Introduction
Schwann cells (SCs) are the predominant cell type
constituting the structure of peripheral nerves, and are crucial in
repair and regeneration following peripheral nerve injury (PNI)
(1). Following PNI, SCs can
activate macrophages to remove necrotic debris in the early stage,
and provide a channel for regeneration (2). SCs not only form the Büngner band to
guide axonal regeneration, they also secrete neurotrophic factors,
extracellular matrix and cell adhesion molecules to promote nerve
regeneration (3). However,
autologous SCs are difficult to obtain and expand to sufficient
numbers in a short duration; this is due to limitations in tissue
availability, morbidity of the donor site and the sacrifice one or
more functioning nerves with consequential loss of sensation
(4). The ideal features of
transplantable cells are that they are readily available,
proliferate rapidly in vitro and have characteristics of low
or no immunogenicity.
Mesenchymal stem cells (MSCs) are an attractive cell
source for the regeneration of nerve tissue due to their
self-renewal ability, high growth rate and multipotent
differentiation properties (5).
Bone marrow-derived mesenchymal stem cells (BMMSCs) can
differentiate into an SC phenotype (6), as well as express myelin-associated
markers and remyelinate when transplanted into injured sciatic
nerves of rats (7). However, the
isolation of BMMSCs is an invasive and painful procedure, and the
ratio of MSCs in the bone marrow is relatively low (<1/100,000)
(8). Therefore, an alternative
cell source is in urgent demand. Adipose-derived stem cells (ADSCs)
have similar phenotypic and gene expression profiles to BMMSCs.
ADSCs also have unique advantages: They can be readily harvested
using a safe and conventional liposuction procedure from
subcutaneous fat tissue; the ratio of ADSCs in adipose tissue is
higher than in BMMSCs (~1–2%); and ADSCs proliferate significantly
faster than BMMSCs (9). It has
also been reported that ADSCs can be transdifferentiated to exhibit
an SC phenotype (10).
In the present study, the transdifferentiation of
rat ADSCs into Schwann-like cells was performed, and
immunofluorescence, western blot and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
experiments were performed to detect glial fibrillary acidic
protein (GFAP), S100 and p75. The mitotic feature of Schwann-like
cells was also assessed. The present study aimed to provide a
foundation for future experiments regarding the suitable selection
of seed cells for nerve tissue engineering in the treatment of
PNI.
Materials and methods
Animals
A total of four male Wistar rats (age, 3–4 weeks)
were obtained from the Experimental Animal Centre of China Medical
University (Shenyang, China; no. SYXK Liao 2013-0001). The rats
were housed in plastic cages at 24°C, 50% humidity, under a 12-h
light/dark cycle with access to food and water ad libitum.
In the present study, the mice were anesthetized using 10% chloral
hydrate (350g/kg) and sacrificed using chloral hydrate (700g/kg)
following adipose tissue sampling. The study protocol was approved
by the Animal Experimental Committee of the China Medical
University.
ADSC isolation and culture
The ADSCs were carefully isolated from rat inguinal
fat pads, dissected and minced under aseptic conditions.
Subsequently, the tissue was enzymatically dissociated at 37°C for
60 min using 0.1% collagenase I (Invitrogen; Thermo Fisher
Scientific, Inc.). The dissociated cells were cultured in
Dulbecco's modified Eagle's medium/F12 (DMEM/F12; Genview,
Tallahassee, FL, USA) containing 10% fetal bovine serum (FBS;
Biological Industries, Kibbutz Beit Haemek, Israel) and 1% (v/v)
penicillin/streptomycin solution for 48 h at 37°C in 5%
CO2. The non-adherent cells were removed on replacement
of the medium every 2–3 days when the cells reached 80%
confluence.
ADSC multilineage differentiation
potential
Rat ADSCs at the fourth passage were seeded into
6-well plates at a density of 1×105 cells/ml and divided
into experimental and control groups. Once the cells reached 80%
confluence, the experimental group was cultured in induction
medium. To induce osteogenic differentiation, the ADSCs were
cultured at 37°C in 5% CO2 for 28 days in DMEM/F12
supplemented with 10% FBS, 0.1 µM dexamethasone, 10
µM β-glycerol phosphate and 50 µM ascorbate, and
calcium deposition was visualized by staining with Alizarin Red
(Sigma-Aldrich, St. Louis, MO, USA). To induce adipogenic
differentiation, the ADSCs were cultured for 14 days in DMEM/F12
supplemented with 10% FBS, 1 µM dexamethasone, 5
µg/ml insulin, 0.5 mM isobutylmethylxanthine (IBMX) and 100
µM indomethacin, and adipogenesis was evaluated by observing
the production of intracellular lip droplets, detected using
Oil-Red O (Sigma-Aldrich) and observed with a Nikon Eclipse 80i
microscope (Nikon Corporation, Toyko, Japan).
Characterization of ADSC surface
molecules using flow cytometry
Rat ADSCs at the fourth passage were harvested by
trypsinization, following which the cells were fixed in neutralized
4% paraformaldehyde for 30 min at a density of 1×106
cells/ml, and then incubated with the following antibodies: Hamster
fluorescein isothiocyanate (FITC)-conjugated CD29 (1:100; cat. no.
11-0291), phycoerythrin (PE)-conjugated CD31 (1:100; cat. no.
25-0310), mouse FITC-conjugated CD45 (1:200; cat. no. 11-0461),
mouse allophycocyanin-conjugated CD49 (1:50; cat. no. 17-1490) and
mouse PE-conjugated CD44 (1:400; cat. no. 12-0444), from
eBioscience, Inc. (San Diego, CA, USA), and mouse FITC-conjugated
CD106 (1:100; BD Biosciences, Franklin Lakes, NJ, USA) at 4°C for
30 min. The numbers of immunoreactive cells were detected using BD
FACSDiva 7.0 (BD Biosciences), whereas cells stained with PBS (0.01
M; pH 7.4) served as a control.
Induction of ADSCs into an SC
phenotype
When the rat ADSCs in the fourth passage were
subconfluent, the medium was replaced with fresh medium containing
1 mM β-mercaptoethanol (β-ME; Sigma-Aldrich) for 24 h. The cells
were then cultured in DMEM/F12 supplemented with 10% FBS and 35
ng/ml alltrans-retinoic acid (Sigma-Aldrich) for 72 h at 37°C in 5%
CO2. Subsequently, the cells (1×106 cells/ml)
were incubated in SC-conditioned medium containing 10% FBS, 14
µM forskolin (FSK; Alexis Biochemichals, San Diego, CA,
USA), 5 ng/ml platelet-derived growth factor-AA (PeproTech, Rocky
Hill, NJ, USA), 10 ng/ml basic fibroblast growth factor (bFGF;
PeproTech) and 200 ng/ml recombinant human heregulin-β1 (HRG;
PeproTech) for 12 days at 37°C in 5% CO2. The cells were
passaged with trypsin/EDTA when required, and fresh medium was
added approximately every 72 h.
Identification of Schwann-like cells
Immunofluorescence
For immunocytochemical assessment of the
differentiated ADSC markers, the ADSCs were cultured in
SC-conditioned medium for 12–14 days, and were then fixed in 4%
(W/V) paraformaldehyde for 30 min. The fixed cells were blocked
with goat serum for 1 h at room temperature. The polyclonal primary
antibodies, rabbit polyclonal GFAP (1:200; cat. no. sc-9065), S100
(1:200; cat. no. sc-28533) and p75 NGF receptor protein (P75,
1:200; cat. no. sc-5634), all from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA) were added and incubated at 4°C overnight.
Following incubation, goat anti-rabbit Cy3- (1:100; cat. no.
SA00003-2) or FITC-conjugated (1:100; cat. no. SA00009-2) secondary
antibodies (Proteintech Group, Inc., Chicago, IL, USA) were added
at 37°C for 1 h. The cell nuclei were labeled using DAPI
(Sigma-Aldrich). PBS was substituted for primary antibodies as a
control. The cells were visualized and recorded using a
fluorescence microscope (Nikon Eclipse 80i).
Western blot analysis
Rat ADSCs in the fourth passage were harvested by
trypsinization, and 1×106 cells/ml were fixed in 100
µl protein isolation, followed by centrifugation at 12,000 g
for 30 min at 4°C. The cell lysates were analyzed using a
commercial bicinchoninic acid assay kit (cat. no. BCA01; Beijing
Dingguo Biotech, Co., Ltd. Beijing, China). A total of 30 µg
protein was prepared per sample, and denatured at 95°C for 5 min.
The proteins were resolved by 15 or 12% SDS-PAGE (Beijing Dingguo
Biotech, Co., Ltd.) and transferred onto PVDF membranes. The
membranes were blocked in 5% bovine serum albumin for 1 h, and then
incubated overnight at 4°C with GFAP, S100, P75 and GAPDH primary
antibodies (1:10,000, Proteintech Group, Inc.). The membranes were
then incubated with goat anti-mouse (cat. no. L3032-1/2) and
anti-rabbit (cat. no. L3012-1/2) IgG-horseradish
peroxidase-conjugated secondary antibodies (1:10,000; Signalway
Antibody, College Park, MD, USA) for 1 h at 37°C. The blots were
scanned using a ChemiDoc XRS+ image analysis system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and analyzed using Image J
software (National Institutes of Health, Bethesda, MD, USA).
mRNA extraction and RT-qPCR
analysis
The total mRNA was isolated from rat ADSCs in the
fourth passage using RNA Isolator Total RNA Extraction reagent
(Vazyme, Piscataway, NJ, USA). The mRNA was detected using an
Ace-Hi™ One Step Quantitative Real-Time PCR SYBR Green kit (cat.
no. Q221-01; Vazyme) and RT-qPCR was performed using an LCS 480
real-time PCR system, according to the manufacturers' protocols.
The primers were provided as follows: S-100, forward
5-CTTGATTTGCTTCAGGGATGA-3 and reverse 5-CCCACAGAGTGTTGATTTCG-3;
P75, forward 5-TTTGCTTGCTGTTGGAATGA-3 and reverse 5-ATGCTCCTG
GTCTCTTCA CC-3; GFAP, forward 5-GCTCCAAGATGAAACCAACC-3 and reverse
5-CCAGCGACTCAACCTTCCT-3); GAPDH, forward 5-GGAGCGTGGCTACTCTTTTG-3
and reverse 5-GGCTGGAAGAGTGTCTCAGG-3. The reaction conditions were
as follows: Stage one, reverse transcription involving one cycle of
50°C for 30 min; stage two, predegeneration involving one cycle of
95°C for 10 min; stage three, cyclic reaction involving 40 cycles
of 95°C for 10 sec and 60°C for 30 sec; stage four, melting curve
(one cycle, default). The relative expression value of each mRNA
was calculated using the 2−∆∆Cq method (11), and normalized to that of GAPDH mRNA
for each data point.
Proliferation assay of Schwann-like
cells
3-(4,5-dimethyl-2-
thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay
To assess cell proliferation, an MTT (Sigma-Aldrich)
assay was performed. Briefly, following culture for 1, 3, 5, 7, 9
and 11 days, the Schwann-like cells were seeded into 96-well plates
at a density of 2×104 cells/ml, and cultured for 24 h at
37°C in 5% CO2. Subsequently, MTT (5 mg/ml, 20
µl/well) was added at 37°C for 4 h, following which the
culture medium was carefully removed, and 150 µl dimethyl
sulfoxide was added to each well. The culture plate was then placed
on a shaker and agitated at a low speed for 10 min. The absor bance
density of each well was measured using an enzyme-linked
immunometric meter (Multiskan Ascent, Thermo Fisher Scientific,
Inc.) at 490 nm.
Observation and recording of the growth
state of cells
The growth states of the Schwann-like cells were
observed and recorded under an inverted microscope (BH2; Olympus
Corporation, Tokyo, Japan) every day.
Statistical analysis
The experiments for each group were run in
triplicate. Data were expressed as the mean ± standard deviation.
Statistical analysis was performed using SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA). Student's paired-sample t-test was
used for between-group comparisons of the means. One-way analysis
of variance was used for the comparison of multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
ADSC isolation and identification
After 24 h primary culture, the rat ADSCs had
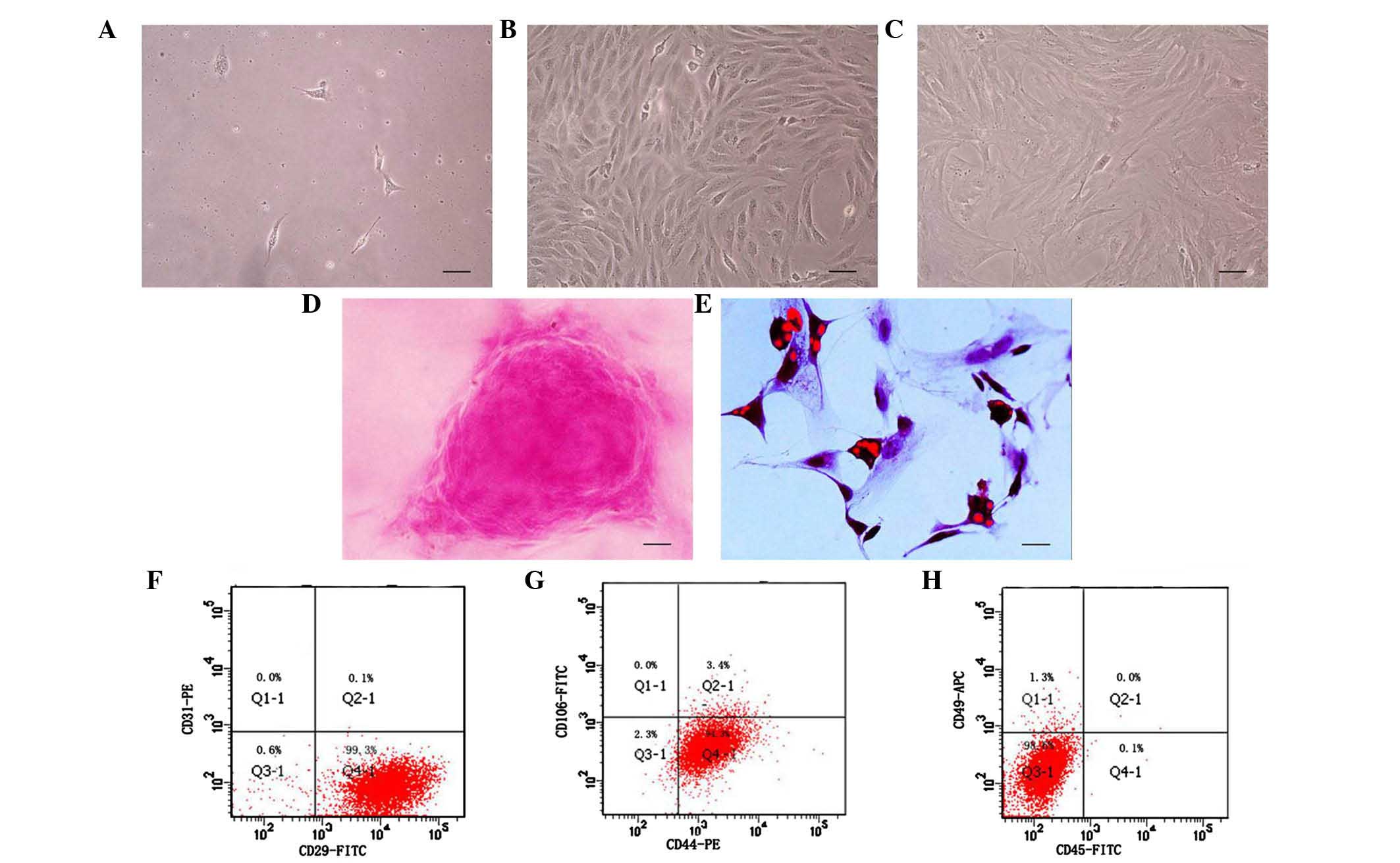
adhered with a short spindle or polygonal shape (Fig. 1A), whereas at 72 h, the cell
density was increased, and fibroblast-like colonies had formed.
After 1 week, the ADSCs exhibited a uniformly aligned monolayer of
fibroblast-like cells, with a swirl or parallel growth style
(Fig. 1B). Between the first and
fifth passages, the growth of the ADSCs was marked. However, the
cells appeared irregular and larger in size at passage 10, and
showed reduced proliferation (Fig.
1C).
The multipotent differentiation characteristic was
demonstrated by culturing the cells with osteogenic medium and
adipogenic medium. As shown in Fig.
1D, a calcific knob was visualized following staining with
Alizarin Red, and small adipose drops had gathered intracellularly,
detected using Oil-Red O, as shown in Fig. 1E. As shown in Fig. 1F–H, the primary cultured ADSCs
expressed positively for CD29 (99.3%) and CD44 (94.3%), but not for
CD 49 (1.3%), CD45 (0.1%), CD106 (0.0%) or CD 31 (0.0%), indicating
that the cells were MSCs, but were not from hematopoietic stem
cells.
Induction of ADSCs to differentiate into
Schwann-like cells
The rat ADSCs were treated with SC-conditioned
medium for 12 days. At the beginning of differentiation, the
morphology of the ADSCs changed from a monolayer of large cells
with a flat morphology to a small number of bipolar or tripolar
spindle-like shaped cells (Fig.
2A). As the cells continued to proliferate, the density of
cells was markedly increased (Fig.
2B). The results of the immunofluorescence (Fig. 2C–E), western blot (Table I, Fig.
2F–I) and RT-qPCR (Fig. 2J)
analyses showed that almost all the differentiated ADSCs were
positive for GFAP, S100 and P75. These characteristics of
differentiated ADSCs were similar to those of SCs.
 | Figure 2ADSC differentiation into cells with
an SC phenotype. (A) Cultured cells at the early stage of
differentiation. (B) ADSCs differentiated into SC phenotype.
Immunofluorescence staining of (C) GFAP, (D) S100 and (E) P75.
Scale bar=100 µm. Cell nuclei were labeled with DAPI (blue).
Detection of (F) GFAP, (G) S100 and (H) P75 proteins using western
blot analysis. (I) Relative protein expression levels of GFAP, S100
and P75 (*P<0.05, vs. ADSCs). 1, ADSCs, 2,
Schwann-like cells. (J) Detection of mRNA expression levels of
GFAP, S100 and P75 using reverse transcription-quantitative
polymerase chain reaction analysis (**P<0.01, vs.
ADSCs). Values are presented as the mean ± standard deviation. SC,
Schwann cell; ADSCs, adipose-derived stem cells; GFAP, glial
fibrillary acidic protein. |
 | Table IProtein expression levels of GFAP,
S100 and P75. |
Table I
Protein expression levels of GFAP,
S100 and P75.
| Group | GFAP | S100 | P75 |
|---|
| Adipose-derived stem
cells | 0.417±0.076 | 0.502±0.150 | 0.431±0.155 |
| Schwann-like
cells | 0.613±0.063a | 0.766±0.134a | 0.721±0.171a |
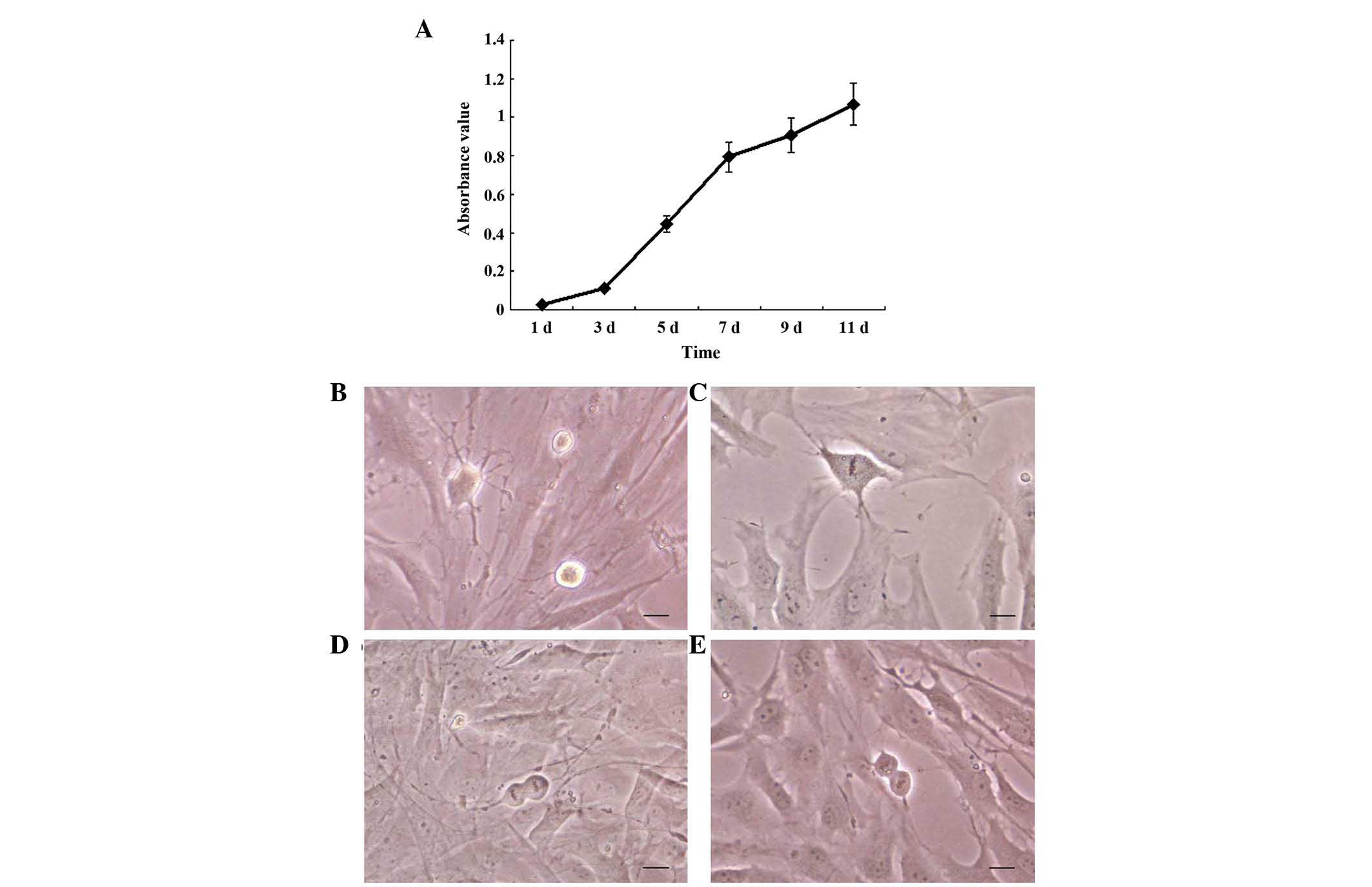
Observation of the proliferation of
Schwann-like cells
In the present study, the viability and
proliferation of the Schwann-like cells were analyzed using an MTT
assay. As shown in Fig. 3A, during
the first 1–3 days, the cells grew slowly. The rate of cell
proliferation increased more rapidly from day 3, and this rapid
growth rate continued until day 7. Between days 9 and 11, the cells
continue to grow, however, the growth rate had declined. During the
experiment, images were captured of mitosis in the Schwann-like
cells in each period. The chromatin filament formed a spiral shape,
resembling a loose knitting ball, during prophase of mitosis
(Fig. 3B). During metaphase
(Fig. 3C), the chromosomes
arranged in order at the equator and, during anaphase (Fig. 3D), the two chromatids of each
chromosome separated and moved in the opposite directions. The
chromatids divided into two groups and the cells were elongated,
exhibiting a dumbbell-like shape. In telophase (Fig. 3E), gradual unwinding of the
chromatids was observed, with one cell dividing into two cells.
Discussion
Considerable attention has been focused on PNI due
to its increased rate of morbidity. Once PNI occurs, the major
physical response of the body is to protect the remaining neurons
around the damage zone and restructure the axon (12). In previous studies of SCs, the
glial cells of the peripheral nervous system, they were shown to be
involved in nerve regeneration by secreting neurotrophic factors
(13). However, Evans et al
(14) demonstrated that the
effects of SCs were also concentration-dependent and
distance-dependent, with more marked regenerative effects on nerve
degeneration with increasing concentration in conduits, and a
larger area of the distal axonal regeneration. Despite this,
cultured SCs have limited clinical application, whereas stem cells
are readily accessible as an alternative cell source for nerve
regeneration.
It has been reported that MSCs can be readily
derived from bone marrow for autologuous transplantation in in
vitro (15) and in vivo
(16) studies. Due to the
complicated procurement and survival of SCs, this alternative cell
source requires further investigation. ADSCs, which are isolated
from adipose tissue, exhibit self-renewal and can differentiate
along several mesenchymal tissue lineages, including adipocytes,
osteoblasts, myocytes, chondrocytes and endothelial cells (17,18).
Liposuction is a common and safe surgical procedure, enabling a
substantial number of cells to be obtained with minimal risk
(19). Furthermore the ratio of
ADSCs in adipose tissue is higher than that of BMMSCs, and ADSCs
proliferate significantly more rapidly, compared with BMMSCs,.
Therefore, ADSCs may be an idea alternative cell source to SCs. It
has also been reported that ADSCs can be induced into SCs in
vitro (10).
The ADSCs used in the present study were obtained
from the rat inguinal fat pad, and the cells in the third to fifth
passages were positive for the expression of CD29 and CD44, whereas
the expression of CD31 (an endothelial cell marker), CD45 (a
hematopoietic cell marker) and CD106 (a marker of BMMSCs) were
negative.
In addition to measurements of cell surface markers,
whether ADSCs have the potential for multidirectional
differentiation has also been investigated (20). Under the combined function of
Vitamin C, β-sodium glycerophosphate and hexadecadrol, the present
study found the presence of a calcific knob, which stained red
following Alizarin Red staining. This showed that calcification
appeared during the induction of osteogenesis, with extracellular
calcium ion deposition and the formation of calcium salt. No
similar knobs were found in the contrasting group. Under the
combined function of insulin, hexadecadrol, IBMX and indometacin,
it was found that small adipose drops gathered, which appeared red
on Oil-Red O staining. This was not observed in the contrasting
group. All the above experiments demonstrated that the ADSCs
exhibited osteogenic and adipogenic differentiation potential.
In the present study, ADSCs were induced and
differentiated into Schwann-like cells with various cytokines. β-ME
promotes neural differentiation in stem cells by preventing damage
to cells of the peroxidase (21).
The mechanism involves β-ME increasing the rate of synthesis of
glutathione and reducing the cell response to oxygen tension
(22). ATRT has an effect on the
differentiation of neural stem cells through cell surface
receptors, retinoic acid receptors and retinoid X receptors, and
these can regulate the expression of certain coding factors, which
are critical to the development of neural cell differentiation
(23). HRG is a subtype of
neuregulin, regarded as an important axonal signal, which can not
only prevent apoptosis of SC precursors, but also induce neural
crest differentiation into SCs selectively (24). Another study showed that HRG
predominately acts on myelination (25). FSK can elevate the level of
intracellular cAMP, and cAMP elevation can mimic SC responses in
the presence of axons during myelination in vivo (26). bFGF has been reported to be
involved in cell growth and differentiation. Zhu et al
(25) demonstrated that bFGF is a
major regulator for MSC differentiation into the SC phenotype.
In the present study, immunofluorescence revealed
that bipolar, tripolar and even multipolar cells were positive for
GFAP, S-100 and P75. To confirm that the results obtained using
immunofluorescence were not due to any artefact of cell shrinkage,
western blot and RT-qPCR analyses were performed. The Schwann-like
cells showed GFAP, S-100 and P75 immunoreactive bands,
corresponding to molecular weights of 48,10 and 42 kDa
respectively. The results of the RT-qPCR, probed with GFAP, S-100
and P75 primers, also supported the results of the
immunofluorescence and western blot analyses. In addition, a
previous study showed that this differentiation was lasting and
irreversible (27).
In addition, the morphological and phenotypic
characteristics did not posses the functions of SCs derived from
adipose cells, thus, the present study observed the mitosis of the
Schwann-like cells, which increased the numbers of cells in
cascades. The results showed that the Schwann-like cells were fully
confluent in a short duration once attached, which is beneficial
for clinical regeneration following PNI. As for the specific
mechanism underlying Schwann-like cell mitotic proliferation, it
may be associated with cross-talk between the two cell types by
soluble secreted proteins (28),
which requires further investigation. The present study
hypothesized that this may be associated with activation of the
mitogen-activated protein kinase/extracellular signal-regulated
kinase signaling pathway and/or inhibition of the caspase pathway,
which requires investigation in subsequent experiments. Due to the
limitations on research funds and time in the present study, only
the mitosis of Schwann-like cells derived from ADSCs were observed.
Whether SCs cultured directly from the sciatic nerve or brachial
nerve, or from BMMSCs can perform mitosis remains to be elucidated
and warrants further investigation to determine their potential
benefit. The present study provides broad information that may aid
the future development of cell therapy in PNI.
Acknowledgments
This study was supported by the Science and
Technology Department of Liaoning Province (grant no. 2013225049)
and the Education Department of Hebei Province (grant no.
QN2014138).
References
|
1
|
Zhang P, Lu X, Chen J and Chen Z: Schwann
cells originating from skin-derived precursors promote peripheral
nerve regeneration in rats. Neural Regen Res. 9:1696–1702. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nishihara T, Remacle AG, Angert M,
Shubayev I, Shiryaev SA, Liu H, Dolkas J, Chernov AV, Strongin AY
and Shubayev VI: Matrix metalloproteinase-14 both sheds cell
surface neuronal glial antigen 2 (NG2) proteoglycan on macrophages
and governs the response to peripheral nerve injury. J Biol Chem.
290:3693–3707. 2015. View Article : Google Scholar :
|
|
3
|
Fairbairn NG, Meppelink AM, Ng-Glazier J,
Randolph MA and Winograd JM: Augmenting peripheral nerve
regeneration using stem cells: A review of current opinion. World J
Stem Cells. 7:11–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berrocal YA, Almeida VW, Gupta R and Levi
AD: Transplantation of Schwann cells in a collagen tube for the
repair of large, segmental peripheral nerve defects in rats. J
Neurosurg. 119:720–732. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gärtner A, Pereira T, Armada-da-Silva P,
Amado S, Veloso A, Amorim I, Ribeiro J, Santos J, Bárcia R, Cruz P,
et al: Effects of umbilical cord tissue mesenchymal stem cells
(UCX®) on rat sciatic nerve regeneration after neurotmesis
injuries. J Stem Cells Regen Med. 10:14–26. 2014.
|
|
6
|
Park HW, Lim MJ, Jung H, Lee SP, Paik KS
and Chang MS: Human mesenchymal stem cell-derived Schwann cell-like
cells exhibit neurotrophic effects, via distinct growth factor
production, in a model of spinal cord injury. Glia. 58:1118–1132.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dadon-Nachum M, Sadan O, Srugo I, Melamed
E and Offen D: Differentiated mesenchymal stem cells for sciatic
nerve injury. Stem Cell Rev. 7:664–671. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sabatino MA, Santoro R, Gueven S, Jaquiery
C, Wendt DJ, Martin I, Moretti M and Barbero A: Cartilage graft
engineering by co-culturing primary human articular chondrocytes
with human bone marrow stromal cells. J Tissue Eng Regen Med.
9:1394–1403. 2015. View Article : Google Scholar
|
|
9
|
Liu Y, Zhang Z, Qin Y, Wu H, Lv Q, Chen X
and Deng W: A new method for Schwann-like cell differentiation of
adipose derived stem cells. Neurosci Lett. 551:79–83. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Razavi S, Mardani M, Kazemi M, Esfandiari
E, Narimani M, Esmaeili A and Ahmadi N: Effect of leukemia
inhibitory factor on the myelinogenic ability of Schwann-like cells
induced from human adipose-derived stem cells. Cell Mol Neurobiol.
33:283–289. 2013. View Article : Google Scholar
|
|
11
|
Esmaeili A and Zaker SR: Differential
expression of glycine receptor subunit messenger RNA in the rat
following spinal cord injury. Spinal Cord. 49:280–284. 2011.
View Article : Google Scholar
|
|
12
|
Liu Y, Nie L, Zhao H, Zhang W, Zhang YQ,
Wang SS and Cheng L: Conserved dopamine neurotrophic
factor-transduced mesenchymal stem cells promote axon regeneration
and functional recovery of injured sciatic nerve. PLoS One.
9:e1109932014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jonsson S, Wiberg R, McGrath AM, Novikov
LN, Wiberg M, Novikova LN and Kingham PJ: Effect of delayed
peripheral nerve repair on nerve regeneration, Schwann cell
function and target muscle recovery. PLoS One. 8:e564842013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Evans GR, Brandt K, Katz S, Chauvin P,
Otto L, Bogle M, Wang B, Meszlenyi RK, Lu L, Mikos AG and Patrick
CW Jr: Bioactive poly (L-lactic acid) conduits seeded with Schwann
cells for peripheral nerve regeneration. Biomaterials. 23:841–848.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang H, Zhang H, Liu M and Wang N: Distal
segment extracts of the degenerated rat sciatic nerve induce bone
marrow stromal cells to express Schwann cell markers in vitro.
Neurosci Lett. 544:89–93. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ladak A, Olson J, Tredget EE and Gordon T:
Differentiation of mesenchymal stem cells to support peripheral
nerve regeneration in a rat model. Exp Neurol. 228:242–252. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ferroni L, Gardin C, Tocco I, Epis R,
Casadei A, Vindigni V, Mucci G and Zavan B: Potential for neural
differentiation of mesenchymal stem cells. Adv Biochem Eng
Biotechnol. 129:89–115. 2013.
|
|
18
|
Ghidoni I, Chlapanidas T, Bucco M, Crovato
F, Marazzi M, Vigo D, Torre ML and Faustini M: Alginate cell
encapsulation: New advances in reproduction and cartilage
regenerative medicine. Cytotechnology. 58:49–56. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Harasymiak-Krzyżanowska I, Niedojadło A,
Karwat J, Kotuła L, Gil-Kulik P, Sawiuk M and Kocki J: Adipose
tissue-derived stem cells show considerable promise for
regenerative medicine applications. Cell Mol Biol Lett. 18:479–493.
2013. View Article : Google Scholar
|
|
20
|
Hagmann S, Moradi B, Frank S, Dreher T,
Kämmerer PW, Richter W and Gotterbarm T: FGF-2 addition during
expansion of human bone marrow-derived stromal cells alters MSC
surface marker distribution and chondrogenic differentiation
potential. Cell Prolif. 46:396–407. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reid AJ, Sun M, Wiberg M, Downes S,
Terenghi G and Kingham PJ: Nerve repair with adipose-derived stem
cells protects dorsal root ganglia neurons from apoptosis.
Neuroscience. 199:515–522. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barnabé GF, Schwindt TT, Calcagnotto ME,
Motta FL, Martinez G Jr, de Oliveira AC, Keim LM, D'Almeida V,
Mendez-Otero R and Mello LE: Chemically-induced RAT mesenchymal
stem cells adopt molecular properties of neuronal-like cells but do
not have basic neuronal functional properties. PLoS One.
4:e52222009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lainey E, Wolfromm A, Sukkurwala AQ, Micol
JB, Fenaux P, Galluzzi L, Kepp O and Kroemer G: EGFR inhibitors
exacerbate differentiation and cell cycle arrest induced by
retinoic acid and vitamin D3 in acute myeloid leukemia cells. Cell
Cycle. 12:2978–2991. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee HJ, Shin YK and Park HT: Mitogen
activated protein kinase family proteins and c-jun signaling in
injury-induced Schwann cell plasticity. Exp Neurobiol. 23:130–137.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu H, Yang A, Du J, Li D, Liu M, Ding F,
Gu X and Liu Y: Basic fibroblast growth factor is a key factor that
induces bone marrow mesenchymal stem cells towards cells with
Schwann cell phenotype. Neurosci Lett. 559:82–87. 2014. View Article : Google Scholar
|
|
26
|
Guo L, Moon C, Niehaus K, Zheng Y and
Ratner N: Rac1 controls Schwann cell myelination through cAMP and
NF2/merlin. J Neurosci. 32:17251–17261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li P, Sun H, Du M, Fa Z, Qin K, Xu W,
Zhang R, Chen L, Yao C, Xiao Z, et al: Adult rat hippocampus
soluble factors: A novel transplantation model mimicking
intracranial microenvironment for tracing the induction and
differentiation of adipose-derived stromal cells in vitro. Neurosci
Lett. 542:5–11. 2013. View Article : Google Scholar
|
|
28
|
Wei Y, Gong K, Zheng Z, Liu L, Wang A,
Zhang L, Ao Q, Gong Y and Zhang X: Schwann-like cell
differentiation of rat adipose-derived stem cells by indirect
co-culture with Schwann cells in vitro. Cell Prolif. 43:606–616.
2010. View Article : Google Scholar : PubMed/NCBI
|