eckel J: ERS Meeting
Report. Metabolic aspects of obstructive sleep apnoea syndrome. Eur
Respir Rev. 18:113–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
González-Mariscal L, Tapia R and Chamorro
D: Crosstalk of tight junction components with signaling pathways.
Biochim Biophys Acta. 1778:729–756. 2008. View Article : Google Scholar
|
|
13
|
Feng J, Wang QS, Chiang A and Chen BY: The
effects of sleep hypoxia on coagulant factors and hepatic
inflammation in emphysematous rats. PLoS One. 5:e132012010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chiu CJ, McArdle AH, Brown R, Scott HJ and
Gurd FN: Intestinal mucosal lesion in low-flow states. I A
morphological, hemodynamic, and metabolic reappraisal. Arch Surg.
101:478–483. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Lavie L: Obstructive sleep apnoea syndrome
- an oxidative stress disorder. Sleep Med Rev. 7:35–51. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng X, Mao Y, Cai J, Li Y, Liu W, Sun P,
Zhang JH, Sun X and Yuan H: Hydrogen-rich saline protects against
intestinal ischemia/reperfusion injury in rats. Free Radic Res.
43:478–484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang R, Gallo DJ, Baust JJ, Watkins SK,
Delude RL and Fink MP: Effect of hemorrhagic shock on gut barrier
function and expression of stress-related genes in normal and
gnotobiotic mice. Am J Physiol Regul Integr Comp Physiol.
283:R1263–R1274. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ban K, Peng Z and Kozar RA: Inhibition of
ERK1/2 worsens intestinal ischemia/reperfusion injury. PLoS One.
8:e767902013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou W, Li S, Wan N, Zhang Z, Guo R and
Chen B: Effects of various degrees of oxidative stress induced by
intermittent hypoxia in rat myocardial tissues. Respirology.
17:821–829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan G, Khan SA, Luo W, Nanduri J, Semenza
GL and Prabhakar NR: Hypoxia-inducible factor 1 mediates increased
expression of NADPH oxidase-2 in response to intermittent hypoxia.
J Cell Physiol. 226:2925–2933. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nanduri J, Vaddi DR, Khan SA, Wang N,
Makarenko V, Semenza GL and Prabhakar NR: HIF-1α activation by
intermittent hypoxia requires NADPH oxidase stimulation by xanthine
oxidase. PLoS One. 10:e01197622015. View Article : Google Scholar
|
|
23
|
Yuan G, Nanduri J, Khan S, Semenza GL and
Prabhakar NR: Induction of HIF-1alpha expression by intermittent
hypoxia: Involvement of NADPH oxidase, Ca2+ signaling,
prolyl hydroxylases, and mTOR. J Cell Physiol. 217:674–685. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nanduri J, Yuan G, Kumar GK, Semenza GL
and Prabhakar NR: Transcriptional responses to intermittent
hypoxia. Respir Physiol Neurobiol. 164:277–281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peng YJ, Yuan G, Ramakrishnan D, Sharma
SD, Bosch-Marce M, Kumar GK, Semenza GL and Prabhakar NR:
Heterozygous HIF-1alpha deficiency impairs carotid body-mediated
systemic responses and reactive oxygen species generation in mice
exposed to intermittent hypoxia. J Physiol. 577:705–716. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ryan S, Taylor CT and McNicholas WT:
Systemic inflammation: A key factor in the pathogenesis of
cardiovascular complications in obstructive sleep apnoea syndrome?
Thorax. 64:631–636. 2009.PubMed/NCBI
|
|
27
|
Ryan S, McNicholas WT and Taylor CT: A
critical role for p38 map kinase in NF-kappaB signaling during
intermittent hypoxia/reoxygenation. Biochem Biophys Res Commun.
355:728–733. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Greenberg HE, Sica AL, Scharf SM and
Ruggiero DA: Expression of c-fos in the rat brainstem after chronic
intermittent hypoxia. Brain Res. 816:638–645. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peterson LW and Artis D: Intestinal
epithelial cells: Regulators of barrier function and immune
homeostasis. Nat Rev Immunol. 14:141–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hering NA, Fromm M and Schulzke JD:
Determinants of colonic barrier function in inflammatory bowel
disease and potential therapeutics. J Physiol. 590:1035–1044. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Saeedi B, Kendrick A, Schwisow K, Bayless
A, Colgan S and Glover L: A role for hypoxia inducible factor in
the junctional integrity and barrier function of intestinal
epithelial cells (60.1). FASEB J. 28:S60.12014.
|
|
32
|
Furuta GT, Turner JR, Taylor CT, Hershberg
RM, Comerford K, Narravula S, Podolsky DK and Colgan SP:
Hypoxia-inducible factor 1-dependent induction of intestinal
trefoil factor protects barrier function during hypoxia. J Exp Med.
193:1027–1034. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guma M, Stepniak D, Shaked H, Spehlmann
ME, Shenouda S, Cheroutre H, Vicente-Suarez I, Eckmann L, Kagnoff
MF and Karin M: Constitutive intestinal NF-κB does not trigger
destructive inflammation unless accompanied by MAPK activation. J
Exp Med. 208:1889–1900. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fischer A, Gluth M, Pape UF, Wiedenmann B,
Theuring F and Baumgart DC: Adalimumab prevents barrier dysfunction
and antagonizes distinct effects of TNF-α on tight junction
proteins and signaling pathways in intestinal epithelial cells. Am
J Physiol Gastrointest Liver Physiol. 304:G970–G979. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ye D, Ma I and Ma TY: Molecular mechanism
of tumor necrosis factor-alpha modulation of intestinal epithelial
tight junction barrier. Am J Physiol Gastrointest Liver Physiol.
290:G496–G504. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tang Y, Clayburgh DR, Mittal N, Goretsky
T, Dirisina R, Zhang Z, Kron M, Ivancic D, Katzman RB, Grimm G, et
al: Epithelial NF-kappaB enhances transmucosal fluid movement by
altering tight junction protein composition after T cell
activation. Am J Pathol. 176:158–167. 2010. View Article : Google Scholar :
|
|
37
|
Chen ML, Ge Z, Fox JG and Schauer DB:
Disruption of tight junctions and induction of proinflammatory
cytokine responses in colonic epithelial cells by Campylobacter
jejuni. Infect Immun. 74:6581–6589. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Al-Sadi R, Ye D, Boivin M, Guo S, Hashimi
M, Ereifej L and Ma TY: Interleukin-6 modulation of intestinal
epithelial tight junction permeability is mediated by JNK pathway
activation of claudin-2 gene. PLoS One. 9:e853452014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Taylor CT and Colgan SP: Hypoxia and
gastrointestinal disease. J Mol Med Berl. 85:1295–1300. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Colgan SP and Taylor CT: Hypoxia: An alarm
signal during intestinal inflammation. Nat Rev Gastroenterol
Hepatol. 7:281–287. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Eltzschig HK and Carmeliet P and Carmeliet
P: Hypoxia and inflammation. N Engl J Med. 364:656–665. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sitkovsky M and Lukashev D: Regulation of
immune cells by local-tissue oxygen tension: HIF1 α and adenosine
receptors. Nat Rev Immunol. 5:712–721. 2005. View Article : Google Scholar : PubMed/NCBI
|