Introduction
Gwakhyangjeonggi-san (GHJGS) is a traditional Korean
herbal formula composed of the following 13 medicinal herbs,
Agastache rugosa, Perilla frutescens, Angelica
dahurica, Areca catechu, Poria cocos, Magnolia
officinalis, Atractylodes macrocephala, Citrus
reticulata, Pinelliaternata, Platycodon
grandiflorum, Glycyrrhiza uralensis, Ziziphus
jujuba and Zingiber officinale. It has been used for
treating diarrhea-predominant irritable bowel syndrome (1). In addition, GHJGS has been identified
as an effective treatment for allergies (2), respiratory (3) and cardiovascular (4) diseases, and bacterial infections
(5). However, to the best of our
knowledge, there have been no reports to date on the
anti-inflammatory effect of GHJGS.
Inflammation is a protective response against
various harmful stimuli, such as pathogens, damaged cells and
irritants (6). This response is
controlled by production of proinflammatory biomolecules (7,8).
Overproduction of the proinflammatory cytokines, tumor necrosis
factor-α (TNF-α) and interleukin-6 (IL-6), and the proinflammatory
mediator, prostaglandin E2 (PGE2) may result
in inflammatory disorders accompanied by fever, tissue destruction
or pain (7,8). Therefore, targeting these
proinflammatory cytokines or PGE2 is considered to be a
potential therapeutic approach for treating inflammatory disorders.
Mitogen-activated protein kinase (MAPK) and/or nuclear factor-κB
(NF-κB) signaling pathways are important in the regulation of
inflammatory responses, including triggering the initiation of
proinflammatory cytokine production (9). Additionally, previous studies have
reported a link between anti-inflammatory and antioxidative
regulation using various natural products through activation of
heme oxygenase-1 (HO-1), an enzyme with antioxidant effects
(10–12).
In the present study, the anti-inflammatory and
antioxidant activity of GHJGS was investigated using the murine
macrophage cell line, RAW 264.7. The inflammatory reaction was
induced by lipopolysaccharide (LPS) stimulation and the production
of TNF-α, IL-6, and PGE2 was examined using
enzyme-linked immunosorbent assays (ELISAs). In addition, the
effects of GHJGS on activation of MAPK and NF-κB signaling
pathways, and the expression of HO-1 in RAW 264.7 cells were
investigated.
Materials and methods
Plant materials
The 13 herbs that form GHJGS were purchased from
Kwangmyungdang Medicinal Herbs (Ulsan, South Korea). The taxonomic
classification of the 13 herbs was verified by Professor Je-Hyun
Lee from Dongguk University (Gyeongju, South Korea). Voucher
specimens (2012-KE32-1 to KE32-13) were deposited at the K-herb
Research Center, Korea Institute of Oriental Medicine (Daejeon,
South Korea).
Chemicals and reagents
Liquiritin, glycyrrhizin and 6-gingerol were
purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Hesperidin and rosmarinic acid were purchased from Acros Organics
(Morris, NJ, USA) and Sigma-Aldrich (St. Louis, MO, USA),
respectively. The purity of each component was determined to be
≥98% using high-performance liquid chromatography (HPLC) analysis.
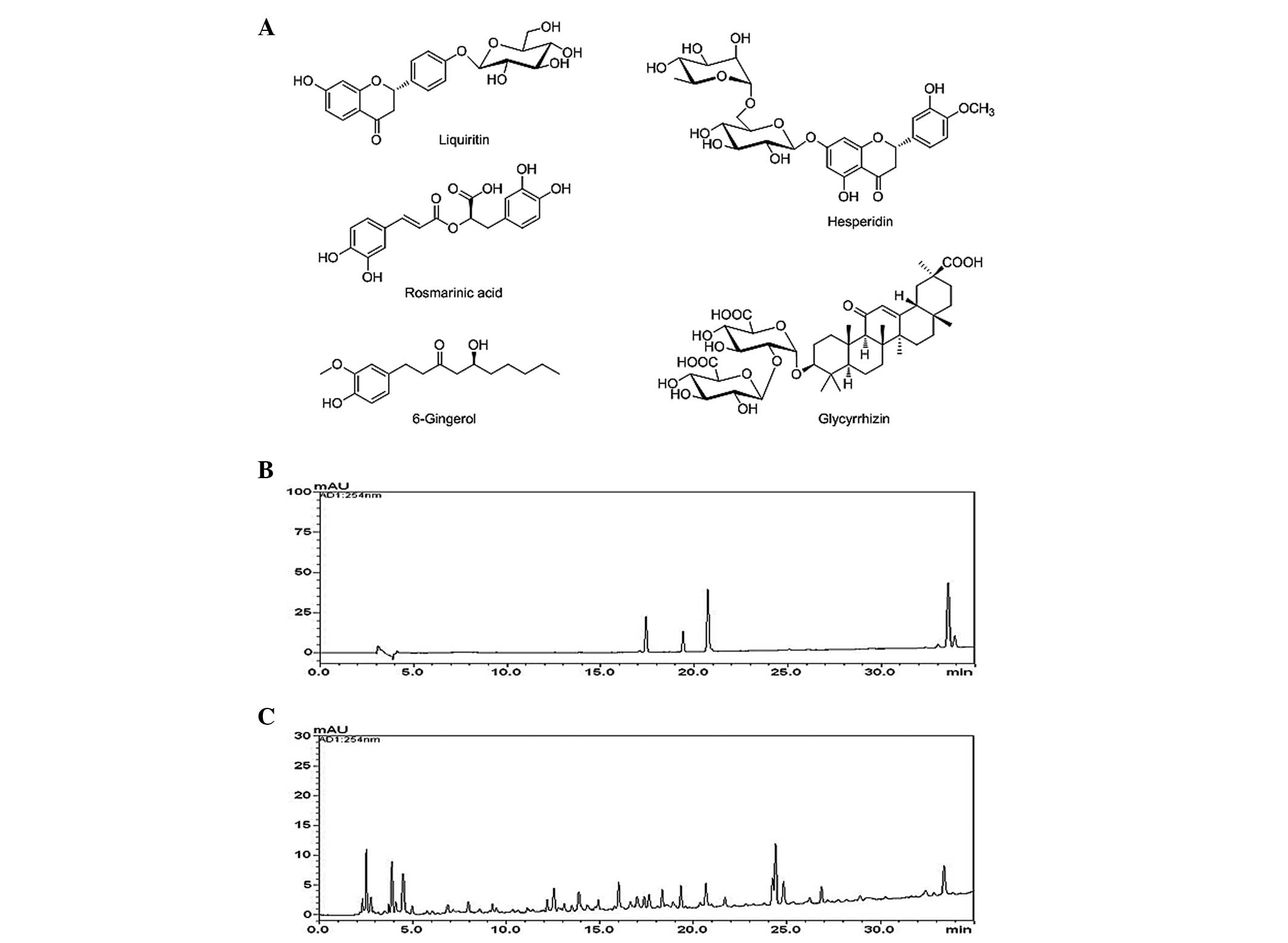
The chemical structures of the five marker compounds are presented
in Fig. 1A. HPLC-grade reagents,
methanol, acetonitrile and distilled water were obtained from J.T.
Baker; Avanto Performance Materials (Phillipsburg, NJ, USA). Acetic
acid was obtained from Merck KGaA (Darmstadt, Germany).
Preparation of GHJGS decoction
GHJGS was composed of 13 herbs (Table I; total weight, 5.0 kg, ~148.15
times the composition of a single dose) and extracted in distilled
water at 100°C for 2 h under 98 kPa pressure using a COSMOS-660
electric extractor (KyungSeo Machine Co., Incheon, South Korea).
The extracted solution was filtered using a standard sieve (no.
270; mesh size, 53 µm; Chung Gye Sang Gong Sa, Seoul, Korea)
and freeze-dried. The yield of the extract was 12.89% (644.5 g).
The lyophilized GHJGS extract (40 mg) was dissolved in 50% methanol
(20 ml) and mixed for quantitative analysis. The solution was
filtered through a 0.2-µm SmartPor GHP syringe filter (Woong
Ki Science Co., Ltd., Seoul, South Korea) prior to being injected
into a HPLC column.
 | Table IComposition of
Gwakhyangjeonggi-san. |
Table I
Composition of
Gwakhyangjeonggi-san.
| Latin name | Scientific
name | Quantity (g) | Origin |
|---|
| Agastachis
Herba | Agastache
rugosa (Fisch. et Meyer) O. Kuntze | 833.30 | Andong, South
Korea |
| Perillae Herba | Perilla
frutescens var. crispa (Thunb.) H. Deane | 555.56 | Yeongcheon, South
Korea |
| Angelicae Dahuricae
Radix | Angelica
dahurica Benth. et Hook. f. | 277.78 | Uljin, South
Korea |
| Arecae
Pericarpium | Areca
catechu L. | 277.78 | China |
| Hoelen | Poria cocos
F. A. Wolf | 277.78 | Pyeongchang, South
Korea |
| Magnoliae
Cortex | Magnolia
officinalis Rehd. et E. H. Wils. | 277.78 | China |
| Atractylodis
Rhizoma Alba | Atractylodes
macrocephala Koidz. | 277.78 | China |
| Citri Unshius
Pericarpium | Citrus
reticulata Blanco | 277.78 | Jeju, South
Korea |
| Pinelliae
Tuber | Pinellia
ternata Breit. | 277.78 | China |
| Platycodi
Radix | Platycodon
grandiflorum A. DC. | 277.78 | Andong, South
Korea |
| Glycyrrhizae Radix
et Rhizoma | Glycyrrhiza
uralensis Fisch. | 277.78 | China |
| Zizyphi
Fructus | Ziziphus
jujuba var. inermis (Bunge) Rehder | 555.56 | Yeongcheon, South
Korea |
| Zingiberis Rhizoma
Crudus | Zingiber
officinale Rosc. | 555.56 | Ulsan, South
Korea |
| Total | | 5,000 | |
Quantitative analysis of GHJGS
The quantitative determination was performed using a
Prominence LC-20A series HPLC system (Shimadzu Corporation, Kyoto,
Japan) consisting of a solvent delivery unit (LC-20AT), online
degasser (DGU-20A3), column oven (CTO-20A), auto sample injector
(SIL-20AC), and photodiode array (PDA) detector (SPD-M20A). Data
were collected and processed using LC solution software (version
1.24; Shimadzu Corporation). A Gemini C18 column (250 mm × 4.6 mm;
particle size, 5 µm; Phenomenex, Inc., Torrance, CA, USA)
was used for separation of the marker compounds and maintained at
40°C. The mobile phases consisted of 1.0% (v/v) acetic acid in
distilled water (designated as A) and 1.0% (v/v) acetic acid in
acetonitrile (designated as B). The gradient flow was as follows:
5–70% B for 0–40 min; 70–100% B for 40–50 min; 100% B for 50–55
min; and 100-5% B for 55–60 min. The analysis was conducted at 1.0
ml/min with PDA detection at 254 nm (glycyrrhizin), 280 nm
(liquiritin, hesperidin and 6-gingerol), and 330 nm (rosmarinic
acid). The sample injection volume was 10 µl.
Cell culture
The murine macrophage cell line, RAW 264.7, was
obtained from the American Type Culture Collection (Rockville, MD,
USA). The cells were cultured in Dulbecco's modified Eagle's medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 5.5% heat-inactivated fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.), penicillin (100 U/ml; HyClone
Laboratories, Inc., Logan, UT, USA), and streptomycin (100
µg/ml; HyClone Laboratories, Inc.) in an incubator with 5%
CO2 at 37°C.
Cytotoxicity assay
Cell viability assay was performed to determine the
cytotoxicity of GHJGS using a Cell Counting Kit-8 (CCK-8; Dojindo
Molecular Technologies, Inc., Kumamoto, Japan). Cells were plated
onto a 96-well microplate at 3×103 cells/well and
treated with 0, 15.625, 31.25, 62.5, 125, 250, 500 or 1,000
µg/ml GHJGS for 24 h. Following incubation with CCK-8
reagent for 4 h, optical density (OD) at a wavelength of 450 nm was
determined using a Benchmark plus microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Cell viability was
calculated using the following equation: Cell viability (%) = mean
ODGHGJS-treated cells / mean ODuntreated
cells ×100
ELISAs for TNF-α, IL-6, and
PGE2
Cells were pretreated with 0, 250, 500 or 1,000
µg/ml GHJGS for 4 h and stimulated with LPS (1 µg/ml)
for an additional 20 h. Production of TNF-α, IL-6 and
PGE2 in the culture supernatants was measured using
commercial ELISA kits from R&D Systems, Inc. (Minneapolis, MN,
USA), BD Biosciences (San Jose, CA, USA) and Cayman Chemical
Company (Ann Arbor, MI, USA), respectively. Indomethacin (2.5
ng/ml; Sigma-Aldrich) was used as a positive control.
Western blotting
Whole cell extract (WCE) was prepared by suspending
cells using the Mammalian Cell Lysis kit (Sigma-Aldrich) containing
protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN,
USA). Nuclear extract (NE) was isolated using NE-PER®
Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher
Scientific, Inc., Rockford, IL, USA) according to the
manufacturer's protocol. The protein concentration was determined
using the Bio-Rad Protein Assay kit II (Bio-Rad Laboratories,
Inc.). Equal quantities of cell extract (30 µg) were
resolved by 4–20% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (Bio-Rad Laboratories, Inc.) at 100 v for 1 h and
transferred to a polyvinylidene fluoride membrane (GE Healthcare
Life Sciences, Piscataway, NJ, USA). The membrane was incubated
with blocking solution [5% skimmed milk in Tris-buffered saline
containing Tween-20 (TBST); DyneBio, Seongnam, Korea], followed by
an overnight incubation at 4°C with the appropriate primary
antibody, including rabbit polyclonal phosphorylated (p)-p38 MAPK
(1:1,000 dilution; cat. no. 9211; Cell Signaling Technology, Inc.,
Danvers, MA, USA), rabbit polyclonal p-extracellular
signal-regulated kinase (ERK; 1:1,000 dilution; cat. no. 9101; Cell
Signaling Technology, Inc.), rabbit polyclonal p-c-Jun N-terminal
kinase (JNK; 1:1,000 dilution; cat. no. 9251; Cell Signaling
Technology, Inc.), mouse monoclonal HO-1 (1:1,000 dilution; cat.
no. ab13248; Abcam, Boston, MA, USA), rabbit polyclonal NF-κB p65
(1:1,000; cat. no. sc-372; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) and mouse monoclonal β-actin (1:5,000; cat. no. sc-47778;
Santa Cruz Biotechnology, Inc.). The membranes were washed three
times with TBST, and then incubated with polyclonal horseradish
peroxidase-conjugated goat anti-mouse IgG (cat. no. 115-001-003;
Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA;
1:2,000 dilution) and goat anti-rabbit IgG (cat. no. 111-001-003;
Jackson ImmunoResearch Laboratories, Inc.; 1:2,000 dilution)
secondary antibodies for 1 h at room temperature. The membranes
were washed three times with TBST, and developed using the
SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher
Scientific, Inc.). Image capture was performed using Chemi-Doc
(Bio-Rad Laboratories, Inc.).
Reactive oxygen species (ROS)
staining
To examine the generation of ROS, the ROS-ID™ Total
ROS Detection kit (Enzo Life Sciences, Inc., Plymouth Meeting, PA,
USA) was used. The effect of GHJGS on ROS generation was examined
by immunofluorescence staining. Cells were plated on
µ-Dishes 35 mm (Ibidi, Aarhus, Denmark), treated with GHJGS
(1,000 µg/ml) and LPS (1 µg/ml) for 30 min, and fixed
in 4% paraformaldehyde (Sigma-Aldrich) and 100% acetone
(Sigma-Aldrich). The ROS detection solution was loaded to the cells
and incubated at room temperature for 1 h. Following the addition
of a mounting medium (Vector Laboratories Inc., Burlingame, CA,
USA), the stained cells were visualized using a FLUOVIEW FV10i
confocal microscope (Olympus Corporation, Tokyo, Japan).
Statistical analysis
The data are expressed as the mean ± standard error
of the mean. Data were analyzed using one-way analysis of variance
and Dunnett's multiple comparisons test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Quantitative determination of the five
marker compounds in GHJGS
The novel HPLC-PDA method was applied for
simultaneous quantification of the five marker compounds in GHJGS.
The typical chromatogram patterns for standard compounds and the
GHJGS decoction are presented in Fig.
1B and C. The retention times of the liquiritin, hesperidin,
rosmarinic acid, glycyrrhizin and 6-gingerol were ~ 17.35, 19.35,
20.66, 33.39, and 33.88 min, respectively. The concentrations of
the five components were between 1.18 and 3.16 mg/g (Table II).
 | Table IIConcentration of the five marker
compounds in Gwakhyangjeonggi-san by high-performance liquid
chromatography. |
Table II
Concentration of the five marker
compounds in Gwakhyangjeonggi-san by high-performance liquid
chromatography.
| Compound | Concentration
| Source |
|---|
| Mean (mg/g;
n=3) | SD | RSD (%) |
|---|
| Liquiritin | 1.18 | 0.01 | 1.08 | Glycyrrhiza
uralensis |
| Hesperidin | 3.16 | 0.01 | 0.27 | Camellia
reticulata |
| Rosmarinic
acid | 1.45 | 0.01 | 0.74 | Agastache
rugosa and Perilla frutescens |
| Glycyrrhizin | 3.03 | 0.02 | 0.58 | Glycyrrhiza
uralensis |
| 6-Gingerol | 1.35 | 0.02 | 1.13 | Zingiber
officinale |
GHJGS inhibits production of TNF-α, IL-6
and PGE2 in LPS-stimulated RAW 264.7 cells
Cytotoxicity of GHJGS was evaluated using RAW 264.7
cells. Cells were treated with serial dilutions of GHJGS for 24 h.
Fig. 2 demonstrates that no
cytotoxic effect was observed up to 1,000 µg/ml GHJGS
treatment. For the subsequent assays, cell treatment with GHJGS was
performed in the nontoxic concentration range (250-1,000
µg/ml).
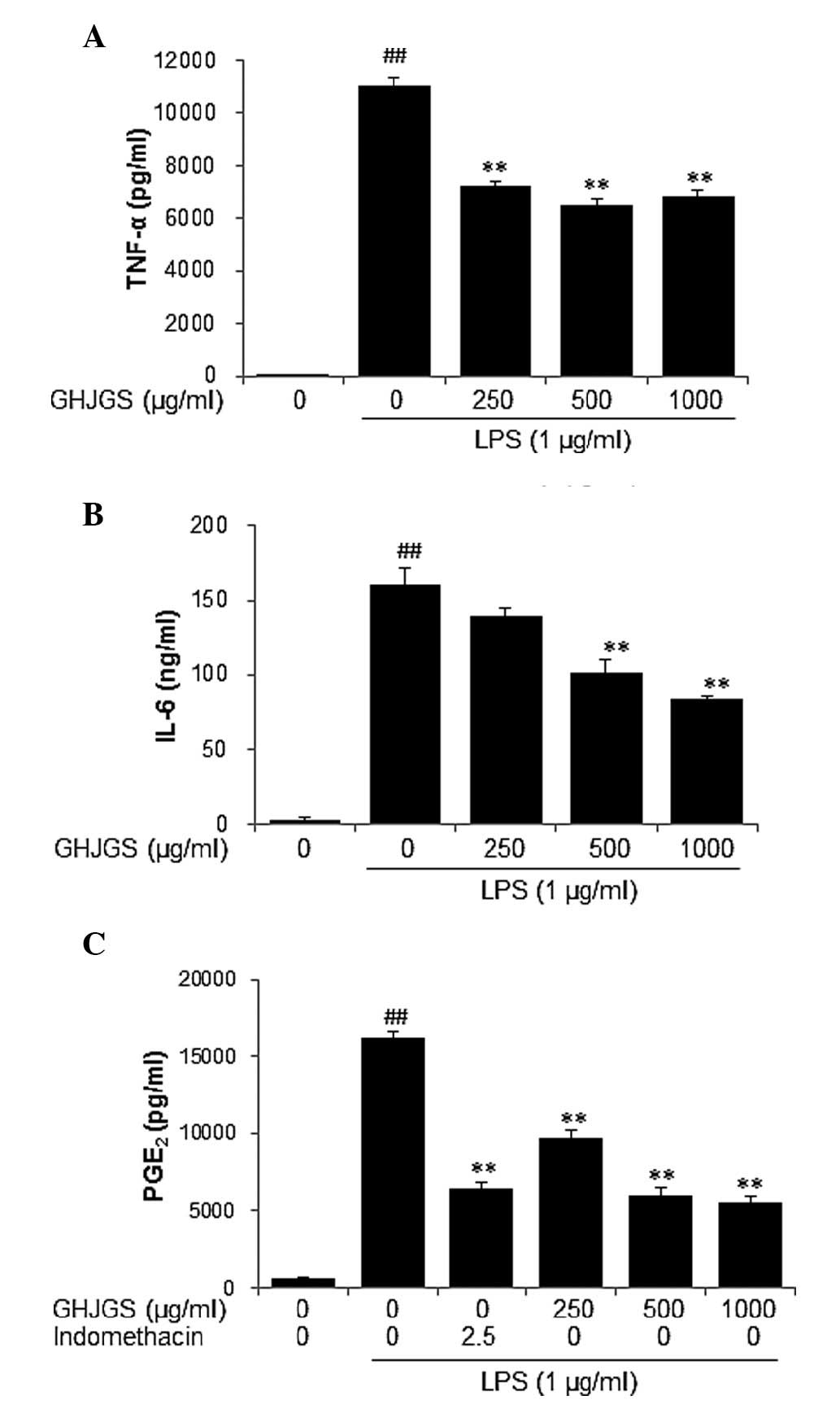
GHJGS reduces the levels of TNF-α. IL-6
and PGE2 in LPS-stimulated RAW 264.7 cells
To examine the anti-inflammatory effect of GHJGS,
production of TNF-α and IL-6 was assessed in LPS-stimulated RAW
264.7 cells. LPS stimulation significantly increased levels of
TNF-α and IL-6 in RAW 264.7 cells, compared with untreated
controls. By contrast, GHJGS treatment significantly reduced
LPS-induced production of TNF-α and IL-6 (P<0.01; Fig. 3A and B). The quantity of
PGE2 was also determined and indomethacin served as a
positive control. The level of PGE2 was significantly
increased in cells treated with LPS alone. By contrast, GHJGS
treatment significantly reduced PGE2 production by LPS
stimulation (P<0.01; Fig.
3C).
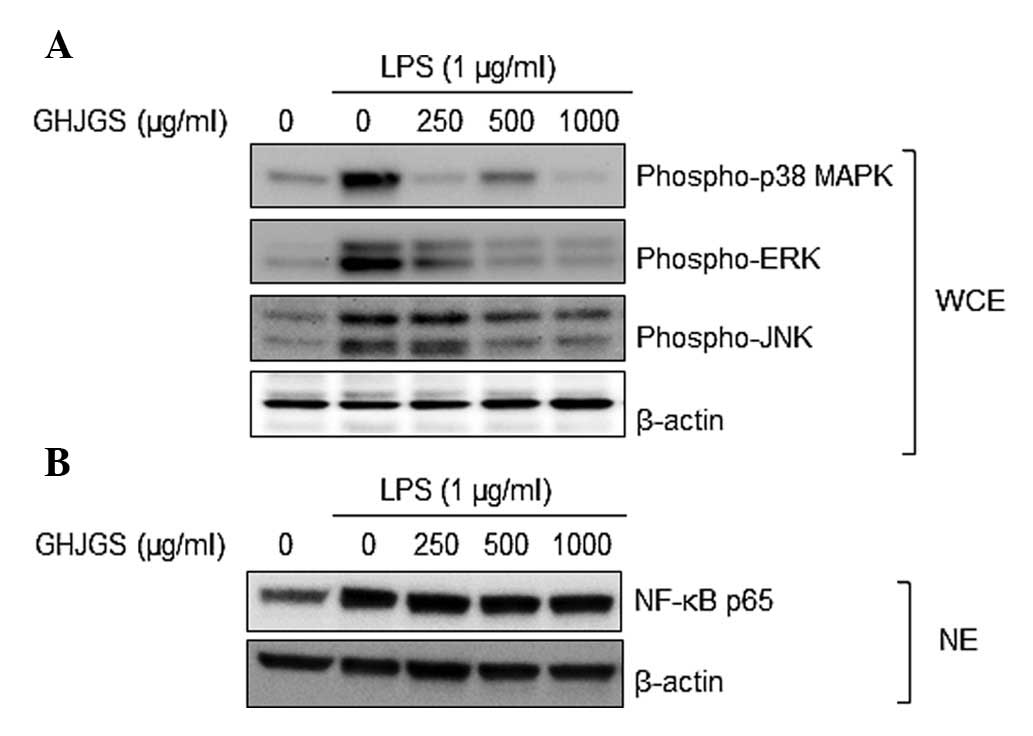
GHJGS suppresses phosphorylation of MAPK
family proteins in LPS-stimulated RAW 264.7 cells
As demonstrated by Fig.
4A, LPS markedly enhanced phosphorylation of p38 MAPK, ERK and
JNK in RAW 264.7 cells, compared with untreated controls.
LPS-induced activation of MAPKs was inhibited when cells were
pretreated with GHJGS. NF-κB p65 expression in the nucleus of RAW
264.7 cells was also analyzed. LPS increased the expression level
of NF-κB p65, compared with that of untreated controls. When cells
were exposed to LPS and GHJGS, the GHJGS exerted no influence on
NF-κB p65 in the LPS-stimulated RAW 264.7 cells (Fig. 4B).
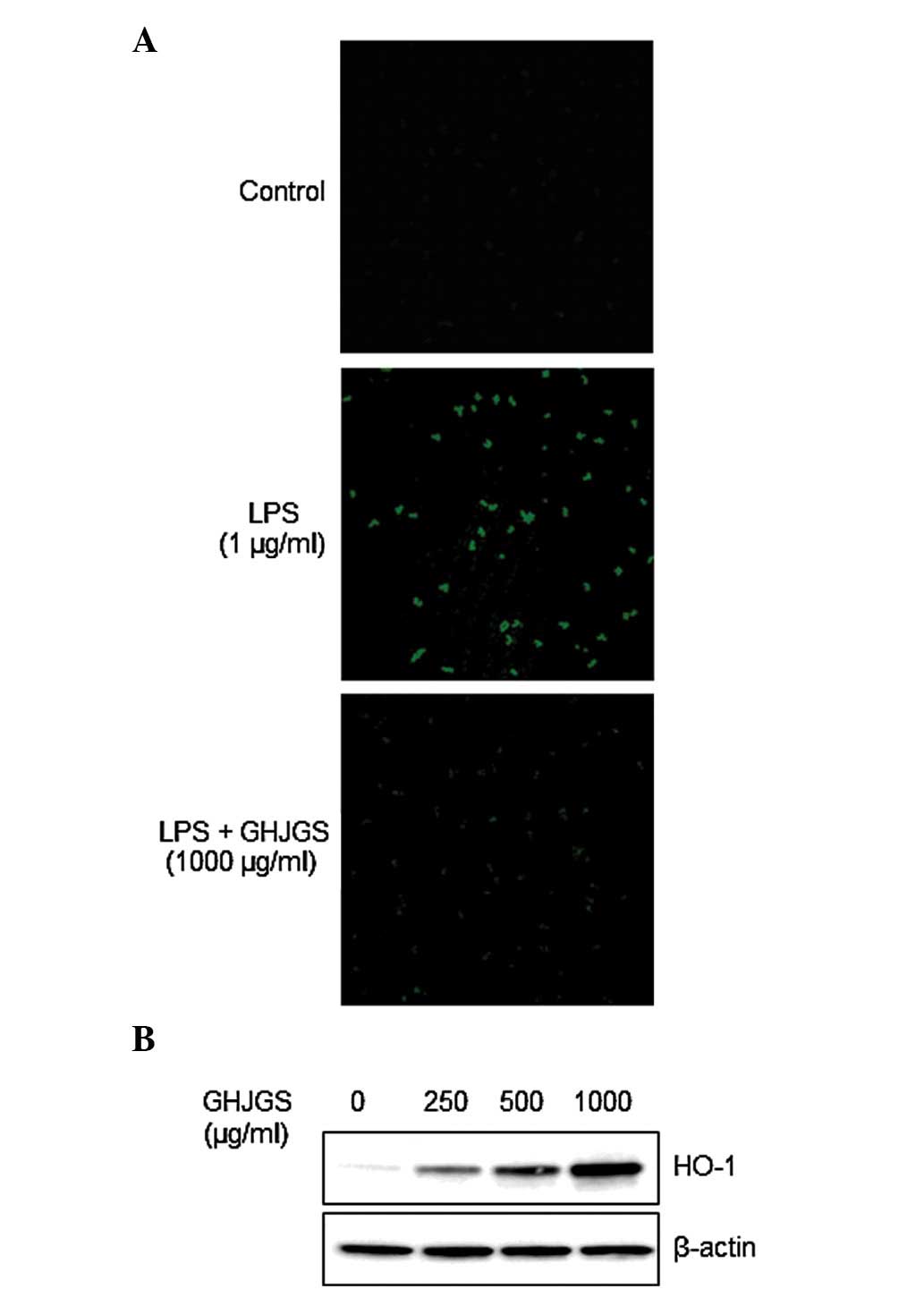
GHJGS induces HO-1 expression and blocks
LPS-induced ROS generation in RAW 264.7 cells
Cells distinctly stained with green fluorescence
were observed in LPS-stimulated cells compared with the
undifferentiated control (Fig.
5A). By contrast, GHJGS treatment blocked LPS-mediated ROS
generation (Fig. 5A).
Additionally, GHJGS induced HO-1 expression in a dose-dependent
manner (Fig. 5B).
Discussion
Although the global market share for synthetic
therapeutic agents is gradually increasing annually, various
negative features, including toxicity and severe side effects, may
limit their therapeutic efficacies and result in reduced quality of
life (13,14). To overcome the issues associated
with synthetic therapeutic agents, natural products (including
herbal medicines) have been considered as a valuable source for
establishing novel remedies for numerous years (15,16).
Therefore, the present study aimed to determine the
anti-inflammatory and antioxidant effects of the herbal formula,
GHJGS using in vitro experimental models. GHJGS suppressed
LPS-stimulated production of TNF-α, IL-6 and PGE2, and
inhibited LPS-mediated phosphorylation of MAPKs. GHJGS increased
the scavenging activity of
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) and
di(phenyl)-(2,4,6-trinitrophenyl)iminoazanium radicals, and reduced
low-density lipoprotein oxidation (data not shown). In addition,
GHJGS enhanced the level of HO-1 expression and reduced the
LPS-induced generation of ROS in R AW 264.7 macrophages.
Table I presents
the relative quantities and origin of the 13 herbs that form GHJGS.
To improve quality control of GHJGS, simultaneous analysis of the
marker compounds in GHJGS were conducted using the HPLC-PDA method.
The primary active ingredients of each herb are as follows:
Phenylpropanoids (e.g. rosmarinic acid) from A. rugose
(17), phenylpropanoids (e.g.
rosmarinic acid) and flavonoids (e.g. luteolin) from P.
frutescens (18, 19), coumarins (e.g. imperatorin) from
A. dahurica (20),
coumarins (e.g. catechin) from A. catechu (21), triterpenoids (e.g. pachymic acid)
from P. cocos (22),
lignans (e.g. magnolol) from M. officinalis (23), sesquiterpenoids (e.g.
atractylenolide I) from A. macrocephala (24), flavonoids (e.g. hesperidin) from
C. reticulate (25),
phenolic acid (e.g. homogentisic acid) and phenolic aldehyde (e.g.
3,4-dihydroxybenzaldehyde) from P. ternate (26), triterpenoids (e.g. platycodin D)
from P. grandiflorum (27),
triterpene saponin (e.g. glycyrrhizin) and flavonoids (e.g.
liquiritin and liquiritigenin) from G. uralensis (28), flavonoids (e.g. spinosin and
6‴-feruloylspinosin) from Z. jujube (29), and phenols (e.g. 6-gingerol) from
Z. officinale (30). Among
those components, five compounds were investigated, including
liquiritin and glycyrrhizin (G. uralensis), hesperidin
(C. reticulata), rosmarinic acid (A. rugosa and P.
frutescens), and 6-gingerol (Z. officinale) using
HPLC-PDA. Consequently, hesperidin (3.16 mg/g) and glycyrrhizin
(3.03 mg/g), marker compounds of C. reticulata and G.
uralensis, respectively, were identified as the predominant
components.
The inhibitory effects of GHJGS on inflammatory
response were evaluated using the murine macrophage cell line, RAW
264.7. Macrophages are involved in the initiation, maintenance and
resolution of inflammation (31,32),
and thus considered to be useful for inflammation-associated
studies. Activated macrophages stimulate production of
proinflammatory cytokines, such as TNF-α and IL-6 during
pathological conditions of inflammatory disease (33). Significant increases in the
production of TNF-α and IL-6 in LPS-stimulated RAW 264.7 cells were
observed in the current study. GHJGS significantly inhibited TNF-α
and IL-6 production induced by LPS treatment. PGE2, a
proinflammatory mediator, is produced through inflammatory
stimulation of cyclooxygenase-2 (34). LPS stimulation significantly
increased the level of PGE2, whereas GHJGS treatment
markedly reduced LPS-mediated PGE2 production in R AW
264.7 cells. These results indicate the anti-inflammatory
properties of GHJGS.
Production of proinflammatory factors, including
TNF-α, IL-6, and PGE2, is regulated by numerous
intracellular signaling pathways at the transcription and
post-transcription level (9).
Inflammatory stimuli, such as LPS, activate MAPK and/or NF-κB
signaling pathways associated with inflammatory cytokine production
(35–37). In the present study, LPS
stimulation markedly enhanced the levels of p-p38 MAPK, p-ERK and
p-JNK, and nuclear expression levels of NF-κB p65 in R AW 264.7
cells. By contrast, GHJGS suppressed LPS-induced phosphorylation of
p38 MAPK, ERK and JNK. However, NF-κB activation was not altered by
GHJGS in LPS-treated RAW 264.7 cells.
Inflammation is associated with oxidative stress.
During inflammation, ROS generation is a critical event in the
elimination of pathogens (38),
and induces production of proinflammatory cytokines and molecules
(32,39). Thus, the present study examined
whether GHJGS has an inhibitory effect on ROS generation and
determined that GHJGS suppressed LPS-induced ROS generation in
macrophages. HO-1, a stress-inducible and redox-sensitive enzyme,
is important during the inflammatory response (40). HO-1 negatively regulates production
of proinflammatory cytokines, such as TNF-α, IL-1β and IL-6 in
activated macrophages (41). Thus,
HO-1 is a potential molecular target against inflammation and
oxidative stress (42). The
current study identified that GHJGS increased the expression of
HO-1 in a dose-dependent manner.
In conclusion, the findings of the present study
demonstrate that GHJGS inhibits LPS-stimulated production of
proinflammatory biomarkers, TNF-α, IL-6 and PGE2 through
suppression of the MAPK signaling pathway. Furthermore, GHJGS
inhibited ROS generation and enhanced HO-1 expression. Overall,
these findings confirm the anti-inflammatory and antioxidant
actions of GHJGS, thus presenting it as a potential candidate for
targeting inflammatory diseases and oxidative stress-associated
diseases.
Acknowledgments
The present study was supported by the Korea
Institute of Oriental Medicine (grant no. K15250).
References
|
1
|
Yun HS, Ryu BH, Park DW and Ryu KW:
Experimental comparative studies on the effects of
Kwakhyangjeonggisan and Souminkwakhyang-jeonggisan. K H Univ O Med
J. 21:197–211. 1998.In Korean.
|
|
2
|
Chuan-xing Y and Ling Z: Experimental
researches on inhibitory effect of Huoxiang Zhengqi liquid on
histamine release. Chin J Integr Med. 9:276–280. 2003.In Chinese.
View Article : Google Scholar
|
|
3
|
Xie C, Wang XF, Qi XJ, Lu LL and Chan HC:
Effect of Huoxiang-zhengqi liquid on HCO3- secretion by intact
porcine distal airway epithelium. Sheng Li Xue Bao. 60:90–96.
2008.In Chinese. PubMed/NCBI
|
|
4
|
Koo CM, Sun JK, Kim HH and Nam CG: Effects
of GwakHyangJungGiSan on the arterial contraction in rabbit. Kor
Orient Int Med. 24:260–268. 2003.In Korean.
|
|
5
|
Zhang HK, Huang Y, Li K and Wu SH:
Antibacterial material basis and quality control of Huoxiang
Zhengqi tincture. Chin Tradit Herbal Drugs. 43:1349–1354. 2012.In
Chinese.
|
|
6
|
Ferrero-Miliani L, Nielsen OH, Andersen PS
and Girardin SE: Chronic inflammation: Importance of NOD2 and NALP3
in interleukin-1beta generation. Clin Exp Immunol. 147:227–235.
2007.PubMed/NCBI
|
|
7
|
Huerre MR and Gounon P: Inflammation:
Patterns and new concepts. Res Immunol. 47:417–434. 1996.
View Article : Google Scholar
|
|
8
|
Kalinski P: Regulation of immune responses
by prostaglandin E2. J Immunol. 188:21–28. 2012. View Article : Google Scholar :
|
|
9
|
Saklatvala J, Dean J and Clark A: Control
of the expression of inflammatory response genes. Biochem Soc Symp.
95–106. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Motterlini R, Foresti R, Bassi R and Green
CJ: Curcumin, an antioxidant and anti-inflammatory agent, induces
heme oxygenase-1 and protects endothelial cells against oxidative
stress. Free Radic Biol Med. 28:1303–1312. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu H and Talalay P: Relevance of
anti-inflammatory and antioxidant activities of exemestane and
synergism with sulforaphane for disease prevention. Proc Natl Acad
Sci USA. 110:19065–19070. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee MY, Lee JA, Seo CS, Ha H, Lee H, Son
J-K and Shin H-K: Anti-inflammatory activity of Angelica dahurica
ethanolic extract on RAW264.7 cells via upregulation of heme
oxygenase-1. Food Chem Toxic. 49:1047–1055. 2011. View Article : Google Scholar
|
|
13
|
Gurney SM, Scott KS, Kacinko SL, Presley
BC and Logan BK: Pharmacology, toxicology, and adverse effects of
synthetic cannabinoid drugs. Forensic Sci Rev. 26:53–78.
2014.PubMed/NCBI
|
|
14
|
He M and Guo QL: Drug-induced fatal
adverse effects in the United States from 1999 to 2004. Zhong Nan
Da Xue Xue Bao Yi Xue Ban. 33:1060–1065. 2008.In Chinese.
PubMed/NCBI
|
|
15
|
Szychowski J, Truchon JF and Bennani YL:
Natural products in medicine. Transformational outcome of synthetic
chemistry. 57:9292–9308. 2014.
|
|
16
|
Koeberle A and Werz O: Multi-target
approach for natural products in inflammation. 19:1871–1882.
2014.
|
|
17
|
Kim HK, Kim YA, Chun JM and Ko BS: Pattern
analysis of Agastachis Herba and Pogostemonis Herba. Korean J
Pharmacogn. 34:274–277. 2003.In Korean.
|
|
18
|
Kim BY, Jeong JS, Kwon HJ, Lee JH and Hong
SP: Determination of rosmarinic acid and caffeic acid from Perilla
frutescens var. japonica and var. acuta by reversed-phase HPLC. Kor
J Herbology. 23:67–72. 2008.In Korean.
|
|
19
|
Jeon IH, Kim HS, Kang HJ, Lee HS, Jeong
SI, Kim SJ and Jang SI: Anti-inflammatory and antipruritic effects
of luteolin from Perilla (P. frutescens L.) leaves. Molecules.
19:6941–6951. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li B, Zhang X, Wang J, Zhang L, Gao B, Shi
S, Wang X, Li J and Tu P: Simultaneous characterisation of fifty
coumarins from the roots of Angelica dahurica by off-line
two-dimensional high-performance liquid chromatography coupled with
electrospray ionisation tandem mass spectrometry. Phytochem Anal.
25:229–240. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu Q, Yang Y and Simon JE: Qualitative and
quantitative HPLC/MS determination of proanthocyanidins in areca
nut (Areca catechu). Chem Biodivers. 4:2817–2826. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li G, Xu ML, Lee CS, Woo MH, Chang HW and
Son JK: Cytotoxicity and DNA topoisomerases inhibitory activity of
constituents from the sclerotium of Poria cocos. Arch Pharm Res.
27:829–833. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang Y, Vaysse J, Gilard V, Balayssac S,
Déjean S, Malet-Martino M, David B, Fiorini C and Barbin Y: Quality
assessment of commercial Magnoliae officinalis Cortex by
1H-NMR-based metabolomics and HPLC methods. Phytochem
Anal. 23:387–395. 2012. View
Article : Google Scholar
|
|
24
|
Tsai CJ, Liang JW and Lin HR:
Sesquiterpenoids from Atractylodes macrocephala act as farnesoid X
receptor and progesterone receptor modulators. Bioorg Med Chem
Lett. 22:2326–2329. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu EH, Zhao P, Duan L, Zheng GD, Guo L,
Yang H and Li P: Simultaneous determination of six bioactive
flavonoids in Citri Reticulatae Pericarpium by rapid resolution
liquid chromatography coupled with triple quadrupole electrospray
tandem mass spectrometry. Food Chem. 141:3977–3983. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Han JH, Jo SG, Lee MJ, Baek SH and Park
SH: Contents of homogentisic acid and 3,4-dihydroxybenzaldehyde in
the Pinellia ternate by various processing method and its safety
estimate. J Orient Physiol Pathol. 18:846–853. 2004.In Korean.
|
|
27
|
Ha YW and Kim YS: Preparative isolation of
six major saponins from Platycodi Radix by high-speed
counter-current chromatography. Phytochem Anal. 20:207–213. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Q and Ye M: Chemical analysis of the
Chinese herbal medicine Gan-Cao (licorice). J Chromatogr A.
1216:1954–1969. 2009. View Article : Google Scholar
|
|
29
|
Niu C and Zhang J: Quantitative analysis
and chromatographic fingerprinting of the semen zizyphi spinosae by
ultra-high-performance liquid chromatography coupled with
diode-array detector. J Sep Sci. 34:2989–2996. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee HR, Lee JH, Park CS, Ra KR, Ha JS, Cha
MH, Kim SN, Choi Y, Hwang J and Nam JS: Physicochemical properties
and antioxidant capacities of different parts of Ginger (Zingiber
officinale Roscoe). J Korean Soc Food Sci Nutr. 43:1369–1379.
2014.In Korean. View Article : Google Scholar
|
|
31
|
Ahmed JS and Mehlhorn H: Review: The
cellular basis of the immunity to and immunopathogenesis of
tropical theileriosis. Parasitol Res. 85:539–549. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fujiwara N and Kobayashi K: Macrophages in
inflammation. Curr Drug Targets Inflamm Allergy. 4:281–286. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bottomley MJ, Webb NJ, Watson CJ, Holt PJ,
Freemont AJ and Brenchley PE: Peripheral blood mononuclear cells
from patients with rheumatoid arthritis spontaneously secrete
vascular endothelial growth factor (VEGF): Specific up-regulation
by tumour necrosis factor-alpha (TNF-alpha) in synovial fluid. Clin
Exp Immunol. 117:171–176. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Crofford LJ, Wilder RL, Ristimäki AP, Sano
H, Remmers EF, Epps HR and Hla T: Cyclooxygenase-1 and -2
expression in rheumatoid synovial tissues. Effects of interleukin-1
beta, phorbol ester, and corticosteroids. J Clin Invest.
93:1095–1101. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim KS, Cui X, Lee DS, Sohn JH, Yim JH,
Kim YC and Oh H: Anti-inflammatory effect of neoechinulin a from
the marine fungus Eurotium sp. SF-5989 through the suppression of
nf-κb and p38 MAPK Pathways in lipopolysaccharide-stimulated
RAW2647 macrophages. Molecules. 18:13245–13259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kwon DJ, Bae YS, Ju SM, Youn GS, Choi SY
and Park J: Salicortin suppresses lipopolysaccharide-stimulated
inflammatory responses via blockade of NF-κB and JNK activation in
RAW 264.7 macrophages. BMB Rep. 47:318–323. 2014. View Article : Google Scholar :
|
|
37
|
Shao J, Li Y, Wang Z, Xiao M, Yin P, Lu Y,
Qian X, Xu Y and Liu J: 7b, a novel naphthalimide derivative,
exhibited anti-inflammatory effects via targeted-inhibiting TAK1
following down-regulation of ERK1/2- and p38 MAPK-mediated
activation of NF-κB in LPS-stimulated RAW264.7 macrophages. Int
Immunopharmacol. 17:216–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Winyard PG, Ryan B, Eggleton P, Nissim A,
Taylor E, Lo Faro ML, Burkholz T, Szabó-Taylor KE, Fox B, Viner N,
et al: Measurement and meaning of markers of reactive species of
oxygen, nitrogen and sulfur in healthy human subjects and patients
with inflammatory joint disease. Biochem Soc Trans. 39:1226–1232.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Singh KB, Maurya BK and Trigun SK:
Activation of oxidative stress and inflammatory factors could
account for histopathological progression of aflatoxin-B1 induced
hepatocarcinogenesis in rat. Mol Cell Biochem. 401:185–196. 2015.
View Article : Google Scholar
|
|
40
|
Ryter SW, Alam J and Choi AM: Heme
oxygenase-1/carbon monoxide: From basic science to therapeutic
applications. Physiol Rev. 86:583–650. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Southan GJ and Szabó C: Selective
pharmacological inhibition of distinct nitric oxide synthase
isoforms. Biochem Pharmacol. 51:383–394. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zabalgoitia M, Colston JT, Reddy SV, Holt
JW, Regan RF, Stec DE, Rimoldi JM, Valente AJ and Chandrasekar B:
Carbon monoxide donors or heme oxygenase-1 (HO-1) overexpression
blocks interleukin-18-mediated NF-kappaB-PTEN-dependent human
cardiac endothelial cell death. Free Radic Biol Med. 44:284–298.
2008. View Article : Google Scholar : PubMed/NCBI
|