Introduction
Granulosa cell tumors (GCTs) are malignant
neoplasms, which originate from the sex cord. GCT is a relatively
rare disease, but commonly occurs in the ovary. GCTs frequently
occur in young women, and the majority of GCT cases are diagnosed
in the late stages due to early stage GCT exhibiting few clinical
symptoms, leading to a poor prognosis (1,2). Due
to the current lack of understanding of this particular disease,
the clinical management of GCTs is similar to that of other types
of epithelial ovarian cancer. However, unlike other types of
ovarian cancer, the prognostic assessment for GCT predominantly
relies on clinicopathological variables, including stage and grade,
although these do not provide biological insight into the disease
(3). Thus, early diagnosis is
critical for the successful management of numerous types of human
cancer, including ovarian GCTs.
The identification of molecular or epigenetic
markers may provide biological insight into GCT and a serve as a
critical tool in successful treatment of the disease. Current
knowledge indicates that the development and progression of cancer
are driven by the accumulation of genetic abnormalities and
epigenetic alterations, which include gene mutations and silencing,
and epigenetic modifications of genomic DNA, including methylation
of DNA CpG islands or covalent modification of histone tails
(4,5). Gene promoter methylation silences the
expression of tumor suppressor genes, which is one of the key
mechanisms in tumor development (6,7).
Aberrant gene methylation is also one of the earliest molecular
alterations occurring in tumorigenesis, and is considered a
biomarker for early tumor detection or a potential treatment
strategy (8–10). Histone modification also regulates
genetic programs in normal cells, but is altered in cancer cells
(11). Therefore, the pathogenesis
of GCT may follow a similar trend to other types of human
cancer.
In the present study, the methylation status of
three putative tumor suppressor genes, cadherin 13 (CDH13),
dickkopf WNT signaling pathway inhibitor 3 (DKK3) and
forkhead box L2 (FOXL2), and expression of enhancer of zeste
homolog 2 (EZH2) were assessed in GCT tissue samples and compared
with follicular cyst tissues. The aim of the present study was to
screen and identify tumor markers for the early detection of GCT.
As GCTs are rare and reports are limited, the present study focused
on CDH13, DKK3 and FOXL2, as these are putative tumor
suppressor genes, whose functions and expression are associated
with the ovary, including ovary development, function maintenance.
The present study aimed to provide novel information to improve
current understanding of the development of GCT, and to potentially
identify biomarkers for the early detection of GCT.
Materials and methods
Patients and samples
In the present study, 31 patients with GCTs were
recruited from Shandong University Qilu Hospital (Jinan, China) and
Shanxian Central Hospital (Heze, China) between 2010 and 2013. All
patients were diagnosed histologically with GCT, and all tissue
specimens were reconfirmed by pathologists in the Department of
Pathology, Qilu Hospital, which resulted in 30 cases being
available for use in the study. The present study also included
tissues from 30 patients with follicular cysts, which were selected
as a control. Clinicopathological data from each patient, including
age, tumor size, Federation of Gynecology and Obstetrics (FIGO)
stage and postoperative recurrence, were collected from medical
records. The present study was approved by the Ethics Committee of
Shandong University School of Medicine (Jinan, China) and the
patients or their guardians provided signed informed consent prior
to involvement in the investigation. Written informed consent was
obtained from patients.
Methylation-specific polymerase chain
reaction (MSP)
A total of 31 patients with GCTs were recruited from
Shandong University Qilu Hospital (Jinan, China) and Shanxian
Central Hospital (Heze, China) between 2010 and 2013. All patients
were diagnosed histologically with GCT, and all tissue specimens
were reconfirmed by pathologists in the Department of Pathology,
Qilu Hospital. One tissue wax block was not large enough, which
resulted in only 30 cases being available for use in the present
study. For MSP, two 8-µm tissue sections were prepared from
the 30 paraffin-embedded tissue blocks and deparaffinized in
xylene, following which tumor cells were dissected from sections
for genomic DNA extraction. Specifically, genomic DNA was extracted
using a Genomic DNA Purification kit (Qiagen, Hilden, Germany) and
subjected to bisulfite conversion using a CpGenome DNA Modification
kit (Intergen Co., Purchase, NY, USA), according to the
manufacturer's protocols. Subsequently, 2 µl of the modified
DNA (50 ng) was subjected to PCR amplification in a 50 µl
volume reaction [0.25 µl Taq polymerase, 5 µl 10X PCR
buffer; 4 µl dNTP mix (2.5 mM); 0.5 µl forward and
reverse primers; 50 ng DNA template made up to the volume in Milli
Q water] under the following conditions: 45 cycles at an annealing
temperature of 58°C for 45 sec (CDH13), 60°C for 45 sec
(DKK3) or 58°C for 45 sec (FOXL2), and primer
extension at 72°C. All PCR amplifications were performed with
positive controls for unmethylated and methylated alleles, and
DNA-free empty controls. The PCR amplification kit was purchased
from Eppendorf AG (Hamburg, Germany). The primers (Sangon Biotech.,
Co., Ltd., Shanghai, China) for each gene promoter methylation were
designed according to previous reports (12–14)
by first identifying the methylated- and unmethylated-specific
sequences, respectively, and subsequent synthesis for MSP
amplification of the CDH13, DKK3 and FOXL2 genes
(Table I). The PCR products were
then separated on 3% agarose gels and visualized using ethidium
bromide staining under an UV-3000 ultraviolet light box.
 | Table IPrimer sequences for PCR
amplification of multiple tumor suppressor genes and
methylation-specific PCR analysis of gene promoter methylation. |
Table I
Primer sequences for PCR
amplification of multiple tumor suppressor genes and
methylation-specific PCR analysis of gene promoter methylation.
| Gene | Sequence | Amplicon (bp) | Temperature
(°C) |
|---|
| CDH13-M-F |
5′-TCGCGGGGTTCGTTTTTCGC-3′ | 243 | 58 |
| CDH13-M-R |
5′-GACGTTTTCATTCATACACGCG-3′ | | |
| CDH13-U-F |
5′-TTGTGGGGTTTGTTTTTTGT-3′ | 242 | |
| CDH13-U-R |
5′-AACTTTTCATTCATACACACA-3′ | | |
| DKK3-M-F |
5′-GGGGCGGGCGGCGGGGC-3′ | 120 | 60 |
| DKK3-M-R |
5′-ACATCTCCGCTCTACGCCCG-3′ | | |
| DKK3-U-F |
5′-TTAGGGGTGGGTGGTGGGGT-3′ | 126 | |
| DKK3-U-R |
5′-CTACATCTCCACTCTACACCCA-3′ | | |
| FOXL2-M-F |
5′-GTTATAATATTTTTTCGGTTGTTC G-3′ | 211 | 58 |
| FOXL2-M-R |
5′-CTAACTCCACGACCTATACTCGAT-3′ | | |
| FOXL2-U-F |
5′-AGGTTATAATATTTTTTTGGTTGTTTG-3′ | 214 | |
| FOXL2-U-R |
5′-CCTAACTCCACAACCTATACTCAAT-3′ | | |
Immunohistochemistry
Immunohistochemistry was performed to detect the
protein expression of EZH2 in the tissue samples. In brief,
3-µm thick tissue sections were prepared from the
paraffin-embedded tissue blocks, deparaffinized in xylene and
rehydrated in a series of ethanol. The sections were then incubated
with 0.5% TritonX-100 for 30 min at room temperature to ensure that
the antibody entered the nuclei. The sections then underwent
epitope retrieval in a steam cooker in 0.01 M citric buffer (pH
6.0) for 15 min at 100°C. The slides were subsequently washed with
phosphate-buffered saline (pH 7.4) three times for 5 min. The
slides were immersed in 3% H2O2 methanol
solution (freshly prepared) for 10 min. The slides were
subsequently washed as above. Following being blocked in normal
serum for 30 min, the sections were incubated with anti-EZH2
antibody at a dilution of 1:100 (cat. no. 5246; Cell Signaling
Technology, Inc., Danvers, MA, USA) at 4°C overnight. The following
day, the sections were washed with phosphate-buffered saline (PBS)
three times and further incubated with a rabbit anti-human
secondary antibody Dako (Glostrup, Denmark; cat. no. K5007) at room
temperature for 30 min. The positive signal was visualized using
diaminobenzidine as the chromogen. Breast cancer tissue sections
(Shanxian Central Hospital; patient was diagnosed as infiltrating
ductal carcinoma) were used as a positive control, and PBS was used
as a negative control. The stained sections were reviewed and
scored under a light microscope (BX43; Olympus, Tokyo, Japan) by
two investigators for staining intensity and percentage of staining
(15). The staining intensity
score was recorded as follows: Absent, 0; weak, 1; moderate, 2;
strong, 3. The percentage of positive cells was recorded as
follows: Absent, 0; ≤10%, 1; 11–50%, 2; 51-≤80%, 3; >80 %, 4.
These two scores were then multiplied to obtain a staining index.
If the staining index was ≥3, the case was considered positive.
Statistical analysis
All statistical analyses were performed using SAS
version 9.1 software (SAS Institute, Inc., Cary, NC, USA). The
frequencies of methylation were compared using χ2 test
or Fisher's exact test. P<0.05 was considered to indicate a
statistically significant difference. All tests were two-sided.
Results
Differential methylation status of CDH13,
DKK3 and FOXL2 promoters in GCTs
In the present study, the methylation status of the
CDH13, DKK3 and FOXL2 promoters in the GCT
tissue specimens were assessed and compared with those in
follicular cyst specimens. Table
II summarizes the methylation rates of these three genes in the
GCTs, compared with the follicular cysts. Representative examples
of the MSP data are shown in Fig.
1. Significant differences were found in the methylation of the
CDH13, DKK3 and FOXL2 promoters in the GCT
tissues, compared with the follicular cyst tissues (P<0.001).
The associations between gene methylation and clinicopathological
parameters, including age, tumor size, FIGO stage and postoperative
recurrence, were also analyzed (Table III), however, no significant
associations were observed.
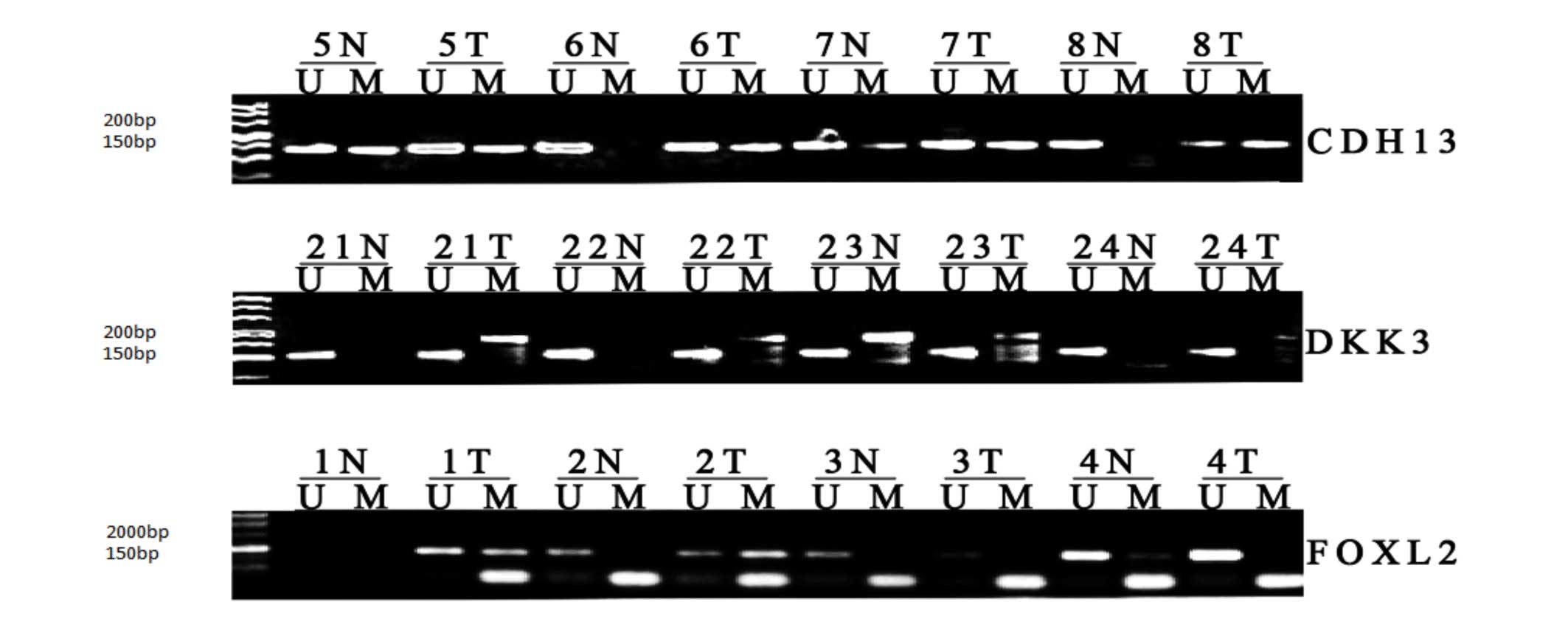 | Figure 1MSP analysis of CDH13, DKK3
and FOXL2 promoter methylation in GCT and follicular cyst
tissues. The MSP products in the M lanes indicate the presence of
methylated alleles, whereas those in the U lanes indicate the
presence of unmethylated alleles. MSP, methylation-specific
polymerase chain reaction; T, GCT; N, follicular cyst; CDH13,
cadherin 13; DKK3, dickkopf WNT signaling pathway inhibitor 3;
FOXL2, forkhead box L2; U, unmethylated; M, methylated. |
 | Table IIComparison of methylation rates of
CDH13, CKK3 and FOXL-2 promoters between GCT
and follicular cyst tissues. |
Table II
Comparison of methylation rates of
CDH13, CKK3 and FOXL-2 promoters between GCT
and follicular cyst tissues.
| Gene | GCT (n=30) | Follicular cyst
(n=30) | χ2 | P-valuea |
|---|
| CDH13 | 86.67 (26) | 23.33 (7) | 21.70 | <0.001 |
| DKK3 | 80 (24) | 26.67 (8) | 17.14 | <0.001 |
| FOXL2 | 70 (21) | 20 (6) | 17.38 | <0.001 |
 | Table IIIAssociation between CDH13,
DKK3 and FOXL2 methylation and clinicopathological
data from patients with granulosa cell tumors. |
Table III
Association between CDH13,
DKK3 and FOXL2 methylation and clinicopathological
data from patients with granulosa cell tumors.
| | DKK3 (M)
| CDH13(M)
| FOXL2(M)
| EZH2 (M)
|
|---|
| Clinical
feature | n | n (%) | P-valuea | n (%) | P-valuea | n (%) | P-valuea | n (%) | P-valuea |
|---|
| Age (years) | | | 1.00 | | 0.55 | | 1.00 | | 1.00 |
|
<40 | 8 | 7
(87.5) | | 8 (100.0) | | 6
(75.0) | | 3 (37.5) | |
|
≥40 | 22 | 17 (77.3) | | 18 (81.8) | | 15 (68.2) | | 8 (36.4) | |
| Tumor size
(cm) | | | 0.37 | | 1.00 | | 0.68 | | 0.69 |
|
≥10 | 19 | 14 (73.7) | | 16 (84.2) | | 14 (73.3) | | 6 (31.6) | |
|
<10 | 11 | 10 (90.9) | | 10 (90.9) | | 7
(63.6) | | 5 (45.5) | |
| FIGO stage | | | 0.57 | | 0.55 | | 1.00 | | 0.64 |
| I | 24 | 20 (83.3) | | 20 (83.3) | | 17 (70.8) | | 8 (33.3) | |
|
II–III | 6 | 4
(66.7) | | 6 (100.0) | | 4
(66.7) | | 3 (50.0) | |
| Recurrence | | | 0.50 | | 1.00 | | 0.53 | | 0.53 |
|
Yes | 3 | 2
(66.7) | | 3 (100.0) | | 3 (100.0) | | 2 (44.4) | |
| No | 27 | 22 (81.5) | | 23 (85.2) | | 18 (66.7) | | 9 (33.3) | |
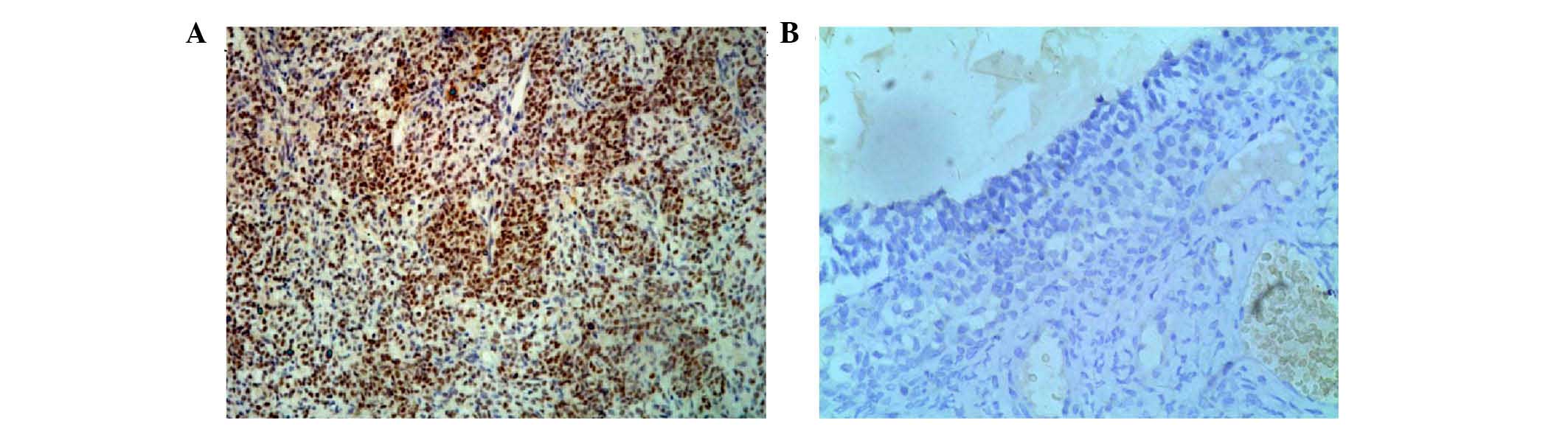
Differential protein expression of EZH2
in GCT tissues
The immunohistochemical staining showed that EZH2
protein was localized in the nuclei of the positive tumor cells.
Representative examples of the immunohistochemical data are shown
in Fig. 2. It was found that the
expression of EZH2 was higher in the GCT tissues, compared with the
follicular cysts (Table IV),
however, no associations were found between the expression of EZH2
and the clinicopathological parameters of the patients with GCT
with respect to age, tumor size, FIGO stage and metastasis
(Table II). The present study
then examined the associations between the expression of EZH2 and
methylation of the CDH13, DKK3 and FOXL2 gene
promoters, however, no positive associations were found (Table V).
 | Table IVComparison of the expression of
enhancer of zeste homolog 2 between GCT and follicular cyst
tissues. |
Table IV
Comparison of the expression of
enhancer of zeste homolog 2 between GCT and follicular cyst
tissues.
| EZH2
| | | |
|---|
| Tissue | + (n) | − (n) | % | χ2 | P-valuea |
|---|
| GCT | 11 | 19 | 36.7 | | |
| Follicular
cyst | 0 | 30 | 0 | 13.5 | <0.001 |
 | Table VAssociation between the expression of
EZH2 and methylation of the CDH13, DKK3 and
FOXL2 promoters. |
Table V
Association between the expression of
EZH2 and methylation of the CDH13, DKK3 and
FOXL2 promoters.
| DKK3
| | CDH13
| | FOXL2
| |
|---|
| EZH2 | M | U | P-valuea | M | U | P-valuea | M | U | P-valuea |
|---|
| Positive (n) | 9 | 2 | | 9 | 2 | | 9 | 2 | |
| Negative (n) | 15 | 4 | 1.00 | 17 | 2 | 0.61 | 12 | 7 | 0.41 |
Discussion
GCTs are a relatively rare type of malignancy in the
ovary, and are inconsistent in size, ranging between small spots
and large masses, with an average diameter of 10 cm. Although the
clinical appearance, symptoms and management are similar to those
of epithelial ovarian tumors, the mechanism underlying the
development and progression of GCT may be different from other
types of ovarian cancer (1). Thus,
an improved understanding of the mechanism underlying the
development and progression of GCT may lead to improved options for
the early detection, prevention and treatment of GCT clinically
(16). To date, clinical
prognostic indicators rely predominantly on clinicopathological
variables, including patient age, tumor stage and grade. Thus, the
identification of molecular or epigenetic markers may provide
biological insight and serve as a critical tool in the successful
treatment of GCT. The present study assessed the methylation status
of the CDH13, DKK3 and FOXL2 promoters, and
found that the promoters of these three genes were significantly
hypermethylated in the GCT tissues, compared with the follicular
cyst tissues. Expression of the EZH2 protein was also high in the
GCT tissues, however, no associations were found between these
alterations and the clinicopathological data from the patients with
GCT. Further investigations with a larger sample size are required
to confirm these findings. As GCT is a relatively rare type of
tumor, a consortium of different cancer centers or hospitals may be
required to obtain sufficient numbers of tissue samples to
facilitate further molecular investigations.
Although the cause and pathogenesis of GCTs remain
to be elucidated, GCTs are similar to the majority of other types
of human cancer, the development of which involves gene mutation
and promoter methylation, and epigenetic modifications of genomic
DNA. The accumulation of genetic abnormalities and epigenetic
alterations lead to the malignant transformation of normal cells.
To date, the known primary human epigenetic modifications include
alterations of DNA methylation status in CpG islands and covalent
modifications of histone tails. Accumulating evidence suggests that
more genes are affected by aberrant epigenetic alterations than
genetic mutations in human carcinogenesis (6,7,17).
Thus, promoter methylation-induced silencing of tumor suppressor
genes has been suggested as a key mechanism in the development of
several types of human cancer. Aberrant gene methylation is also
one of the earliest molecular alterations occurring during
tumorigenesis and may be used as a marker for early tumor
detection. As methylation of the promoter region is a reversible
process, the detection of gene methylation levels may provide
guidance for individualized chemotherapy. The present study showed
for the first time, to the best of our knowledge, methylation of
the CDH13, DKK3 and FOXL2 gene promoters in
GCT tissue samples, with no similar findings reported previously.
Friedrichs et al (15)
first reported a specific pattern of CpG island hypermethylation in
different types of human cancer. In GCTs, the detection of
different gene promoter methylation has been shown in cell lines
and in a limited number of tumor tissues, with the most frequently
methylated gene promoters being p16 and ER-α (40%), BRCA1 and
RASSF1A (36%), MGMT (32.5%) and hMLH1 and FHIT (28%) (16,18,19).
CDH13 is a cell adherence protein of a unique
cadherin superfamily member and functions to mediate intracellular
signaling in vascular cells. Emerging evidence indicates that
CDH13 is a candidate tumor suppressor in several types of
human cancer, including breast and lung cancer (12,20–26),
colorectal cancer (21,27), hepatocellular carcinoma (28), bladder cancer (29), cervical cancer (30) and ovarian cancer (31–34).
Previous studies have showed that CDH13 promoter methylation
is a frequent event in cancer, which is associated with unfavorable
tumor features, increased risk of recurrence and poorer survival
rates, and has been suggested as an independent predictor for tumor
recurrence and progression (26,29).
DKK3 is a secreted protein, which is involved in embryonic
development through its interactions with the Wnt signaling
pathway. The expression of DKK3 is reduced in a variety of
cancer cell lines and may function as a tumor suppressor gene by
antagonizing Wnt signaling (35–37).
Epigenetic silencing of DKK3 has been observed to disrupt
normal Wnt/β-catenin signaling and apoptosis regulation (38). DKK3 methylation has been
frequently detected in a broad range of types of cancer and appears
to be important in tumor development (13,37–42).
FOXL2 is a member of the forkhead transcription factor
family and functions as an essential regulator of ovarian
maintenance. FOXL2 protein is expressed in the pituitary
gonadotrope, thyrotrope cells and ovarian granulosa cells, and is
required for commitment to ovary differentiation (43,44).
FOXL2 mutations are associated with syndromic and
non-syndromic ovarian failure, and occurs in ovary GSTs with a
mutation rate at FOXL2 (402 C->G) of 97% in adult GCT
(45–50). Tran et al showed that the
CpG island of the murine FOXL2 proximal promoter was
differentially methylated in primary and immortalized cells
(51). The FOXL2 promoter
was also abnormally methylated in non-small cell lung cancer
(52). In the present study, the
methylation statuses of three gene promoters in GCT tissues were
detected, and the results demonstrated that promoter methylation
was associated with the development of GCT, but not with its
progression. Further investigations aim to investigate the
underlying molecular mechanism for silencing the expression of
these three genes in GCT.
Histone modifications are considered to regulate
genetic programs in normal cells, but are altered in cancer cells.
The methylation of histone H3 at lysine 27 silences gene
expression, which induces transcriptional repression and is thus
involved in controlling gene expression patterns (53,54).
EZH2 is a methyltransferase and a component of the polycomb
repressive complex 2, which is essential in the epigenetic
maintenance of the H3K27me3 repressive chromatin mark (55). The abnormal expression of EZH2 has
been associated with aggressive tumor subgroups, disease-free
survival rates and overall survival rates in patients with
cutaneous melanoma, and in cancer of the endometrium, prostate,
breast, colorectal and ovary (11,56–59).
The present study was the first, to the best of our knowledge, to
detect the expression of EZH2 in GCT and found 11 positive cases in
30 GCT tissue samples (36.7%), compared with follicular cyst tissue
samples, in which no positive cases were found. In addition, a
previous study linked EZH2 to gene silencing in association with
the maintenance of DNA methylation (58). EZH2 may affect DNA methylation by
direct interaction with DNA methyltransferases, however, the
majority of H3K27me3-marked genes lack DNA methylation in embryonic
stem cells, indicating that EZH2 recruitment may not be sufficient
to promote DNA methylation (60).
In the present study, no associations were found between the
expression of EZH2 and methylation of the CDH13, DKK3
and FOXL2 promoters. Thus, further investigation is required
to assess the functions of the EZH2 protein in GCTs.
The results of the present study were
proof-of-principle, and future investigations with a larger sample
size are required to verify the findings. Future investigations aim
to assess how the methylation of the CDH13, DKK3 and
FOXL2 gene promoters affects the expression of their
proteins, and how these proteins contribute to the development of
GCT. Whether the altered methylation status of these gene promoters
can be detected as biomarkers for the early detection of GCT also
requires investigation.
Acknowledgments
The authors would like to thank Medjaden Bioscience
Ltd. (Hong Kong, China) for their assistance with manuscript
editing.
References
|
1
|
Sun HD, Lin H, Jao MS, Wang KL, Liou WS,
Hung YC, Chiang YC, Lu CH, Lai HC and Yu MH: A long-term follow-up
study of 176 cases with adult-type ovarian granulosa cell tumors.
Gynecol Oncol. 124:244–249. 2012. View Article : Google Scholar
|
|
2
|
Lee YK, Park NH, Kim JW, Song YS, Kang SB
and Lee HP: Characteristics of recurrence in adult-type granulosa
cell tumor. Int J Gynecol Cancer. 18:642–647. 2008. View Article : Google Scholar
|
|
3
|
Hashem IAT, Yaqoob I, Anuar NB, Mokhtar S,
Gani A and Ullah Khan S: The rise of 'big data' on cloud computing:
Review and open research issues. Inform Syst. 47:98–115. 2015.
View Article : Google Scholar
|
|
4
|
Gomes A, Reis-Silva M, Alarcão A, Couceiro
P, Sousa V and Carvalho L: Promoter hypermethylation of DNA repair
genes MLH1 and MSH2 in adenocarcinomas and squamous cell carcinomas
of the lung. Rev Port Pneumol. 20:20–30. 2014. View Article : Google Scholar
|
|
5
|
Amente S, Lania L and Majello B:
Epigenetic reprogramming of Myc target genes. Am J Cancer Res.
1:413–418. 2011.PubMed/NCBI
|
|
6
|
Nishida N, Kudo M, Nagasaka T, Ikai I and
Goel A: Characteristic patterns of altered DNA methylation predict
emergence of human hepatocellular carcinoma. Hepatology.
56:994–1003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ling Q, Shi W, Huang C, Zheng J, Cheng Q,
Yu K, Chen S, Zhang H, Li N and Chen M: Epigenetic silencing of
dual oxidase 1 by promoter hypermethylation in human
hepato-cellular carcinoma. Am J Cancer Res. 4:508–517. 2014.
|
|
8
|
Sun D, Zhang Z, Van Do N, Huang G, Ernberg
I and Hu L: Aberrant methylation of CDH13 gene in nasopharyngeal
carcinoma could serve as a potential diagnostic biomarker. Oral
Oncol. 43:82–87. 2007. View Article : Google Scholar
|
|
9
|
Kim JS, Han J, Shim YM, Park J and Kim DH:
Aberrant methylation of H-cadherin (CDH13) promoter is associated
with tumor progression in primary nonsmall cell lung carcinoma.
Cancer. 104:1825–1833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Putku M, Kals M, Inno R, Kasela S, Org E,
Kožich V, Milani L and Laan M: CDH13 promoter SNPs with pleiotropic
effect on cardiometabolic parameters represent methylation QTLs.
Hum Genet. 134:291–303. 2015. View Article : Google Scholar
|
|
11
|
Yoo KH and Hennighausen L: EZH2
methyltransferase and H3K27 methylation in breast cancer. Int J
Biol Sci. 8:59–65. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kontic M, Stojsic J, Jovanovic D,
Bunjevacki V, Ognjanovic S, Kuriger J, Puumala S and Nelson HH:
Aberrant promoter methylation of CDH13 and MGMT genes is associated
with clinicopathologic characteristics of primary non-small-cell
lung carcinoma. Clin Lung Cancer. 13:297–303. 2012. View Article : Google Scholar
|
|
13
|
Kloten V, Becker B, Winner K, Schrauder
MG, Fasching PA, Anzeneder T, Veeck J, Hartmann A, Knüchel R and
Dahl E: Promoter hypermethylation of the tumor-suppressor genes
ITIH5, DKK3, and RASSF1A as novel biomarkers for blood-based breast
cancer screening. Breast Cancer Res. 15:R42013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou XY, Sun JF, He YH, Zhang HY, Yu J,
Guo SC, Cai Y, Hu XC and Zhu JD: Correlation of the methylation
status of CpG islands in the promoter region of 10 genes with the
5-Fu chemosensitivity in 3 breast cancer cell lines. Zhonghua Zhong
Liu Za Zhi. 32:328–333. 2010.PubMed/NCBI
|
|
15
|
Friedrichs K, Gluba S, Eidtmann H and
Jonat W: Overexpression of p53 and prognosis in breast cancer.
Cancer. 72:3641–3647. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dhillon VS, Young AR, Husain SA and Aslam
M: Promoter hypermethylation of MGMT, CDH1, RAR-beta and SYK tumour
suppressor genes in granulosa cell tumours (GCTs) of ovarian
origin. Br J Cancer. 90:874–881. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen H, Zhang C, Sheng Y, Yao S, Liu Z,
Zhang C and Zhang T: Frequent SOCS3 and 3OST2 promoter methylation
and their epigenetic regulation in endometrial carcinoma. Am J
Cancer Res. 5:180–190. 2014. View Article : Google Scholar
|
|
18
|
Dhillon VS, Aslam M and Husain SA: The
contribution of genetic and epigenetic changes in granulosa cell
tumors of ovarian origin. Clin Cancer Res. 10:5537–5545. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dhillon VS, Shahid M and Husain SA: CpG
methylation of the FHIT, FANCF, cyclin-D2, BRCA2 and RUNX3 genes in
Granulosa cell tumors (GCTs) of ovarian origin. Mol Cancer.
3:332004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Toyooka KO, Toyooka S, Virmani AK,
Sathyanarayana UG, Euhus DM, Gilcrease M, Minna JD and Gazdar AF:
Loss of expression and aberrant methylation of the CDH13
(H-cadherin) gene in breast and lung carcinomas. Cancer Res.
61:4556–4560. 2001.PubMed/NCBI
|
|
21
|
Toyooka S, Toyooka KO, Harada K, Miyajima
K, Makarla P, Sathyanarayana UG, Yin J, Sato F, Shivapurkar N,
Meltzer SJ and Gazdar AF: Aberrant methylation of the CDH13
(H-cadherin) promoter region in colorectal cancers and adenomas.
Cancer Res. 62:3382–3386. 2002.PubMed/NCBI
|
|
22
|
Xu J, Shetty PB, Feng W, Chenault C, Bast
RC Jr, Issa JP, Hilsenbeck SG and Yu Y: Methylation of HIN-1,
RASSF1A, RIL and CDH13 in breast cancer is associated with clinical
characteristics, but only RASSF1A methylation is associated with
outcome. BMC Cancer. 12:2432012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhai X and Li SJ: Methylation of RASSF1A
and CDH13 genes in individualized chemotherapy for patients with
non-small cell lung cancer. Asian Pac J Cancer Prev. 15:4925–4928.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhai X and Li SJ: Methylation of RASSF1A
and CDH13 genes in individualized chemotherapy for patients with
non-small cell lung cancer. Asian Pac J Cancer Prev. 15:4925–4928.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhong YH, Peng H, Cheng HZ and Wang P:
Quantitative assessment of the diagnostic role of CDH13 promoter
methylation in lung cancer. Asian Pac J Cancer Prev. 16:1139–1143.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xue R, Yang C, Zhao F and Li D: Prognostic
significance of CDH13 hypermethylation and mRNA in NSCLC. Onco
Targets Ther. 7:1987–1996. 2014.PubMed/NCBI
|
|
27
|
Hibi K, Kodera Y, Ito K, Akiyama S and
Nakao A: Aberrant methylation of HLTF, SOCS-1 and CDH13 genes is
shown in colorectal cancers without lymph node metastasis. Dis
Colon Rectum. 48:1282–1286. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Riou P, Saffroy R, Chenailler C, Franc B,
Gentile C, Rubinstein E, Resink T, Debuire B, Piatier-Tonneau D and
Lemoine A: Expression of T-cadherin in tumor cells influences
invasive potential of human hepatocellular carcinoma. FASEB J.
20:2291–2301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin YL, Xie PG and Ma JG: Aberrant
methylation of CDH13 is a potential biomarker for predicting the
recurrence and progression of non muscle invasive bladder cancer.
Med Sci Monit. 20:1572–1577. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abudukadeer A, Bakry R, Goebel G,
Mutz-Dehbalaie I, Widschwendter A, Bonn GK and Fiegl H: Clinical
relevance of CDH1 and CDH13 DNA-methylation in serum of cervical
cancer patients. Int J Mol Sci. 13:8353–8363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu Q, Lothe RA, Ahlquist T, Silins I,
Tropé CG, Micci F, Nesland JM, Suo Z and Lind GE: DNA methylation
profiling of ovarian carcinomas and their in vitro models
identifies HOXA9, HOXB5, SCGB3A1, and CRABP1 as novel targets. Mol
Cancer. 6:452007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Makarla PB, Saboorian MH, Ashfaq R,
Toyooka KO, Toyooka S, Minna JD, Gazdar AF and Schorge JO: Promoter
hypermeth-ylation profile of ovarian epithelial neoplasms. Clin
Cancer Res. 11:5365–5369. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rathi A, Virmani AK, Schorge JO, Elias KJ,
Maruyama R, Minna JD, Mok SC, Girard L, Fishman DA and Gazdar AF:
Methylation profiles of sporadic ovarian tumors and nonmalignant
ovaries from high-risk women. Clin Cancer Res. 8:3324–3331.
2002.PubMed/NCBI
|
|
34
|
Kawakami M, Staub J, Cliby W, Hartmann L,
Smith DI and Shridhar V: Involvement of H-cadherin (CDH13) on 16q
in the region of frequent deletion in ovarian cancer. Int J Oncol.
15:715–720. 1999.PubMed/NCBI
|
|
35
|
Yu J, Tao Q, Cheng YY, Lee KY, Ng SS,
Cheung KF, Tian L, Rha SY, Neumann U, Röcken C, et al: Promoter
methylation of the Wnt/beta-catenin signaling antagonist Dkk-3 is
associated with poor survival in gastric cancer. Cancer. 115:49–60.
2009. View Article : Google Scholar
|
|
36
|
Ying J, Li H, Yu J, Ng KM, Poon FF, Wong
SC, Chan AT, Sung JJ and Tao Q: WNT5A exhibits tumor-suppressive
activity through antagonizing the Wnt/beta-catenin signaling, and
is frequently methylated in colorectal cancer. Clin Cancer Res.
14:55–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hayashi T, Asano H, Toyooka S, Tsukuda K,
Soh J, Shien T, Taira N, Maki Y, Tanaka N, Doihara H, et al: DNA
methylation status of REIC/Dkk-3 gene in human malignancies. J
Cancer Res Clin Oncol. 138:799–809. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Voorham QJ, Janssen J, Tijssen M,
Snellenberg S, Mongera S, van Grieken NC, Grabsch H, Kliment M,
Rembacken BJ, Mulder CJ, et al: Promoter methylation of
Wnt-antagonists in polypoid and nonpolypoid colorectal adenomas.
BMC Cancer. 13:6032013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yin DT, Wu W, Li M, Wang QE, Li H, Wang Y,
Tang Y and Xing M: DKK3 is a potential tumor suppressor gene in
papillary thyroid carcinoma. Endocr Relat Cancer. 20:507–514. 2013.
View Article : Google Scholar
|
|
40
|
Kang WS, Cho SB, Park JS, Lee MY, Myung
SC, Kim WY, Lee SH, Kim DH and Lee EJ: Clinico-epigenetic
combination including quantitative methylation value of DKK3
augments survival prediction of the patient with cervical cancer. J
Cancer Res Clin Oncol. 139:97–106. 2013. View Article : Google Scholar
|
|
41
|
Liang L, He H, Lv R, Zhang M, Huang H, An
Z and Li S: Preliminary mechanism on the methylation modification
of Dkk-1 and Dkk-3 in hepatocellular carcinoma. Tumour Biol.
36:1245–1250. 2015. View Article : Google Scholar
|
|
42
|
Tao L, Huang G, Chen Y and Chen L: DNA
methylation of DKK3 modulates docetaxel chemoresistance in human
nonsmall cell lung cancer cell. Cancer Biother Radiopharm.
30:100–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ottolenghi C, Omari S, Garcia-Ortiz JE,
Uda M, Crisponi L, Forabosco A, Pilia G and Schlessinger D: Foxl2
is required for commitment to ovary differentiation. Hum Mol Genet.
14:2053–2062. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Schmidt D, Ovitt CE, Anlag K, Fehsenfeld
S, Gredsted L, Treier AC and Treier M: The murine winged-helix
transcription factor Foxl2 is required for granulosa cell
differentiation and ovary maintenance. Development. 131:933–942.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rosario R, Araki H, Print CG and Shelling
AN: The transcriptional targets of mutant FOXL2 in granulosa cell
tumours. PLoS One. 7:e462702012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shah SP, Köbel M, Senz J, Morin RD, Clarke
BA, Wiegand KC, Leung G, Zayed A, Mehl E, Kalloger SE, et al:
Mutation of FOXL2 in granulosa-cell tumors of the ovary. N Engl J
Med. 360:2719–2729. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jamieson S, Butzow R, Andersson N,
Alexiadis M, Unkila-Kallio L, Heikinheimo M, Fuller PJ and Anttonen
M: The FOXL2 C134W mutation is characteristic of adult granulosa
cell tumors of the ovary. Mod Pathol. 23:1477–1485. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim MS, Hur SY, Yoo NJ and Lee SH:
Mutational analysis of FOXL2 codon 134 in granulosa cell tumour of
ovary and other human cancers. J Pathol. 221:147–152. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
D'Angelo E, Mozos A, Nakayama D, Espinosa
I, Catasus L, Muñoz J and Prat J: Prognostic significance of FOXL2
mutation and mRNA expression in adult and juvenile granulosa cell
tumors of the ovary. Mod Pathol. 24:1360–1367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Oseto K, Suzumori N, Nishikawa R,
Nishikawa H, Arakawa A, Ozaki Y, Asai H, Kawai M, Mizuno K,
Takahashi S, et al: Mutational analysis of FOXL2 p.C134W and
expression of bone morphogenetic protein 2 in Japanese patients
with granulosa cell tumor of ovary. J Obstet Gynaecol Res.
40:1197–1204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tran S, Wang Y, Lamba P, Zhou X, Boehm U
and Bernard DJ: The CpG island in the murine foxl2 proximal
promoter is differentially methylated in primary and immortalized
cells. PLoS One. 8:e766422013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhao Y, Zhou H, Ma K, Sun J, Feng X, Geng
J, Gu J, Wang W, Zhang H, He Y, et al: Abnormal methylation of
seven genes and their associations with clinical characteristics in
early stage non-small cell lung cancer. Oncol Lett. 5:1211–1218.
2013.PubMed/NCBI
|
|
53
|
Sharma S, Kelly TK and Jones PA:
Epigenetics in cancer. Carcinogenesis. 31:27–36. 2010. View Article : Google Scholar :
|
|
54
|
Viré E, Brenner C, Deplus R, Blanchon L,
Fraga M, Didelot C, Morey L, Van Eynde A, Bernard D, Vanderwinden
JM, et al: The Polycomb group protein EZH2 directly controls DNA
methylation. Nature. 439:871–874. 2006. View Article : Google Scholar
|
|
55
|
Cao R and Zhang Y: The functions of
E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr
Opin Genet Dev. 14:155–164. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hoffmann MJ, Engers R, Florl AR, Otte AP,
Muller M and Schulz WA: Expression changes in EZH2, but not in
BMI-1, SIRT1, DNMT1 or DNMT3B are associated with DNA methylation
changes in prostate cancer. Cancer Biol Ther. 6:1403–1412. 2007.
View Article : Google Scholar
|
|
57
|
Bachmann IM, Halvorsen OJ, Collett K,
Stefansson IM, Straume O, Haukaas SA, Salvesen HB, Otte AP and
Akslen LA: EZH2 expression is associated with high proliferation
rate and aggressive tumor subgroups in cutaneous melanoma and
cancers of the endometrium, prostate, and breast. J Clin Oncol.
24:268–273. 2006. View Article : Google Scholar
|
|
58
|
Wang J, Yu L, Cai J, Jia J, Gao Y, Liang M
and Wang Z: The role of EZH2 and DNA methylation in hMLH1 silencing
in epithelial ovarian cancer. Biochem Biophys Res Commun.
433:470–476. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kodach LL, Jacobs RJ, Heijmans J, van
Noesel CJ, Langers AM, Verspaget HW, Hommes DW, Offerhaus GJ, van
den Brink GR and Hardwick JC: The role of EZH2 and DNA methylation
in the silencing of the tumour suppressor RUNX3 in colorectal
cancer. Carcinogenesis. 31:1567–1575. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Rush M, Appanah R, Lee S, Lam LL, Goyal P
and Lorincz MC: Targeting of EZH2 to a defined genomic site is
sufficient for recruitment of Dnmt3a but not de novo DNA
methylation. Epigenetics. 4:404–414. 2009. View Article : Google Scholar : PubMed/NCBI
|