Introduction
Cholangiocellular carcinoma (CCC) is a relatively
rare malignant tumor of the bile duct epithelium. In Europe,
approximately 10,000 new cases of CCC are diagnosed every year
(1). CCC is classified into
intrahepatic, perihilar and distal cholangiocarcinoma according to
anatomical location. Currently, complete surgical resection is the
only means for cure in patients with CCC at the early stage
(2). However, most patients are
initially diagnosed at the advanced stage, losing the opportunity
of radical surgical resection (3).
Even following surgery, the 5-year recurrence rate is in the range
of 60–90% (4). The overall 5 year
survival rate of patients with CCC is <5% and median survival
time is ~12–30 months (5). Rapid
invasion and metastatic capabilities of CCC contribute to the poor
prognosis and resistance to the clinical therapeutic strategies
(6). Thus, it is necessary to
understand the precise mechanisms of this process to elucidate
novel therapeutic modalities and improve the prognosis of patients
with CCC.
Ras homolog family member C (RHOC) is a member of
the ras superfamily of GTP-binding proteins, which act as molecular
switches between active GTP-bound and inactive GDP-bound states
(7). The family of RHO genes,
which are important for cell proliferation and motility, have
previously been implicated in tumorigenesis and metastatic
progression (8). The RHO subfamily
includes RHOA, RHOB and RHOC, which share 85% amino acid sequence
identity (9). Despite this
similarity, each protein has differing affinities for various
downstream effectors and demonstrate different subcellular
localization, suggesting that they have distinct functions in
normal cellular activities and during tumor pathogenesis (10). Overexpression of RHOA has
previously been reported to promote the invasiveness of tumor cells
in several types of malignancy (11). By contrast, RHOB was previously
reported as a suppressor or negative modifier of cancer progression
(12).
A previous study demonstrated RHOC to be correlated
with the metastasis of various types of tumor (13). Other studies have demonstrated that
RHOC expression is associated with aggressive phenotypes in a human
cholangiocarcinoma cell line (14,15).
However, the precise molecular mechanisms involved remain unclear.
Thus, the current study aimed to investigate the pathological
function of RHOC and the potential molecular mechanisms associated
with cholangiocarcinoma.
Materials and methods
Patients and clinicopathological
data
Clinical and pathological data were collected from
24 patients that underwent surgical resection of pathologically
confirmed CCC between March 10, 2011 and May 15, 2014 at the First
Affiliated Hospital of Henan Science and Technology University
(Luoyang, China). Demographic data and pathological results were
collected for each patient. The study was approved by Ethics
Committee of the First Affiliated Hospital of Henan Science and
Technology University in March 2011. All patients provided signed
informed consent. Additionally, 24 samples of adjacent nontumorous
bile duct tissues (NBD) were obtained as controls. All fresh
samples were obtained from surgical resection and immediately
preserved in liquid nitrogen. Clinicopathological staging was
determined by the TNM classification of the 7th edition American
Joint Committee on Cancer (16).
Cells lines and cell culture
RBE and HCCC-9810 human cholangiocarcinoma cell
lines were purchased from the Shanghai Institutes for Biological
Sciences (Shanghai, China) and Wuhan Boster Biological Technology,
Ltd. (Wuhan, China), respectively. QBC939 and SK-ChA-1 human
cholangiocarcinoma cell lines were kindly provided by Dr. Chundong
Yu (Xiamen University, Xiamen, China). All cell lines were cultured
in Dulbecco's modified Eagle's medium or RPMI 1640 medium
supplemented with 10% fetal bovine serum (FBS), streptomycin (100
µg/ml) and penicillin (100 units/ml) purchased from Hyclone;
Thermo Fisher Scientific, Inc. (Logan, UT, USA) at 37°C in 5%
CO2 atmosphere.
Lentivirus vector and cell
transfection
Lentiviral-mediated RHOC short hairpin RNA (shRNA)
and negative control shRNA were packaged and produced by Shanghai
GenePharma Co., Ltd. (Shanghai, China). The RHOC shRNA target
sequence (NM_175744, NCBI GenBank accession number) was cloned into
the pGLV-3/H1/GFP + puro lentiviral vector (Shanghai GenePharma
Co., Ltd.), which specifically expresses RHOC shRNA. The RHOC shRNA
target sequence was as follows:
5′-GATCCCGCTATATTGCGGACATTGAGTTCAAGAGACTCAATGTCCGCAATATAGTTTTTTGGAAA-3′,
as described by Wu et al (17). The shRNA nontarget sequence,
5′-TTCTCCGAACGTGTCACGT-3′ was also cloned into a pGLV-3/H1/GFP +
puro lentiviral vector as a negative control. The recombinant
lentivirus RHOC shRNA (Lv-shRHOC) and control shRNA (Lv-shCTRL)
were packaged in 293T cells (Shanghai GenePharma Co., Ltd.) using a
lentivector expression system. DNA sequencing results revealed that
the shRNA interference sequence targeting the RHOC gene was
successfully inserted into the recombinant lentivirus. For cell
infection, 30–50% confluent RBE and HCCC-9810 negative control
shRNA and RHOC shRNA cell lines were incubated with lentivirus for
72–96 h. Untransfected controls were used in preliminary shRNA
experiments, and demonstrated no significant difference compared
with the negative control shRNA group. Thus, negative control shRNA
was used to represent normal controls in the subsequent experiments
The expression of green fluorescent protein (GFP) was examined
under an Eclipse Ti fluorescence microscope (Nikon Corporation,
Tokyo, Japan), and the intensity of green fluorescence indicated
the transduction efficiency. The CCC cells were transfected with
high titres of Lv-shRHOC and Lv-shCTRL particles (2×108
TU/ml; MOI of 30 transfection concentration) according to the
instructions of the lentivirus manufacturer. Stable knockdown of
RHOC and negative control shRNA transfectants were obtained by
continuous treatment with 2 µg/ml puromycin (Shanghai
GenePharma Co., Ltd.) in transfected RBE and HCCC-9810 cell
lines.
Western blotting assay
Proteins were extracted from the cells using
radioimmunoprecipitation assay lysis buffer (Nanjing KeyGen Biotech
Co., Ltd., Nanjing, China). Lysates were centrifuged at 4°C at
4,000 × g for 20 min, and the supernatants were collected. Protein
concentration was quantified using a Bradford assay (Beyotime
Institute of Biotechnology, Haimen, China). Lysates (50 µg)
were separated by 10% sodium dodecyl sulfate-polyacrylamide gel
(Beyotime Institute of Biotechnology) electrophoresis and
transferred onto nitrocellulose membrane (EMD Millipore, Billerica,
MA, USA). Membranes were blocked by 5% non-fat milk in
Tris-buffered saline Tween 20 (TBST) then incubated with primary
antibodies at 4–8°C overnight. The primary antibodies used were
monoclonal mouse anti-RHOC (1:300 dilution; cat. no. AT3636a;
Abgent, Inc., San Diego, CA, USA), monoclonal mouse anti-MMP2, MMP3
and MMP9 (all 1:1,000 dilution; cat. nos. ab-86607, ab-17790 and
ab-58803, respectively; Abcam, Cambridge, MA, USA), monoclonal
mouse anti-MMP14 (1:2,000 dilution; cat. no. ab-78738; Abcam),
polyclonal rabbit anti-E-cadherin, Vimentin, Snail and Slug (all
1:500 dilution; cat. nos. WL-01482, WL-01960, WL-01863 and
WL-01508, respectively; Wanleibio Co., Ltd., Shenyang, China) and
monoclonal mouse anti-β-actin (1:5,000 dilution; cat. no. sc-47778;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Membranes were
washed with TBST 3 times for 10 min then incubated with horseradish
peroxidase-conjugated goat anti-rabbit (cat. no. sc-2004) and goat
anti-mouse (cat. no. sc-2005) secondary antibodies (1:3,000
dilution; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. Protein levels were determined by normalizing to
β-actin. The proteins were visualized by enhanced chemiluminescence
(Advansta, Inc., Menlo Park, CA, USA) and detected using Bio-Rad
ChemiDoc MP imaging system (ImageLab 4.1 software; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The experiments were
performed in triplicate.
In vitro invasion assay
For the invasion assay, 3×104 cells were
added to the cell culture inserts with microporous membrane and
Matrigel coating (BD Biosciences, Franklin Lakes, NJ, USA). Medium
containing 10% FBS was added to the bottom chamber. The cells were
then incubated for 48 h at 37°C and the upper chamber was removed.
The cells on the bottom surface of the upper chambers fixed in 95%
ethanol (Sangon Biotech Co., Ltd., Shanghai, China) for 15 min and
stained with 0.1 mg/ml crystal violet solution and the number of
cells was counted under a Eclipse Ti microscope (magnification,
×200). Individual experiments had triplicate inserts and five
randomly selected fields were counted per insert.
Scratch assay
For wound-healing assays, RBE and HCCC9810 cells
were plated at 2×106 cells/dish density in 60
mm-diameter dishes. Wounds were created in the confluent cells
using a 200 µl pipette tip after cells had reached
confluency. The cells were then rinsed with medium to remove
free-floating cells and debris. Medium with 1% FBS was added and
the culture plates were incubated at 37°C for 48 h. Different
stages of wound healing were observed along the scrape line and
representative scrape lines were imaged with an Eclipse Ti
microscope. Wound closure was measured using AxioVision software
version 4.7 (Zeiss GmbH Jena, Germany). Quantification was
performed by measuring the uncovered areas compared with the
controls. Each experiment was repeated in triplicate.
Statistical analysis
All data were analyzed for statistical significance
using SPSS software, version 18.0 (SPSS, Inc., Chicago, IL, USA).
The data are expressed as the mean ± standard deviation. The
protein expression levels were measured using densitometry with
ImageLab 4.1 software. Two-tailed Student's t-test was used for
comparisons of two independent groups, and analysis of variance
followed by Dunnett's test was used for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
RHOC is highly expressed in human CCC
tissues and cell lines
To evaluate the expression of RHOC in CCC, western
blotting was performed to assess the levels of RHOC protein in 24
tumor and 24 adjacent NBD tissues. As presented in Fig. 1 and Table I, the levels of RHOC protein were
significantly upregulated in 21 CCC specimens, including 12
extrahepatic CCC and 9 intrahepatic CCC samples, compared with NBD
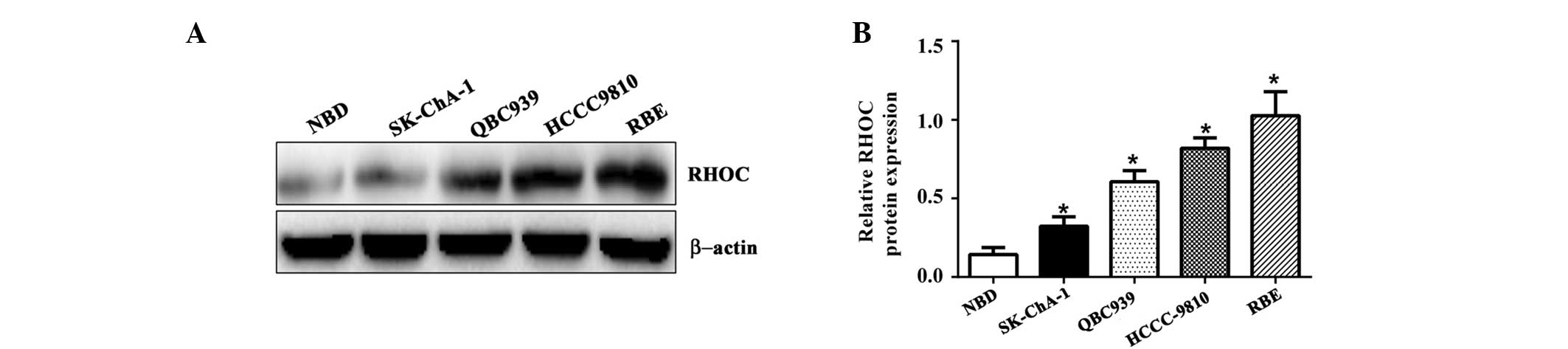
specimens (P=0.000). Additionally, RHOC expression was
significantly increased in CCC cell lines, including HCCC9810,
QBC939, RBE and SK-ChA-1, compared with NBD epithelium (Fig. 2; P=0.006). Thus, the overexpression
of RHOC in CCC specimens and CCC cell lines suggests that RHOC may
be important for the tumorigenesis of CCC.
 | Table IClinicopathological information of
cholangiocellular carcinoma cases studied and RHOC expression in
cholangiocellular carcinoma tissue. |
Table I
Clinicopathological information of
cholangiocellular carcinoma cases studied and RHOC expression in
cholangiocellular carcinoma tissue.
| Case no. | Gender | Age years | Type | Stageb |
Differentiation | RHOC expression
(Fold changea) |
|---|
| 1 | Male | 65 | Intrahepatic | T2aNxMx | NA | 1.81 |
| 2 | Female | 45 | Extrahepatic | T3N1M0 | NA | 1.93 |
| 3 | Male | 47 | Intrahepatic | T1N0M0 | NA | 1.02 |
| 4 | Male | 34 | Extrahepatic | T3N2M0 | NA | 6.21 |
| 5 | Male | 29 | Intrahepatic | T4N2M0 | NA | 9.45 |
| 6 | Female | 67 | Extrahepatic | T3N0M0 | Well | 0.67 |
| 7 | Male | 71 | Extrahepatic | NA | Poor | 3.15 |
| 8 | Female | 58 | Intrahepatic | T2aNxMx | Poor | 5.17 |
| 9 | Male | 49 | Extrahepatic | T2bN0M0 | Well | 1.10 |
| 10 | Male | 44 | Intrahepatic | T3N1M0 | Moderately | 4.23 |
| 11 | Male | 73 | Extrahepatic | T1N0M0 | Well | 0.77 |
| 12 | Female | 54 | Extrahepatic | T2aN2M0 | Moderately | 4.89 |
| 13 | Male | 65 | Extrahepatic | T1N1M0 | Moderately | 3.78 |
| 14 | Male | 41 | Intrahepatic | TxN1M0 | Poor | 2.56 |
| 15 | Male | 38 | Intrahepatic | T1N1M0 | Moderately | 2.66 |
| 16 | Female | 44 | Extrahepatic | NA | Poor | 5.66 |
| 17 | Male | 57 | Extrahepatic | T2bN0M0 | Well | 0.57 |
| 18 | Female | 55 | Extrahepatic | NA | Moderately | 3.40 |
| 19 | Male | 63 | Extrahepatic | T2bN2M0 | Moderately | 7.34 |
| 20 | Female | 68 | Intrahepatic | T3N1M1 | Moderately | 2.91 |
| 21 | Male | 36 | Extrahepatic | T2bN1M0 | Moderately | 3.35 |
| 22 | Female | 47 | Intrahepatic | T4N2M0 | Poor | 6.23 |
| 23 | Male | 36 | Extrahepatic | T2aN1M0 | Well | 1.88 |
| 24 | Female | 42 | Extrahepatic | T1N0M0 | Moderately | 1.67 |
RHOC protein expression is inhibited by
lentiviral-mediated shRNA interference in RBE and HCCC-9810 cell
lines
Analysis of western blotting results demonstrated
that the relative protein expression levels of RHOC were 1–3-fold
higher in RBE and HCCC-9810 cells compared with QBC939 and SK-ChA-1
cell lines (Fig. 2). Thus, the RBE
and HCCC-9810 cell lines were selected for use in knockdown
experiments. The cells were transfected with Lv-shRHOC and
Lv-shCTRL lentiviral vectors. The transfection efficiency of
>90% was determined by detecting the expression of GFP 96 h
after infection (Fig. 3A). Western
blotting indicated RHOC was significantly downregulated in RBE and
HCCC-9810 cells transfected with Lv-shRHOC vector compared with
Lv-shCTRL. In comparison with Lv-shCTRL, the protein levels of RHOC
were decreased by 83.73±9.09% and 81.2±8.53% in
Lv-shRHOC-transfected RBE and HCCC-9810 cells, respectively
(P<0.003, Fig. 3B).
RHOC silencing impairs CCC cell invasion
and migration in vitro
The results of the Matrigel invasion assay indicated
that inhibition of RHOC reduced the invasive ability of RBE and
HCCC-9810 cells compared with Lv-shCTRL-transfected cells
(P<0.016; Fig. 3C). The effects
of RHOC on the migration of CCC cell lines were assessed by scratch
assay. The results demonstrated that compared with
Lv-shCTRL-transfected cells, RHOC knockdown significantly reduced
cell migration in RBE and HCCC-9810 cells (P<0.003; Fig. 3D and E). Collectively, these
findings provided evidence that elevated RHOC expression levels
were involved in promoting the migratory and invasive phenotype of
CCC cells.
Effects of RHOC knockdown on the
expression of tumor invasion-associated molecules
As the invasive ability of tumor cells is often
correlated with the production of secretory proteases, the effect
of RHOC knockdown on the expression of tumor invasion-associated
molecules was determined. As demonstrated in Fig. 4A, western blotting analysis
indicated that downregulation of RHOC reduced the protein
expression level of MMP2, 3 and 9, whereas, no change was observed
in the expression level of MMP14 (Fig.
4A).
Effects of RHOC knockdown on the
expression of epithelial mesenchymal transition (EMT)-associated
genes in CCC cell lines
To further investigate the mechanism by which RHOC
regulates cell invasion and migration, the protein expression
levels of EMT-associated genes in CCC cells was analyzed. It was
demonstrated that RHOC knockdown decreased the expression of the
mesenchymal marker, vimentin, and the EMT central regulators, Snail
and Slug, compared with Lv-shCTRL-transfected cells. In contrast to
Lv-shCTRL-transfected cells, the expression of epithelial marker
E-cadherin was upregulated in CCC cell lines following RHOC
knockdown (Fig. 4B). Thus, the
results of the current study demonstrated that knockdown of RHOC
altered the expression levels of MMP2, 3 and 9, and EMT-associated
genes, therefore, suppressing the invasive and migratory phenotype
of CCC cells.
Discussion
CCC is a highly metastatic disease characterized by
invasive growth along the lymphangion or perineurium, or direct
invasion into the liver (18).
Thus, intensive studies for potential candidate molecules involved
in the metastatic process are urgently required to provide
effective treatments for patients with advanced CCC.
RHOC has been shown to promote cancer metastasis in
a variety of tumor types, including breast cancer (19), non-small cell lung carcinoma
(20), colon carcinoma (21), malignant melanoma (22), hepatocellular carcinoma (23), head and neck cancer (24) and prostate cancer (25). However, the function of RHOC in
human CCC remains unclear. The current study demonstrated that the
expression levels of RHOC were significantly increased in CCC
tissues and cell lines compared with normal biliary epithelium,
suggesting that RHOC may be involved in the progression of CCC.
Additionally, the present study specifically and efficiently
inhibited RHOC gene expression in CCC cell lines by
lentiviral-mediated shRNA interference, and the results
demonstrated that the invasion and migration capacities of
transfected RBE and HCCC9810 cell lines were significantly
inhibited by RHOC knockdown in vitro. These results
collectively suggested that RHOC may be important in CCC
progression.
To date, the molecular mechanisms by which RHOC
promote tumor development and metastasis are not fully understood
(26). Previous studies have
demonstrated that RHOC promote cancer progression by regulating the
expression of MMP genes (14,23,27).
It is well established that MMPs induce cancer cell invasion and
metastatic spread by degrading the extracellular matrix and other
barriers (28). In hepatocellular
carcinoma, Liao et al (29)
reported that hepatocellular carcinoma cell invasion and migration
are modulated by the genes RHOC, MMP-2 and MMP-9 (29). Wang et al (14) also demonstrated that RHOC promotes
the malignant progression of CCC cells via regulation of MMP
expression levels (14). The
present study demonstrated that RHOC promotes CCC cell invasion
partially via inducing the expression of MMP2, 3 and 9, but not
that of MMP14, which is consistent with previous observations in
other types of tumor (27,30,31).
Further studies are required to determine the precise molecular
mechanisms of the invasion process in CCC.
Emerging evidence has established that EMT is an
important event during carcinoma progression (32). EMT promotes carcinoma progression
by increasing migratory and invasive properties, and cancer stem
cell-like phenotype of cells, which may be prerequisites for cancer
cell metastasis (32). Thus,
understanding the regulatory mechanisms of EMT may provide greater
insight into the signaling programs that control CCC metastasis.
EMT is characterized by decreased expression of epithelial
proteins, such as E-cadherin, and increased expression of
mesenchymal proteins, such as vimentin (32). Additionally, Snail and Slug are
crucial for the transcriptional regulation of EMT (33,34).
Reportedly, either upregulation or increased activity of RHOC
promotes the invasive potential of cancer cells, which is closely
associated with EMT (35).
Bellovin et al (21)
reported that RHOC expression and activation are induced by EMT and
RHOC promotes post-EMT cell migration. In ovarian carcinoma, a
study by Gou et al (36)
indicated that ectopic RHOC expression enhanced migration, invasion
and altered the expression of EMT markers. Similarly, the current
study in CCC demonstrated that RHOC knockdown markedly altered the
expression of EMT-associated genes. These results consistently
suggested that RHOC has an important function during the malignant
progression of CCC by regulating MMPs and EMT.
In summary, the results of the present study
indicate that the expression of RHOC is significantly increased in
CCC cell lines and clinical samples. Furthermore, knockdown of RHOC
expression significantly inhibited CCC cell migration and invasion
in vitro, and regulated the expression of MMPs and
EMT-associated genes. Thus, strategies interfering with RHOC
expression may provide a novel and promising alternative approach
for the treatment of aggressive CCC.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 81172026).
References
|
1
|
Razumilava N and Gores GJ:
Cholangiocarcinoma. Lancet. 383:2168–2179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vogel A, Wege H, Caca K, Nashan B and
Neumann U: The diagnosis and treatment of cholangiocarcinoma. Dtsch
Arztebl Int. 111:748–754. 2014.PubMed/NCBI
|
|
3
|
Ghouri YA, Mian I and Blechacz B: Cancer
review: Cholangiocarcinoma. J Carcinog. 14:12015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Groot Koerkamp B and Fong Y: Outcomes in
biliary malignancy. J Surg Oncol. 110:585–591. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang Y, Li X and Zhao Y: Progression of
targeted therapy in advanced cholangiocarcinoma. Chin J Cancer Res.
27:122–127. 2015.PubMed/NCBI
|
|
6
|
Sirica AE: Cholangiocarcinoma: Molecular
targeting strategies for chemoprevention and therapy. Hepatology.
41:5–15. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Turner SJ, Zhuang S, Zhang T, Boss GR and
Pilz RB: Effects of lovastatin on Rho isoform expression, activity
and association with guanine nucleotide dissociation inhibitors.
Biochem Pharmacol. 75:405–413. 2008. View Article : Google Scholar :
|
|
8
|
Amin E, Dubey BN, Zhang SC, Gremer L,
Dvorsky R, Moll JM, Taha MS, Nagel-Steger L, Piekorz RP, Somlyo AV
and Ahmadian MR: Rho-kinase: Regulation, (dys) function and
inhibition. Biol Chem. 394:1399–1410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schaefer A, Reinhard NR and Hordijk PL:
Toward understanding RhoGTPase specificity: Structure, function and
local activation. Small GTPases. 5:62014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Donnelly SK, Bravo-Cordero JJ and Hodgson
L: Rho GTPase isoforms in cell motility: Don't fret, we have FRET.
Cell Adh Migr. 8:526–534. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
O'Connor K and Chen M: Dynamic functions
of RhoA in tumor cell migration and invasion. Small GTPases.
4:141–147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vega FM, Thomas M, Reymond N and Ridley
AJ: The Rho GTPase RhoB regulates cadherin expression and
epithelial cell-cell interaction. Cell Commun Signal. 13:62015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bravo-Cordero JJ, Hodgson L and Condeelis
JS: Spatial regulation of tumor cell protrusions by RhoC. Cell Adh
Migr. 8:263–267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Q, Tang H, Yin S and Dong C:
Downregulation of microRNA-138 enhances the proliferation,
migration and invasion of cholangiocarcinoma cells through the
upregulation of RhoC/p-ERK/MMP-2/MMP-9. Oncol Rep. 29:2046–2052.
2013.PubMed/NCBI
|
|
15
|
Shi Z, Chen ML, He QL and Zeng JH:
Antisense RhoC gene suppresses proliferation and invasion capacity
of human QBC939 cholangiocarcinoma cells. Hepatobiliary Pancreat
Dis Int. 6:516–520. 2007.PubMed/NCBI
|
|
16
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu M, Wu ZF, Rosenthal DT, Rhee EM and
Merajver SD: Characterization of the roles of RHOC and RHOA GTPases
in invasion, motility and matrix adhesion in inflammatory and
aggressive breast cancers. Cancer. 116:2768–2782. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Skipworth JR, Keane MG and Pereira SP:
Update on the management of cholangiocarcinoma. Dig Dis.
32:570–578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rosenthal DT, Zhang J, Bao L, Zhu L, Wu Z,
Toy K, Kleer CG and Merajver SD: RhoC impacts the metastatic
potential and abundance of breast cancer stem cells. PLoS One.
7:e409792012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shikada Y, Yoshino I, Okamoto T, Fukuyama
S, Kameyama T and Maehara Y: Higher expression of RhoC is related
to invasiveness in non-small cell lung carcinoma. Clin Cancer Res.
9:5282–5286. 2003.PubMed/NCBI
|
|
21
|
Bellovin DI, Simpson KJ, Danilov T,
Maynard E, Rimm DL, Oettgen P and Mercurio AM: Reciprocal
regulation of RhoA and RhoC characterizes the EMT and identifies
RhoC as a prognostic marker of colon carcinoma. Oncogene.
25:6959–6967. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goundiam O, Nagel MD and Vayssade M: Akt
and RhoA inhibition promotes anoikis of aggregated B16F10 melanoma
cells. Cell Biol Int. 36:311–319. 2012. View Article : Google Scholar
|
|
23
|
Xie S, Zhu M, Lv G, Geng Y, Chen G, Ma J
and Wang G: Overexpression of Ras homologous C (RhoC) induces
malignant transformation of hepatocytes in vitro and in nude mouse
xenografts. PLoS One. 8:e544932013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Islam M, Sharma S, Kumar B and Teknos TN:
Atorvastatin inhibits RhoC function and limits head and neck cancer
metastasis. Oral Oncol. 49:778–786. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iiizumi M, Bandyopadhyay S, Pai SK, Watabe
M, Hirota S, Hosobe S, Tsukada T, Miura K, Saito K, Furuta E, et
al: RhoC promotes metastasis via activation of the Pyk2 pathway in
prostate cancer. Cancer Res. 68:7613–7620. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Julian L and Olson MF: Rho-associated
coiled-coil containing kinases (ROCK): Structure, regulation and
functions. Small GTPases. 5:e298462014. View Article : Google Scholar
|
|
27
|
Xue F, Takahara T, Yata Y, Xia Q, Nonome
K, Shinno E, Kanayama M, Takahara S and Sugiyama T: Blockade of
Rho/Rho-associated coiled coil-forming kinase signaling can prevent
progression of hepatocellular carcinoma in matrix
metalloproteinase-dependent manner. Hepatol Res. 38:810–817. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cockett MI, Murphy G, Birch ML, O'Connell
JP, Crabbe T, Millican AT, Hart IR and Docherty AJ: Matrix
metalloproteinases and metastatic cancer. Biochem Soc Symp.
63:295–313. 1998.PubMed/NCBI
|
|
29
|
Liao CG, Kong LM, Zhou P, Yang XL, Huang
JG, Zhang HL and Lu N: miR-10b is overexpressed in hepatocellular
carcinoma and promotes cell proliferation, migration and invasion
through RhoC, uPAR and MMPs. J Transl Med. 12:2342014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Faried A, Faried LS, Kimura H, Nakajima M,
Sohda M, Miyazaki T, Kato H, Usman N and Kuwano H: RhoA and RhoC
proteins promote both cell proliferation and cell invasion of human
oesophageal squamous cell carcinoma cell lines in vitro and in
vivo. Eur J Cancer. 42:1455–1465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ikoma T, Takahashi T, Nagano S, Li YM,
Ohno Y, Ando K, Fujiwara T, Fujiwara H and Kosai K: A definitive
role of RhoC in metastasis of orthotopic lung cancer in mice. Clin
Cancer Res. 10:1192–1200. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kothari AN, Mi Z, Zapf M and Kuo PC: Novel
clinical therapeutics targeting the epithelial to mesenchymal
transition. Clin Transl Med. 3:352014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kaufhold S and Bonavida B: Central role of
Snail1 in the regulation of EMT and resistance in cancer: A target
for therapeutic intervention. J Exp Clin Cancer Res. 33:622014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alves CC, Carneiro F, Hoefler H and Becker
KF: Role of the epithelial-mesenchymal transition regulator slug in
primary human cancers. Front Biosci (Landmark Ed). 14:3035–3050.
2009. View Article : Google Scholar
|
|
35
|
Sequeira L, Dubyk CW, Riesenberger TA,
Cooper CR and van Golen KL: Rho GTPases in PC-3 prostate cancer
cell morphology, invasion and tumor cell diapedesis. Clin Exp
Metastasis. 25:569–579. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gou WF, Zhao Y, Lu H, Yang XF, Xiu YL,
Zhao S, Liu JM, Zhu ZT, Sun HZ, Liu YP, et al: The role of RhoC in
epithelial-to-mesenchymal transition of ovarian carcinoma cells.
BMC Cancer. 14:4772014. View Article : Google Scholar : PubMed/NCBI
|