Introduction
Vibrio vulnificus is a gram-negative
bacterium, is known to cause primary sepsis and gastroenteritis in
humans. Following an infection with V. vulnificus, the
disease proceeds rapidly, resulting in extensive cellular damage.
Additionally, the consumption of contaminated shellfish or wound
infection with V. vulnificus can induce fatal septicemia in
susceptible individuals with chronic liver disease (1). A variety of virulence factors
produced by V. vulnificus can induce septic shock, which is
often fatal. Putative virulence factors, including capsular
polysaccharides (2,3), siderophores (4), hemolysin (5), matrix metalloproteinase (6), flagella (7,8) and
RtxA toxin (9–11) have been reported in vivo and
in vitro. These virulence factors may induce the persistent
production of proinflammatory mediators, such as interleukin
(IL)-1β, IL-6, IL-8, tumor necrosis factor (TNF)-α and nitric oxide
in the host (12,13). Therefore, highly active
antimicrobial agents are required for the efficient treatment of
V. vulnificus infections. In this study, the anti-V.
vulnificus activity of psammaplin A was investigated in
vitro and in vivo.
Psammaplin A is a natural marine product isolated
from sponges, such as Poecillastra sp., Jaspis sp.
and Psammaplysilla sp. (14,15).
Psammaplin A is known to possess antimicrobial (16), antitumor and cytotoxic activities
against several cell lines, including the P388 leukemia cell line
(14,15), as well as lung, ovarian and colon
cancer (17). It was also reported
to have inhibitory activities against DNA gyrase, DNA
topoisomerase, farnesyl protein transferase and leucine
aminopeptidase (16,18–22).
Previous studies showed that psammaplin A possesses an
antimicrobial effect against methicillin-resistant Stapylococcus
aureus (MRSA) (16,23,24).
However, the effects of psammaplin A on V. vulnificus
infection in vitro and in vivo have not been
investigated.
In this study, the antibacterial activity of
psammaplin A against V. vulnificus as well as its
suppressive effects against the cell cytotoxicity induced by V.
vulnificus were examined in vitro and in
vivo.
Materials and methods
Animal cell culture and chemicals
The INT-407 human epithelial cell-line (ATCC CCL-6)
was purchased from the American Type Culture Collection (Manassas,
VA, USA), and maintained at 37°C under 5% CO2 in Minimum
Essential Medium (MEM; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and antibiotics (10 U/ml penicillin G and 10
μg/ml streptomycin) (growth medium). Psammaplin A is a
natural marine product that was isolated from two sponges,
Jaspis sp. and Poecillastra wondoensis (17). The other compounds were isolated
from a sponge-derived fungus Acremonium sp. and their
configurations were determined by CD spectroscopic data, along with
comparison of 1H and 13C spectroscopic data
(25). All chemicals used in the
study were a gift from Professor Jung (College of Pharmacy, Pusan
National University, Busan, Korea). The natural marine products
were dissolved in anhydrous ethanol to make a 10 mg/ml stock
solution. Subsequent dilutions were made in Dulbecco's modified
Eagle's medium.
Bacterial strains and growth
conditions
V. vulnificus strain MO6-24/O used in the
present study was isolated from patients (9,10)
and provided by Professor Sang Ho Choi (Seoul National University,
Seoul, Korea). The V. vulnificus bacteria were grown to log
phase at 30°C in Luria-Bertani medium (produced in the laboratory)
supplemented with 2.0% NaCl LBS medium, after which they were
diluted to 6×108 CFU/ml in LBS medium, and then
centrifuged for 3 min at 2,500 × g and resuspended in
antibiotic-free MEM medium prior to infection of INT-407 cells. The
concentration of bacteria was confirmed via viable colony counting
conducted on LBS agar.
In vitro broth cultures of V.
vulnificus
The V. vulnificus inoculum size was
6×108 CFU/ml. Variable concentrations of natural pure
compounds 1, 4, 6, 8 and 10 (1, 5, 10, 12.5, 20, 25, 40, 50, 75 and
100 μg/ml) were solubilized in 20 ml of growth medium (2%
NaCl LBS) and then tested for their ability to alter bacterial
growth by spectrometry (OD540). This was conducted by
culturing V. vulnificus for 0–13 h in the presence of 50
μg/ml psammaplin A or 0–100 μg/ml psammaplin A for 13
h at 37℃ in 2% NaCl LB medium, and bacterial growth was evaluated
by measuring the optical density at 540 nm (OD540). The
V. vulnificus cultures were then incubated with aeration at
150 rpm using a gyratory shaker for 5 h at 37°C.
Infection protocol
INT-407 human epithelial cells were infected with
V. vulnificus as previously described (9,10).
Briefly, INT-407 cells were grown in growth medium at 37°C in a 5%
CO2 incubator. Next, the cells were seeded onto 6-well
(8×105 cells/well) and 96-well (2×104
cells/well) culture plates and then cultured for 24 h in
antibiotic-free growth medium. Prior to infection, the bacteria
were centrifuged for 3 min at 2,500 × g, resuspended and adjusted
to 6×108 CFU/ml in antibiotic-free MEM medium. The
bacterial suspensions were then added to psammaplin A-treated or
untreated-epithelial cells at various multiplicities of infection
(MOI; the ratio of the number of bacteria to the number of
epithelial cells), after which the infected cells were incubated
for 1–4 h in antibiotic-free growth medium at 37°C under 5%
CO2.
Cytotoxicity assay
The bacteria-infected INT-407 cell cultures were
aliquoted into a 96-well tissue culture plate (Nunc, Roskilde,
Denmark) as previously described (9,10).
The cytotoxicity was then determined by measuring the activity of
lactate hydrogenase (LDH) in the supernatant using a cytotoxicity
detection kit (Roche, Mannheim, Germany). The cytotoxic level was
expressed as a percentage relative to the total LDH activity of
cells that were completely lysed by 1% Triton X-100 (9,10).
Morphological study
INT-407 (8×105 cells/well) cells were
incubated with bacteria in a 6-well plate for 3 h at an MOI of 10,
after which the cells were washed with phosphate-buffered saline
(PBS). The cells were then fixed with 4% para-formaldehyde
(Sigma-Aldrich, St. Louis, MO, USA) for 10 min at room temperature,
washed and completely dried. Next, the cells were stained with
Giemsa solution (Molecular Probes, Thermo Fisher Scientific, Inc.)
for 1 h at room temperature. The cells were then washed twice with
distilled water and dried, after which the images of the specimens
were acquired using a microscope (Olympus IX 71, Tokyo, Japan).
Survival of V. vulnificus-infected
mice
A total of 35 female ICR mice (Samtaco Bio Korea,
Gyounggi-do, Korea; age, 8 weeks; weight, 20–22 g) that were housed
under specific-pathogen free conditions were used for all
experiments. They were maintained at 24°C with a relative humidity
of 50%, under a 12-h light/dark cycle. The mice had access to food
and water ad libitum. The present study was approved by
Korea University (Seoul, Korea). The mice were intraperitoneally
infected with 0.1 ml of 250 μg iron dextran (Sigma-Aldrich)
30 min prior to injection with V. vulnificus. Next, the mice
were intraperitoneally injected with 1×103 CFU/0.1 ml
V. vulnificus. The use of iron dextran produces a useful
model to investigate systemic disease that results from V.
vulnificus infection. The mice were administered 0.2 ml
psammaplin A (DCM 1-9-1) solution (5, 10, 25 or 50 μg per
mouse) or a PBS intraperitoneally (control), after which their
survival status was assessed every hour for 24 h.
Quantitative analysis of bacteria in
tissues
The V. vulnificus-inoculated mice were
sacrificed by cervical dislocation 7 h after infection. A ventral
incision was made to observe the abdomen of the infected mice
treated with or without psammaplin A (Nikon D60; Nikon Corporation,
Tokyo, Japan), and the spleen, liver and small intestine lesions
were then aseptically removed. The removed specimens were
homogenized in 2 ml PBS using glass tissue homogenizers, after
which the homogenates were diluted in PBS and plated on 2% NaCl HI
agar. The samples were then incubated at 37°C for 12 h and
bacterial colonies were counted.
Statistical analysis
The data were analyzed with Microsoft Excel
(Microsoft Corporation, Redmond, WA, USA). Student's t-test and
one-way analysis of variance followed by the Bonferroni method were
employed to identify statistical differences between the values of
the various experimental and control groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Psammaplin A suppresses V.
vulnificus-induced cytotoxicity in human epithelial cells
Twelve pure compounds were isolated from natural
marine products, and their structures were characterized as
previously described (17,25) (Fig.
1). The inhibitory effects of these compounds were determined
on V. vulnificus-induced cytotoxicity (Fig. 2). INT-407 cells were infected with
V. vulnificus at an MOI of 10 for 3 h in the presence or
absence of the 12 marine product-derived compounds. Then, the
cytotoxicities of the compounds were evaluated in cells using LDH
assays. As shown in Fig. 2A, there
was significantly decreased cytotoxicity in cells treated with
compounds 1, 4, 6, 8, 9 and 10 compared with the untreated cells
infected with V. vulnificus, indicating that these compounds
have inhibitory effects on V. vulnificus-induced
cytotoxicity. Treatment with these compounds significantly
inhibited the cytotoxicity of V. vulnificus in a
concentration- and time-dependent manner (Fig. 2B and C). Of these compounds,
psammaplin A (compound 8) had the strongest inhibitory effect on
the V. vulnificus-induced cytotoxicity.
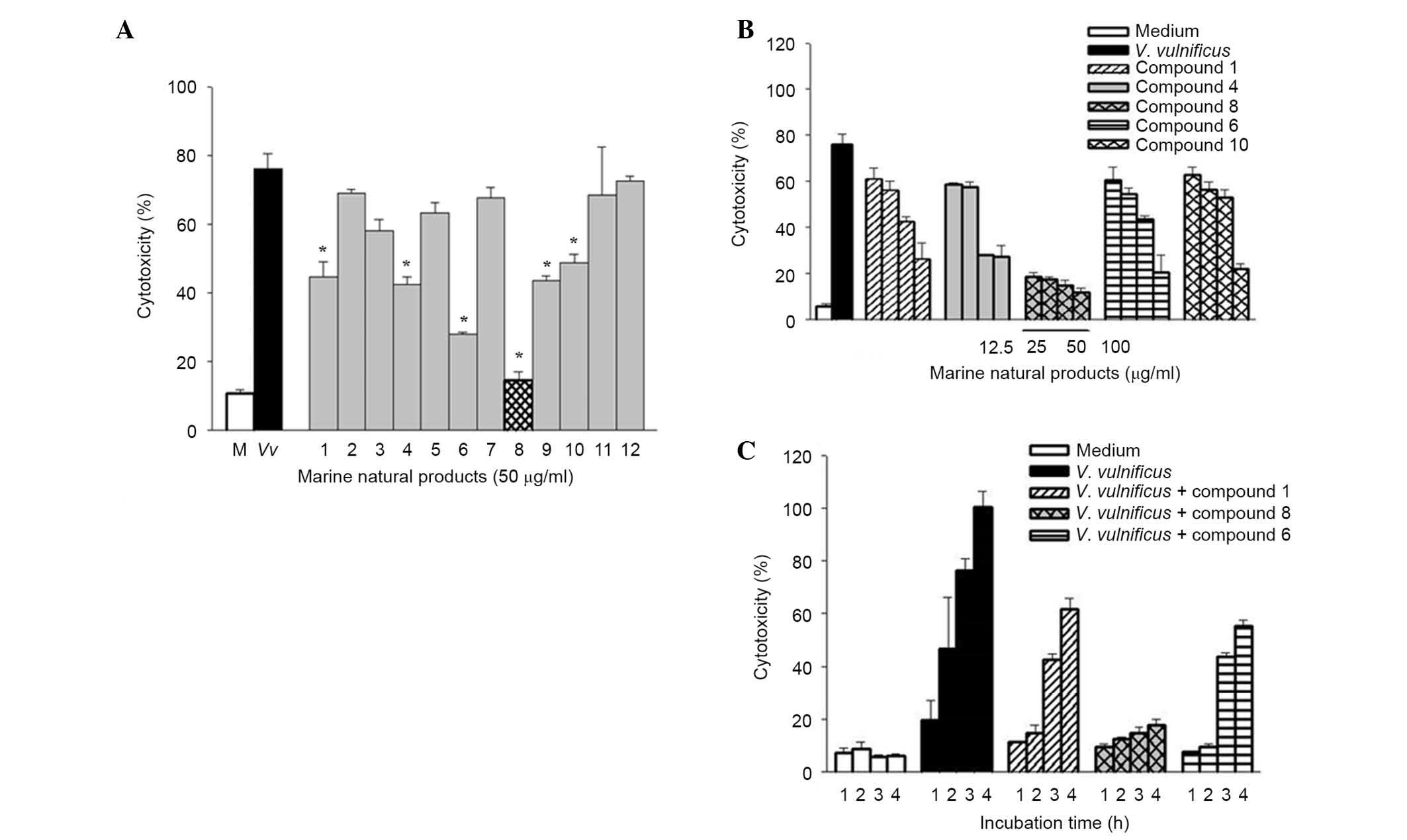 | Figure 2Effects of psammaplin A, a natural
marine product on Vibrio vulnificus-induced cytotoxicity in
human epithelial cells. (A) INT-407 cells were infected with V.
vulnificus for 3 h at an MOI of 10 in the presence or absence
of 12 natural marine products (50 μg/ml), and cytotoxicity
was determined using the lactase dehydrogenase release assay. The
white bars indicate non-infected cells; black, infected but not
treated with natural marine products; grey, infected and treated
with natural marine products; checkered, infected, treated with
psammaplin A. *P<0.05 vs. infected but untreated group. (B)
INT-407 cells were infected with V. vulnificus for 3 h at an
MOI of 10 in the presence of compounds 1, 4, 6, 8 and 10 (0, 12.5,
25, 50 and 100 μg/ml). (C) INT-407 cells were infected with
V. vulnificus at an MOI of 10 for varying times (1, 2, 3 and
4 h) in the presence of compounds 1, 6 and 8. Data are presented as
the mean ± standard error (n=3) for all experiments. MOI,
multiplicity of infection. |
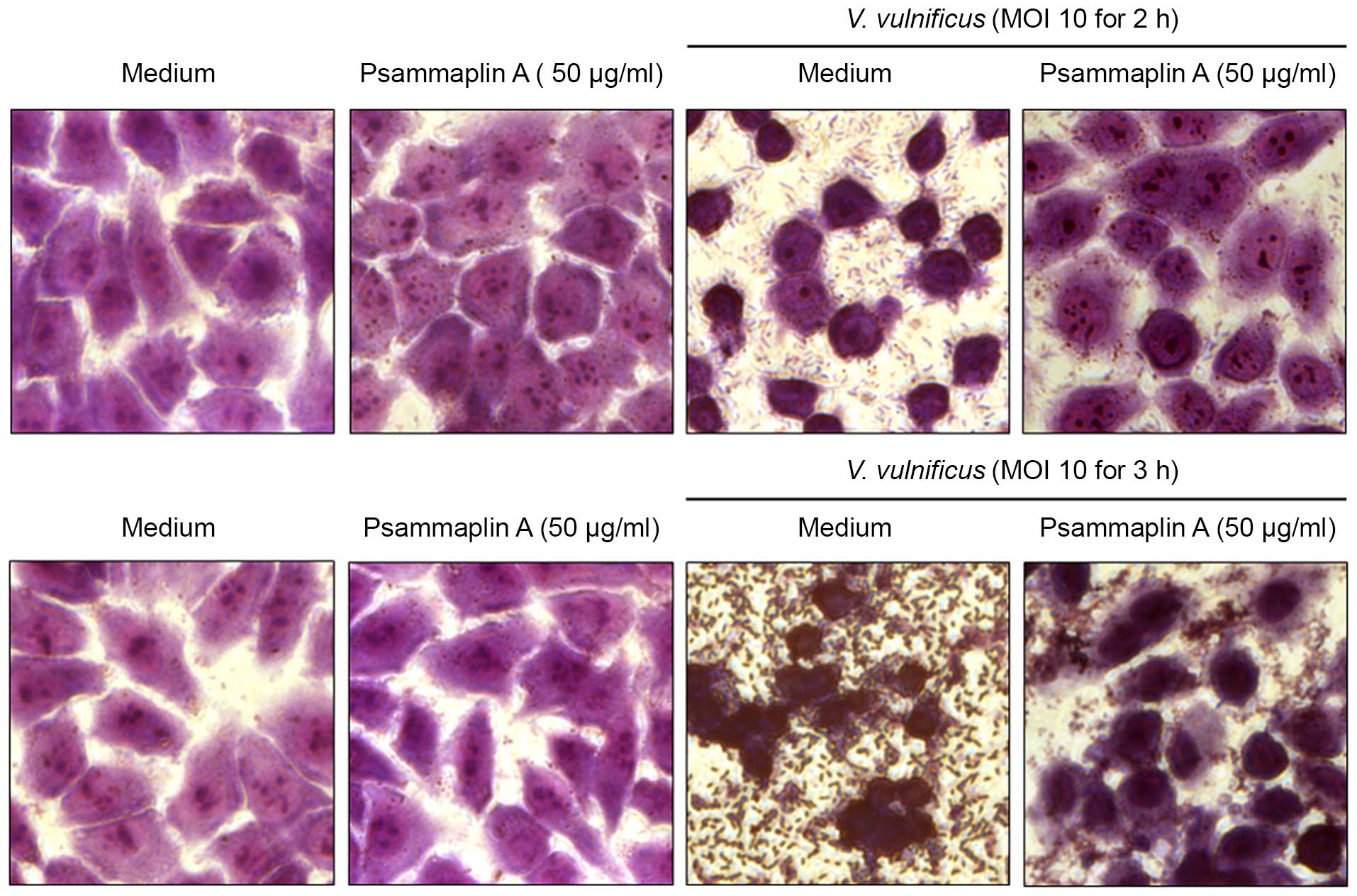
To confirm the inhibitory effects of psammaplin A on
the V. vulnificus-induced cytotoxicity of INT-407 cells, the
size and morphology of nuclei were assessed using a microscope
(Fig. 3). INT-407 cells infected
with V. vulnificus at an MOI of 10 for 2–3 h showed typical
phenotypic features of cell death, such as cytoplasmic loss and
cellular damage, while treatment with psammaplin A reversed that
phenotype. Psammaplin A ameliorated the significant cellular damage
at 3 h after infection with V. vulnificus. These results
suggest that psammaplin A inhibits the cytotoxicity against host
cells induced by V. vulnificus infection.
Psammaplin A treatment prolongs the
survival of V. vulnificus-infected mice
To investigate whether psammaplin A prolonged
survival, mice were infected with V. vulnificus and
administered psammaplin A (0–50 μg per mouse). Mice
inoculated intraperitoneally with 1×103 CFU V.
vulnificus all died within 16 h. However, psammaplin A
treatment of V. vulnificus-infected mice increased the
survival rate. Following psammaplin A treatment, four out of five
mice infected with V. vulnificus (50 μg per mouse)
survived for 24 h (Fig. 4A).
To investigate the effects of psammaplin A treatment
on the growth of V. vulnificus in vivo, mice were
intraperitoneally infected with 1×103 CFU V.
vulnificus and administered psammaplin A (0, 10, 25 and 50
μg per mouse). After 7 h, several tissue samples, including
from the spleen, liver and small intestine were excised from the
mice, and the number of V. vulnificus colonies in each
tissue was evaluated. Fig. 4B
shows that the number of V. vulnificus colonies was
significantly reduced in all tissue samples isolated from
psammaplin A-treated mice compared with the number of V.
vulnificus colonies isolated from untreated controls. In
addition, the necropsy results of V. vulnificus-infected
mice at 7 h post infection showed edema, hemorrhage, vasodilation
and necrosis in the intestines, livers and spleens isolated from
the untreated mice. However, the tissue samples from the psammaplin
A-treated mice did not show any of the symptoms observed in the
tissues of untreated mice (Fig.
4C). These results suggest that psammaplin A significantly
suppresses the growth of V. vulnificus and the associated
pathology in vitro and in vivo.
Psammaplin A strongly inhibits V.
vulnificus growth in vitro
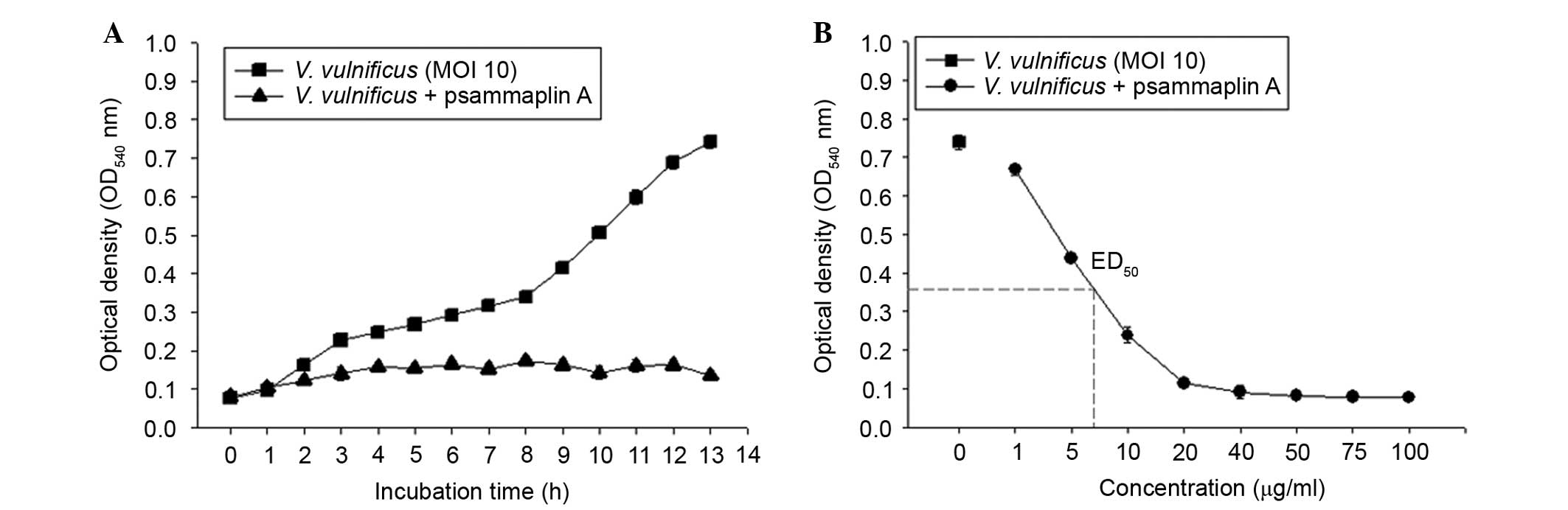
To investigate the antibacterial activities of
psammaplin A against V. vulnificus, V. vulnificus was
incubated in the presence or absence of psammaplin A (0–100
μg/ml) for 0–13 h. As shown in Fig. 5, the bacterial numbers of V.
vulnificus increased in an incubation time-dependent manner.
However, psammaplin A treatment inhibited the growth of V.
vulnificus in a concentration-dependent manner. These findings
suggest that psammaplin A significantly inhibited the growth of
V. vulnificus.
Discussion
V. vulnificus, which is a gram-negative
bacterium, causes fatal septicemia in individuals with liver
cirrhosis, diabetes, hemochromatosis or immunocompromised
conditions (26,27). Infection with V. vulnificus
causes extensive cellular damage and >50% of patients with V.
vulnificus-induced septicemia die. Recent studies revealed that
hemolysin produced by V. vulnificus (VvhA) induces nuclear
factor κ-light-chain-enhancer of activated B cells-dependent
mitochondrial cell death via lipid raft-mediated reactive oxygen
species production in human epithelial cells (28). Therefore, there is an increasing
requirement for effective antimicrobial agents for the treatment of
V. vulnificus infections. Psammaplin A was first isolated
from the Psammaplinaplysilla sponge and it was known to
impede bacterial growth by inhibiting the activities of several key
enzyme-mediated processes in prokaryotic systems including DNA
replication, microbial detoxification and epigenetic control of
gene expression. The results of this study proved that the marine
sponge-derived psammaplin A exerted strong inhibitory activity
against V. vulnificus in epithelial cells and mice.
The 12 pure compounds isolated from natural marine
products were incubated with V. vulnificus-infected
epithelial cells. Among the compounds, psammaplin A exhibited lower
cytotoxicity than the other 11 compounds. In addition, psammaplin A
treatment exerted inhibitory effects on V.
vulnificus-induced cytotoxicity in a concentration- and
time-dependent manner, indicating that it prevented the V.
vulnificus-induced epithelial cell death. Moreover, cytoplasmic
loss and cellular damage were not observed in V.
vulnificus-infected epithelial cells treated with psammaplin A.
Furthermore, administration of psammaplin A to V.
vulnificus-infected mice improved their survival rate compared
with that of untreated mice. The number of V. vulnificus
colonies in the spleens, livers and small intestines of psammaplin
A-treated mice was significantly lower than the number of V.
vulnificus colonies in the untreated mice. Unlike the untreated
mice, there was no edema, hemorrhage, vasodilation or necrosis in
the intestine, liver and spleen isolated from the psammaplin
A-treated mice. Treatment with psammaplin A effectively suppressed
the growth of V. vulnificus throughout the incubation time
in a dose-dependent manner.
The underlying mechanism of the potent anti-V.
vulnificus activity of psammaplin A remains unclear.
Previously, psammaplin A was reported to possess antibacterial
activity against gram-positive bacteria, including MRSA, possibly
by inhibiting DNA synthesis and gyrase activity. The anti-V.
vulnificus activity of psammaplin A warrants further
investigation to determine the specific underlying mechanism.
In conclusion, the results of this study clearly
demonstrated that psammaplin A exerted strong inhibitory activity
against V. vulnificus in vitro and in vivo. These
findings suggest that psammaplin A may be a candidate therapeutic
agent for the treatment of V. vulnificus-related
diseases.
Acknowledgments
This study was supported by the Agriculture, Food
and Rural Affairs Research Center Support Program, Ministry of
Agriculture, Food and Rural Affairs, Republic of Korea (to
Professor Tae Sung Kim).
References
|
1
|
Ikeda T, Kanehara S, Ohtani T and Furukawa
F: Endotoxin shock due to Vibrio vulnificus infection. Eur J
Dermatol. 16:423–427. 2006.PubMed/NCBI
|
|
2
|
Powell JL, Wright AC, Wasserman SS, Hone
DM and Morris JG Jr: Release of tumor necrosis factor alpha in
response to Vibrio vulnificus capsular polysaccharide in vivo and
in vitro models. Infect Immun. 65:3713–3718. 1997.PubMed/NCBI
|
|
3
|
Wright AC, Powell JL, Kaper JB and Morris
JG Jr: Identification of a group 1-like capsular polysaccharide
operon for Vibrio vulnificus. Infect Immun. 69:6893–6901. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Simpson LM and Oliver JD: Siderophore
production by Vibrio vulnificus. Infect Immun. 41:644–649.
1983.PubMed/NCBI
|
|
5
|
Gray LD and Kreger AS: Purification and
characterization of an extracellular cytolysin produced by Vibrio
vulnificus. Infect Immun. 48:67–72. 1985.
|
|
6
|
Kim CM, Park RY, Chun HJ, Kim SY, Rhee JH
and Shin SH: Vibrio vulnificus metalloprotease VvpE is essentially
required for swarming. FEMS Microbiol Lett. 269:170–179. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee JH, Rho JB, Park KJ, Kim CB, Han YS,
Choi SH, Lee KH and Park SJ: Role of flagellum and motility in
pathogenesis of Vibrio vulnificus. Infect Immun. 72:4905–4910.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gulig PA, Bourdage KL and Starks AM:
Molecular pathogenesis of Vibrio vulnificus. J Microbiol.
43:118–131. 2005.PubMed/NCBI
|
|
9
|
Lee JH, Kim MW, Kim BS, Kim SM, Lee BC,
Kim TS and Choi SH: Identification and characterization of the
Vibrio vulnificus rtxA essential for cytotoxicity in vitro and
virulence in mice. J Microbiol. 45:146–152. 2007.PubMed/NCBI
|
|
10
|
Lee BC, Lee JH, Kim MW, Kim BS, Oh MH, Kim
KS, Kim TS and Choi SH: Vibrio vulnificus rtxE is important for
virulence and its expression is induced by exposure to host cells.
Infect Immun. 76:1509–1517. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee BC, Choi SH and Kim TS: Vibrio
vulnificus RTX toxin plays an important role in the apoptotic death
of human intestinal epithelial cells exposed to Vibrio vulnificus.
Microbes Infect. 10:1504–1513. 2008. View Article : Google Scholar
|
|
12
|
Espat NJ, Auffenberg T, Abouhamze A,
Baumhofer J, Moldawer LL and Howard RJ: A role for tumour necrosis
factor-alpha in the increased mortality associated with Vibrio
vulnificus infection in the presence of hepatic dysfunction. Ann
Surg. 223:428–433. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shin SH, Shin DH, Ryu PY, Chung SS and
Rhee JH: Proinflammatory cytokine profile in Vibrio vulnificus
septicemic patients' sera. FEMS Immunol Med Microbiol. 33:133–138.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jung JH, Sim CJ and Lee CO: Cytotoxic
compounds from a two-sponge association. J Nat Prod. 58:1722–1726.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Quiňoà E and Crew CP: Phenolic
constituents of psammaplysilla. Tetrahedron Lett. 28:3229–3233.
1987. View Article : Google Scholar
|
|
16
|
Kim D, Lee IS, Jung JH and Yang SI:
Psammaplin A, a natural bromotyrosine derivative from a sponge,
possesses the antibacterial activity against methicillin-resistant
Staphylococcus aureus and the DNA gyrase-inhibitory activity. Arch
Pharm Res. 22:25–29. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park Y, Liu Y, Hong J, Lee CO, Cho H, Kim
DK, Im KS and Jung JH: New bromotyrosine derivatives from an
association of two sponges, Jaspis wondoensis and Poecillastra
wondoensis. J Nat Prod. 66:1495–1498. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim D, Lee IS, Jung JH, Lee CO and Choi
SU: Psammaplin a natural phenolic compound, has inhibitory effect
on human topoisomerase II and is cytotoxic to cancer cells.
Anticancer Res. 19:4085–4090. 1999.
|
|
19
|
Shin J, Lee HS, Seo Y, et al: New
bromotyrosine metabolites from the sponge Aplysinella rhax.
Tetrahedron. 26:9071–9077. 2000. View Article : Google Scholar
|
|
20
|
Liu S, Fu X, Schmitz FJ and Kelly-Borges
M: Psammaplysin F, a new bromotyrosine derivative from a sponge,
Aplysinella sp. J Nat Prod. 60:614–615. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tabudravu JN, Eijsink VG, Gooday GW,
Jaspars M, Komander D, Legg M, Synstad B and van Aalten DM:
Psammaplin A, a chitinase inhibitor isolated from the Fijian marine
sponge Aplysinella rhax. Bioorganic Med Chem. 10:1123–1128. 2002.
View Article : Google Scholar
|
|
22
|
Piña IC, Gautsch i JT, Wang GY, Sanders
ML, Schmitz FJ, France D, Cornell-Kennon S, Sambucetti LC,
Remiszewski SW, Perez LB, et al: Psammaplins from the sponge
Pseudoceratina purpurea: Inhibition of both histone deacetylase and
DNA methyltransferase. J Org Chem. 68:3866–3873. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nicolaou KC, Hughes R, Pfefferkorn JA,
Barluenga S and Roecker AJ: Combinatorial synthesis through
disulfide exchange: Discovery of potent psammaplin A type
antibacterial agents active against methicillin-resistant
Staphylococcus aureus (MRSA). Chemistry. 7:4280–4295. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nicolaou KC, Hughes R, Pfefferkorn JA and
Barluenga S: Optimization and mechanistic studies of psammaplin A
type antibacterial agents active against methicillin-resistant
Staphylococcus aureus (MRSA). Chemistry. 7:4296–4310. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang P, Bao B, Dong HT, Hong J, Lee HJ,
Yoo ES, Bae KS and Jung JH: Anti-inflammatory sesquiterpenoids from
a sponge-derived fungus Acremonium sp. J Nat Prod. 72:270–275.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Linkous DA and Oliver JD: Pathogenesis of
Vibrio vulnificus. FEMS Microbiol Lett. 174:207–214. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Strom MS and Paranjpye RN: Epidemiology
and pathogenesis of Vibrio vulnificus. Microbes Infect. 2:177–188.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee SJ, Jung YH, Oh SY, Song EJ, Choi SH
and Han HJ: Vibrio vulnificus VvhA induces NF-κB-dependent
mitochondrial cell death via lipid raft-mediated ROS production in
intestinal epithelial cells. Cell Death Dis. 6:16552015. View Article : Google Scholar
|