Introduction
Obesity is a major health obstacle in the
industrialized world, increasing the incidence of several illness,
including hypertension, diabetes and heart disease, and is
characterized by a complex multifactorial chronic disease. An
imbalance between energy intake and expenditure contributes to a
pathological growth of adipocytes (1). It is known that the quantity of
adipose tissue can be regulated by the inhibition of adipogenesis
and by the control of adipocyte size. Obesity is induced by the
abnormal proliferation of adipocytes and recruits the new
adipocytes from precursor cells, two of which are involved in
regulating the differentiation of adipocytes (2).
Berberine is an alkaloid isolated from Chinese herbs
and is currently used as a traditional medicine for the treatment
of bacterial diarrhea, diabetes, hyperlipidemia, cancer, heart and
inflammatory diseases (3–6). Previous studies demonstrate that
berberine presents anticancer activities via the inhibition of cell
proliferation and reproduction of viruses and certain tumorigenic
microorganisms, and the induction of apoptosis in a variety of
cancer cell lines (7–10). Also, it has been reported that
berberine exhibits antiadipogenic effects in several adipocytes,
although its precise mechanism remains to be elucidated (11,12).
Therefore, the present study undertook a detailed study of the
effect of berberine on the differentiation of 3T3-L1 cells.
Micro (mi)RNAs are non-encoding RNA molecules that
regulate gene expression by suppressing the translation of target
genes and degrading target mRNAs (13). miRNAs serve a critical role in a
wide variety of biology processes, including proliferation,
division, survival and apoptosis (14-17).
In addition, miRNA-27 is one of the most important miRNAs and is
associated with the differentiation of adipocytes. A previous study
also suggests that both miRNA-27a and miRNA-27b act as
antiadipogenic miRNAs, at least in part, by suppressing the
proliferation of human adipose tissue-derived stem cells (18).
Previous studies have investigated the effects of
natural compounds on the expression of miRNAs in different cancer
types. Only a few reports on the effect of berberine and miRNAs
have been published, and these effects remain to be fully
understood. Berberine downregulates the expression of miRNA-21 in
human multiple myeloma and ovarian cells, which in turn leads to
apoptosis and inhibition of cell proliferation (19,20).
In the present study, the effects of berberine on
miRNA-27a and miRNA-27b in 3T3-L1 cells were assessed. It was
revealed that berberine increased the levels of miRNA-27a and
miRNA-27b, which led to the enhancement of differentiation
suppression and a reduction in triglyceride contents via the
targeting of peroxisome proliferator-activated receptors
(PPAR)-γ.
Materials and methods
Adipocyte differentiation and
treatments
THe 3T3-L1 cells were obtained from Shanghai
Institute of Cell Biology (Shanghai China). The cells were cultured
in Dulbecco's modified Eagle's medium (DMEM; GE Healthcare,
Piscataway, NJ, USA) and supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at
37°C and 5% CO2. After allowing 2 days for
differentiation, the 3T3-L1 cells were passaged and treated at
confluence with medium in the presence of 0.5 mM
3-isobutyl-1-meth-ylxanthine (Sigma-Aldrich, St. Louis, MO, USA),
0.25 µM dexamethasone (Sigma-Aldrich) and 10 µg/ml
insulin (Sigma-Aldrich) for 24 h. The 3T3-L1 cells were
subsequently treated with berberine (0, 1, 10, 20, 40 and 80
µM) for a further 24 h. The medium was changed to DMEM,
supplemented with 1 µg/ml insulin for 2 days, followed by
DMEM with 10% FBS for 10 days. The medium was replaced on the cells
with DMEM with 10% FBS every 2 days.
Cell viability measurement by cell
counting kit (CCK)-8 following treatment with berberine
Each concentration of berberine used was regarded as
one treatment group, while no berberine was added in the control
group. Each treated or control group contained three parallel
wells. The culture plates were incubated for 0, 24, 48 and 72 h.
The 3T3-L1 cells were subsequently treated with CCK-8 (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan), and the absorbance
at 450 nm was measured for the supernatant of each well with a
Multiskan EX plate reader (Thermo LabSystems, Helsinki,
Finland).
Oil-Red O staining
On day 10 of adipocyte differentiation induction,
the 3T3-L1 cells were stained with 1 mg/ml Oil-Red O dye (Abcam,
Cambridge, MA, USA). The cells were fixed with 70% ethanol and
dehydrated with 100% propylene glycol, and were subsequently
stained with Oil-Red O. The cells were observed under a microscope
(CX41RF; Olympus Corporation, Tokyo, Japan) and fat droplets in the
adipocytes were stained red.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) for
miRNA-27a and miRNA-27b
The total RNA was extracted using TRIzol Reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. cDNA was synthesized using a cDNA
synthesis kit (Thermo Fisher Scientific, Inc.). RT-qPCR was
performed to detect the mRNA expression levels of miRNA-27a and
miRNA-27b, using a One-Step SYBR PrimeScript RT-PCR kit II (Takara
Biotechnology Co., Ltd., Dalian, China) and data collection was
conducted using an ABI 7500 (Thermo Fisher Scientific, Inc.). The
PCR cycling conditions were as follows: 95°C for 10 min, followed
by 40 cycles at 95°C for 15 sec and 60°C for 45 sec, and a final
extension step of 95°C for 15 sec, 60°C for 1 min, 95°C for 15 sec
and 60°C for 15 sec. U6 small nuclear RNA served as an internal
control. The gene expression was calculated using the
2−ΔΔCq method. The primers used were as follows:
Forward, 5′-ACA CTC CAG CTG GGA GGG CTT AGC TGC TTG-3′ and reverse,
5′-CTC AAC TGG TGT CGT GGA GTC GGC AAT TCA GTT GAG TGC TCA-3′ for
miRNA-27a; forward, 5′-ACA CTC CAG CTG GGA GAG CTT AGC TGA TTG-3′
and reverse, 5′-CTC AAC TGG TGT CGT GGA GTC GGC AAT TCY AGTTGA GGT
TCAC-3′ for miRNA-27b; forward, 5′-CTC GCT TCG GCA GCACA-3′ and
reverse, 5′-AAC GCT TCA CGA ATT TGCGT-3′ for U6.
miRNA transfection
The 3T3-L1 cells were transfected with 40 µM
negative control (NC), miRNA-27a and m i R NA-27b, m i R NA-27a and
m i R NA-27b mimics or miRNA-27a and miRNA-27b inhibitors
(anti-miRNA-27a and anti-miRNA-27b) obtained from Beyotime
Institute of Biotechnology (Shanghai, China), which knockdown
miRNA-27a and miRNA-27b, using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
instructions. The 3T3-L1 cells were prepared for further analysis
48 h after transfection.
Triglyceride assay
The content of triglycerides (TGs) were analyzed
using the Triglyceride Quantification assay kit (Abcam), according
to the manufacturer's protocol. Briefly, the 3T3-L1 cells were
collected and resuspended in 0.1 M phosphate-buffered saline.
Following centrifugation for 10 min at 400 × g and 4°C, the cells
were lysed in 1–2% Triton X-100 for 30 min for each assay. The
samples were measured at 546 nm in a plate reader (Multiskan EX;
LabSystems, Helsinki, Finland).
Luciferase reporter assays
PPAR-γ was predicted to interact with miRNA 27a by
bioinformatics analysis using TargetScan, which predicts biological
targets of miRNAs by searching for the presence of 8mer, 7mer, and
6mer sites that match the seed region of each miRNA (21) The 3′-untranslated region (UTR) of
human PPAR-γ predicted to interact with miRNA-27a was synthesized
and immediately inserted downstream of the Renilla luciferase
reporter gene in the pGL3 vector (Promega Corporation, Madison, WI,
USA), yielding pGL3-PPAR-γ. The 3T3-L1 cells were co-transfected
with miRNA-27a mimics or NC using the Lipofectamine 2000. After 24
h, the luciferase activities were examined using the
Dual-Luciferase Reporter assay system (cat. no. E1960; Promega
Corporation). Firefly luciferase activity was normalized against
that of Renilla luciferase activity.
Statistical analysis
The data are presented as the mean ± standard
deviation. One-way analysis of variance, followed by Dunnett's test
was used for statistical analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Berberine inhibits the cell viability of
3T3-L1 cells
Following treatment with concentrations of berberine
(1, 10, 20, 40 and 80 µM) for 72 h, the growth of the 3T3-L1
cells was significantly reduced compared with that of the control
group (P<0.05). The cell viability of 3T3-L1 cells was reduced
by berberine treatment in a dose- and time-dependent manner
(Fig. 1A).
Berberine inhibits the differentiation of
3T3-L1 cells
The present study first examined the antiobesity
potential of berberine by determining pre-adipocyte differentiation
into adipocytes. Cultured 3T3-L1 cells were exposed to berberine at
different doses and cell differentiation was induced using the
differentiation medium. Following reculturing in DMEM with 10% FBS
for 10 days, cell differentiation was terminated and fat droplets
were detected using Oil-Red O staining. As shown Fig. 1B, the 3T3-L1 cells in the control
group exhibited normal differentiation, as indicated by the
appearance of numerous intracellular lipid droplets. However,
treatment of 3T3-L1 cells with berberine at different
concentrations (10 and 20 µM) caused a dramatic reduction in
lipid droplet accumulation dose-dependently. These results
indicated that berberine efficiently inhibited adipocyte
differentiation and may exhibit antiobesity effects in 3T3-L1
cells.
Berberine upregulates the expression
levels of miRNA-27a and miRNA-27b in 3T3-L1 cells
Based on the observed effect of berberine treatment
on cell viability and differentiation, the present study selected
the 10 µM berberine conditions for further mechanistic
studies on miRNA-27a and miRNA-27b changes. RT-qPCR was used to
confirm the expression levels of miRNA-27a and miRNA-27b following
treatment with berberine. Treatment with berberine was observed to
upregulate the expression levels of miRNA-27a and miRNA-27b (1.44-
and 1.87-fold increase; P<0.01; Fig. 1C). Overall, the present findings
provided evidence suggesting that berberine treatment upregulates
the mRNA expression levels of miRNA-27a and miRNA-27b in 3T3-L1
cells.
miRNA-27a and miRNA-27b regulate the
berberine-mediated inhibition of differentiation in 3T3-L1
cells
After determining that miRNA-27a and miRNA-27b were
upregulated by berberine in 3T3-L1 cells, the present study
investigated whether the expression levels of miRNA-27a and
miRNA-27b regulated the differentiation of 3T3-L1 cells after
treatment with berberine. To demonstrate the association between
cell differentiation and miRNA-27a, as well as miRNA-27b, miRNA-27a
and miRNA-27b mimics were transfected into 3T3-L1 cells. The mRNA
expression levels of miRNA-27a and miRNA-27b were subsequently
assessed by RT-qPCR. As shown in Fig.
2A and B, transfecting 40 nM miRNA-27a or miRNA-27b mimics into
3T3-L1 cells resulted in a 1.71- and 1.70-fold increase in the mRNA
expression levels of miRNA-27a and miRNA-27b, respectively. By
contrast, knockdown of miRNA-27a or miRNA-27b by transfecting
anti-miRNA-27a and anti-miRNA-27b into the 3T3-L1 cells resulted in
a 72.2 and 53.8% decrease in the mRNA expression levels of
miRNA-27a and miRNA-27b, respectively (P<0.01; Fig. 2C).
Furthermore, 3T3-L1 cell differentiation was
significantly decreased following treatment with miRNA-27a and
miRNA-27b mimics, combined with berberine treatment alone (Fig. 3A). However, knockdown of miRNA-27a
and miRNA-27b increased the differentiation of 3T3-L1 cells
following treatment with berberine compared with NC (Fig. 3B). These results suggested that the
overexpression of miRNA-27a and miRNA-27b increased the
berberine-mediated inhibition of 3T3-L1 cell differentiation, and
that knockdown of miRNA-27a and miRNA-27b attenuated the inhibition
of cell differentiation.
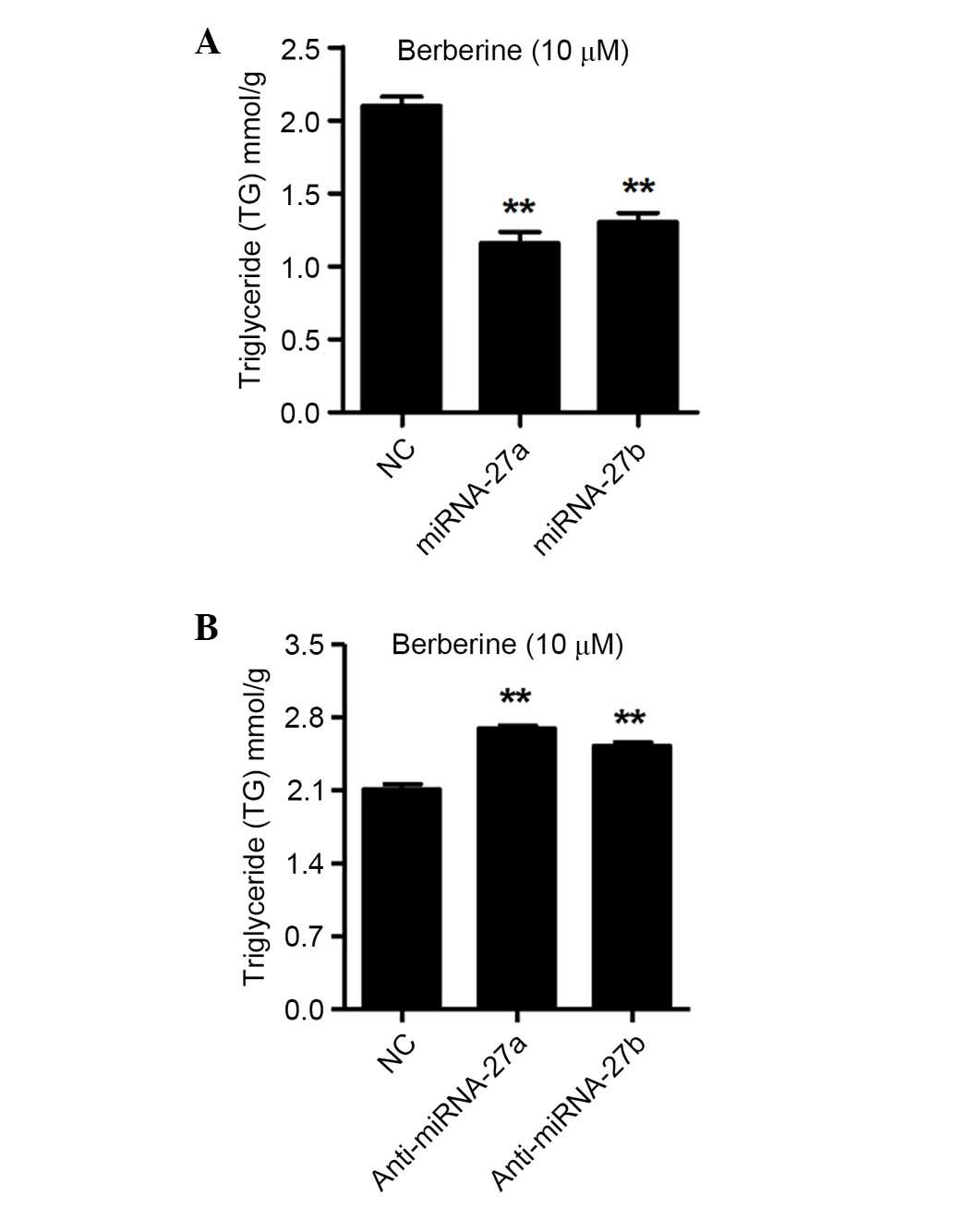
miRNA-27a and miRNA-27b regulate TG
contents in 3T3-L1 cells following treatment with berberine
After determining that the differentiation of 3T3-L1
cells was regulated by miRNA-27a and miRNA-27b, the present study
analyzed the intracellular TG contents in the 3T3-L1 cells
following transfection with miRNA-27a or miRNA-27b mimics, as well
as anti-miRNA-27a and anti-miRNA-27b, and NC for 48 h. As shown in
Fig. 4A, the intracellular TG
contents were decreased by 44.8 and 37.9% by the miRNA-27a and
miRNA-27b mimics transfection in berberine treated 3T3-L1 cells,
respectively (P<0.01). However, as shown in Fig. 4B, the intracellular TG contents
were increased by 27.8 and 19.7% by anti-miRNA-27a and
anti-miRNA-27b transfection in berberine treated 3T3-L1 cells,
respectively (P<0.01). These results indicated that miRNA-27a
and miRNA-27b negatively regulate the intracellular TG contents,
which have been demonstrated to impair the differentiation of
3T3-L1 cells.
miRNA-27a directly targets the PPAR-γ in
3T3-L1 cells
To investigate the regulatory mechanisms of
miRNA-27a, bioinformatics analysis (TargetScan) was used.
TargetScan identified that the mRNA sequence of PPAR-γ contained a
potential binding site for miRNA-27a (Fig. 5A). To confirm PPAR-γ as a target
and that this was regulated by miRNA-27a in 3T3-L1 cells, the
PPAR-γ 3′-UTR was cloned and inserted into a luciferase reporter
vector. The luciferase assay revealed that miRNA-27a significantly
suppressed luciferase activity containing the PPAR-γ 3′-UTR
(Fig. 5B). Western blotting
analysis demonstrated that miRNA-27a overexpression significantly
suppressed endogenous PPAR-γ expression, while inhibition of
miRNA-27a significantly increased the protein expression of PPAR-γ
in 3T3-L1 cells in the absence and presence of berberine (Fig. 5C and D). Together, these results
suggested that PPAR-γ is a target of miRNA-27a and is downregulated
by berberine in 3T3-L1 cells.
Discussion
Berberine serves an essential role in regulating
numerous important cellular processes, including growth,
differentiation, invasion, migration and apoptosis. Previous
studies have suggested that berberine inhibits the proliferation of
breast cancer cell by inducing cell cycle arrest (22) and promoted osteoblast
differentiation by activating Runx2 and p38 mitogen-activated
protein kinase (MAPK) (23). By
contrast, berberine was observed to suppress Th17 and Th1 T cell
differentiation by modulating the activities of
extracellular-regulated kinase, p38 MAPK and c-Jun N-terminal
kinase (24). In the present
study, berberine inhibited the viability (Fig. 1A) and differentiation (Fig. 1B) of 3T3-L1 cells in a dose- and
time-dependent manner. Therefore, the effects of berberine on
different cell types may not be consistent and comparable.
To investigate the mechanisms by which berberine
suppressed the viability and differentiation of 3T3-L1 cells, the
mRNA expression levels of miRNA-27a and miRNA-27b were also
measured by RT-qPCR. The present data showed that miRNA-27a and
miRNA-27b were upregulated in 3T3-L1 cells following treatment with
10 µM berberine (Fig. 1C),
which is in accordance with a recent report by Lo et al
(25) who demonstrated that
berberine treatment upregulated miRNA-21-3p in the HepG2 human
hepatoma cell line (25).
Potential regulatory miRNAs, which were upregulated or
downregulated in 3T3-L1 cells, were recently reported (26,27).
No previous study has experimentally defined the direct association
between berberine and miRNA-27a, as well as miRNA-27b, although
miRNAs have been predicated to be putative targets of berberine.
The present findings provided the first evidence, to the best of
our knowledge, that berberine directly enhances the expression
levels of miRNA-27a and miRNA-27b.
Furthermore, miRNA-27a and miRNA-27b were
subsequently overexpressed and knocked down in 3T3-L1 cells
(Fig. 2). The overexpression of
miRNA-27a and miRNA-27b significantly enhanced the
berberine-mediated inhibition of 3T3-L1 cell differentiation
(Fig. 3A). However, inhibition of
miRNA-27a and miRNA-27b significantly attenuated the
berberine-mediated inhibition of 3T3-L1 cell differentiation
(Fig. 3B). These data indicated
that miRNA-27a and miRNA-27b markedly regulated the differentiation
induced by berberine in 3T3-L1 cells and that berberine exerts an
antidifferentiation activity that is associated with the
upregulation of miRNA-27a and miRNA-27b. Previously, berberine
upregulated the expression levels of two C/EBP inhibitors,
CCAAT-enhancer-binding protein homologous protein (CHOP) and
differentially expressed in chondrocytes-2, while it downregulated
C/EBPα, PPARγ and other adipogenic markers and effectors in
differentiating 3T3-L1 pre-adipocytes and mature adipocytes
(28). Additionally, the
antiadipogenic activity of berberine was notably diminished by
knockdown of CHOP expression or by adjusting the differentiation
culture media (12). As a result
of its antidifferentiation activity in 3T3-L1 cells, miRNA-27a or
miRNA-27b may be act as a potential therapeutic target worth
further investigation.
An elevated TG level is a major marker of obesity,
added to elevated glucose and blood pressure, and reduced
high-density lipoprotein. The adipocyte is cell functioning as an
energy store for TG and cholesterol esters. It also secretes
various adipokines, inducing leptin, adiponectin and resistin,
which regulate pathological processes. Previous studies have
implied that berberine-treated 3T3-L1 cells exhibited significantly
reduced levels of intracellular TGs (29,30).
Therefore, the present study further investigated the effects of
miRNA-27a and miRNA-27b on berberine-mediated reduction of TG
levels in 3T3-L1 cells. As shown in Fig. 4, overexpression of miRNA-27a and
miRNA-27b significantly reduced the accumulation of TGs and, in
contrast, inhibition of miRNA-27a and miRNA-27b increased the
accumulation of TGs in 3T3-L1 cells following treatment with
berberine.
It is necessary to identify miRNA targets in order
to evaluate the roles of miRNAs abnormally expressed in human
cancer and to evolve gene therapies based on miRNAs. miRNA-27a and
miRNA-27b are markedly downregulated in adipocytes. They can target
and, therefore, can potential regulate a variety of genes
associated with adipogenesis. In the present study, PPAR-γ was
predicted as a potential target gene of miRNA-27a by bioinformatics
using TargetScan (Fig. 5A). A
luciferase activity assay indicated that miRNA-27a directly
targeted the 3′-UTR of PPAR-γ mRNA (Fig. 5B). PPAR-γ is an important regulator
of adipocyte differentiation, functioning in systemic lipid and
glucose metabolism (31). It is
widely recognized that a number of miRNAs have antidifferentiation
activity, which may be via the inhibition of PPAR-γ expression.
miRNA-27b targets PPAR-γ to inhibit growth and tumor progression in
neuroblastoma cells (32).
miRNA-130b reduces fat deposition in adipocytes by inhibiting the
expression of PPAR-γ (33).
Consistent with this notion, the present study demonstrated that
miRNA-27a mimics downregulated the expression of PPAR-γ, however,
upregulated the expression of PPAR-γ by anti-miRNA-27a (Fig. 5C). Similar to miRNA-27a mimics,
berberine treatment upregulated miRNA-27a and downregulated PPAR-γ
(Fig. 5D). Notably, berberine and
miRNA-27a mimics produced almost identical effects on cell
differentiation and the accumulation of TGs (Figs. 3 and 4). Therefore, the present study concluded
that the antidifferentiation effect of berberine may be performed,
at least partly, via the suppression of miRNA-27a/PPAR-γ
signaling.
In conclusion, the results of the present study
indicated that berberine inhibited cell viability and
differentiation, and upregulated the mRNA expression levels of
miRNA-27a and miRNA-27b in 3T3-L1 cells. The present data
demonstated that miRNA-27a and miRNA-27b regulate
berberine-mediated inhibition of differentiation and TG
accumulation. In addition, PPAR-γ, a direct and functional target
of miRNA-27a, may mediate miRNA-27a-induced differentiation and TG
accumulation. miRNA-27a/PPAR-γ signaling provided novel insight
towards understanding the underlying mechanisms of the
antidifferentiation activity of berberine and may provide useful
information for targeted therapy.
Acknowledgments
This present study was supported by the Youth
Scientific Research Project of Shanghai Municipal Commission of
Health and Family Planning (no. 20144Y0136), the Scientific
Research Project of shanghai Minhang District Health and Family
Planning commission (no. 2012MW09), the Scientific Research Project
of Shanghai Municipal Commission of Health and Family Planning (no.
20124469) and the Research Project of Shanghai Minhang District
Nature Science and Technology Foundation (no. 2012MHZ033).
References
|
1
|
Hwang JT, Park IJ, Shin JI, Lee YK, Lee
SK, Baik HW, Ha J and Park OJ: Genistein, EGCG, and capsaicin
inhibit adipocyte differentiation process via activating
AMP-activated protein kinase. Biochem Biophys Res Commun.
338:694–699. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shimomura I, Hammer RE, Richardson JA,
Ikemoto S, Bashmakov Y, Goldstein JL and Brown MS: Insulin
resistance and diabetes mellitus in transgenic mice expressing
nuclear SREBP-1c in adipose tissue: Model for congenital
generalized lipodystrophy. Gene Dev. 12:3182–3194. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pandey MK, Sung B, Kunnumakkara AB, Sethi
G, Chaturvedi MM and Aggarwal BB: Berberine modifies cysteine 179
of IkappaBalpha kinase, suppresses nuclear factor-kappaB-regulated
antiapoptotic gene products, and potentiates apoptosis. Cancer Res.
68:5370–5379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shidfar F, Ebrahimi SS, Hosseini S,
Heydari I, Shidfar S and Hajhassani G: The effects of Berberis
vulgaris fruit extract on serum lipoproteins, apoB, apoA-I,
homocysteine, glycemic control and total antioxidant capacity in
type 2 diabetic patients. Iran J Pharm Res. 11:643–652.
2012.PubMed/NCBI
|
|
5
|
Chang XX, Yan HM, Xu Q, Xia MF, Bian H,
Zhu TF and Gao X: The effects of berberine on hyperhomocysteinemia
and hyperlipidemia in rats fed with a long-term high-fat diet.
Lipids Health Dis. 11:862012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim M, Shin MS, Lee JM, Cho HS, Kim CJ,
Kim YJ, Choi HR and Jeon JW: Inhibitory effects of Isoquinoline
Alkaloid Berberine on ischemia-induced apoptosis via activation of
Phosphoinositide 3-kinase/protein Kinase B signaling pathway. Int
Neurourol J. 18:115–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang X, Gu L, Li J, Shah N, He J, Yang L,
Hu Q and Zhou M: Degradation of MDM2 by the interaction between
berberine and DAXX leads to potent apoptosis in MDM2-overexpressing
cancer cells. Cancer Res. 70:9895–9904. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li J, Shen L, Lu FR, Qin Y, Chen R, Li J,
Li Y, Zhan HZ and He YQ: Plumbagin inhibits cell growth and
potentiates apoptosis in human gastric cancer cells in vitro
through the NF-κB signaling pathway. Acta Pharmacol Sin.
33:242–249. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu FS, Yang JS, Lin HJ, Yu CS, Tan TW, Lin
YT, Lin CC, Lu HF and Chung JG: Berberine inhibits WEHI-3 leukemia
cells in vivo. In Vivo. 21:407–412. 2007.PubMed/NCBI
|
|
10
|
Mantena SK, Sharma SD and Katiyar SK:
Berberine inhibits growth, induces G1 arrest and apoptosis in human
epidermoid carcinoma A431 cells by regulating Cdki-Cdk-cyclin
cascade, disruption of mitochondrial membrane potential and
cleavage of caspase 3 and PARP. Carcinogenesis. 27:2018–2027. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu Y and Davies GE: Berberine inhibits
adipogenesis in high-fat diet-induced obesity mice. Fitoterapia.
81:358–366. 2010. View Article : Google Scholar
|
|
12
|
Pham TP, Kwon J and Shin J: Berberine
exerts anti-adipogenic activity through up-regulation of C/EBP
inhibitors, CHOP and DEC2. Biochem Biophys Res Commun. 413:376–382.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer.
94:776–780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu Z, Jian Z, Shen SH, Purisima E and Wang
E: Global analysis of microRNA target gene expression reveals that
miRNA targets are lower expressed in mature mouse and Drosophila
tissues than in the embryos. Nucleic Acids Res. 35:152–164. 2007.
View Article : Google Scholar :
|
|
18
|
Kang T, Lu W, Xu W, Anderson L, Bacanamwo
M, Thompson W, Chen YE and Liu D: MicroRNA-27 (miR-27) targets
prohibitin and impairs adipocyte differentiation and mitochondrial
function in human adipose-derived stem cells. J Biol Chem.
288:34394–34402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu HY, Li KP, Wang XJ, Liu Y, Lu ZG, Dong
RH, Guo HB and Zhang MX: Set9, NF-κB, and microRNA-21 mediate
berberine-induced apoptosis of human multiple myeloma cells. Acta
Pharmacol Sin. 34:157–166. 2013. View Article : Google Scholar
|
|
20
|
Ortiz LM, Lombardi P, Tillhon M and
Scovassi AI: Berberine, an epiphany against cancer. Molecules.
19:12349–12367. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim JB, Yu JH, Ko E, Lee KW, Song AK, Park
SY, Shin I, Han W and Noh DY: The alkaloid Berberine inhibits the
growth of Anoikis-resistant MCF-7 and MDA-MB-231 breast cancer cell
lines by inducing cell cycle arrest. Phytomedicine. 17:436–440.
2010. View Article : Google Scholar
|
|
23
|
Lee HW, Suh JH, Kim HN, Kim AY, Park SY,
Shin CS, Choi JY and Kim JB: Berberine promotes osteoblast
differentiation by Runx2 activation with p38 MAPK. J Bone Miner
Res. 23:1227–1237. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cui G, Qin X, Zhang Y, Gong Z, Ge B and
Zang YQ: Berberine differentially modulates the activities of ERK,
p38 MAPK, and JNK to suppress Th17 and Th1 T cell differentiation
in type 1 diabetic mice. J Biol Chem. 284:28420–28429. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lo TF, Tsai WC and Chen ST:
MicroRNA-21-3p, a berberine-induced miRNA, directly down-regulates
human methionine adenosyltransferases 2A and 2B and inhibits
hepatoma cell growth. PloS One. 8:e756282013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ling HY, Wen GB, Feng SD, Tuo QH, Ou HS,
Yao CH, Zhu BY, Gao ZP, Zhang L and Liao DF: MicroRNA-375 promotes
3T3-L1 adipocyte differentiation through modulation of
extracellular signal-regulated kinase signalling. Clin Exp
Pharmacol Physiol. 38:239–246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Andersen DC, Jensen CH, Schneider M,
Nossent AY, Eskildsen T, Hansen JL, Teisner B and Sheikh SP:
MicroRNA-15a fine-tunes the level of Delta-like 1 homolog (DLK1) in
proliferating 3T3-L1 preadipocytes. Exp Cell Res. 316:1681–1691.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang M and Chen L: Berberine in type 2
diabetes therapy: A new perspective for an old antidiarrheal drug?
Acta Pharm Sin B. 2:379–386. 2012. View Article : Google Scholar
|
|
29
|
Huang C, Zhang Y, Gong Z, Sheng X, Li Z,
Zhang W and Qin Y: Berberine inhibits 3T3-L1 adipocyte
differentiation through the PPARgamma pathway. Biochem Biophys Res
Commun. 348:571–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ko BS, Choi SB, Park SK, Jang JS, Kim YE
and Park S: Insulin sensitizing and insulinotropic action of
berberine from Cortidis rhizoma. Biol Pharm Bull. 28:1431–1437.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Belfort R, Berria R, Cornell J and Cusi K:
Fenofibrate reduces systemic inflammation markers independent of
its effects on lipid and glucose metabolism in patients with the
metabolic syndrome. J Clin Endocrinol Metab. 95:829–836. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee JJ, Drakaki A, Iliopoulos D and Struhl
K: MiR-27b targets PPARγ to inhibit growth, tumor progression and
the inflammatory response in neuroblastoma cells. Oncogene.
31:3818–3825. 2012. View Article : Google Scholar
|
|
33
|
Pan S, Yang X, Jia Y, Li R and Zhao R:
Microvesicle-shuttled miR-130b reduces fat deposition in recipient
primary cultured porcine adipocytes by inhibiting PPAR-γ
expression. J Cell Physiol. 229:631–639. 2014. View Article : Google Scholar
|