Introduction
Autoimmune and inflammatory uveitis are potentially
blinding intraocular diseases that are often associated with
immunological responses to unique retinal proteins (1). Experimental autoimmune uveitis (EAU)
animal models of ocular autoimmunity targeting retinal proteins are
useful to investigate the underlying causes of human uveitis and
have improved the understanding of the basic immunological
mechanisms of uveitis. EAU models act as templates for the
development of novel therapeutic strategies for autoimmune and
inflammatory uveitis (1,2). Previous investigations have used
proteomic profiling to measure changes in EAU rat plasma compared
with control samples, indicating that the pathogenesis of EAU is
associated with the aberrant expression of proteins involved in
multiple signaling pathways (3).
Additionally, the development of EAU is also associated with the
regulation of mRNAs by microRNAs (4).
Previous studies have investigated the changes of
uveitogenic CD4+ T cell populations, particularly Th1
and Th17, during the development of autoimmune uveitis (5,6).
Further studies indicate that alterations to the levels of
cytokines secreted by T cells are correlated with the occurrence
and development of uveitis. Elevated levels of interferon (IFN)-γ,
(interleukin) IL-17 and tumor necrosis factor (TNF)-α are
associated with the exacerbation of uveitis, indicating that these
cytokines are uveitogenic (7).
Furthermore, IL-10 has been demonstrated to ameliorate the clinical
disease scores of EAU and has a protective function in uveitis
(7). Additionally, measuring
changes to the CD4+/CD8+ ratio in aqueous
humor (8) or vitreous fluid
(9,10) may be useful during the diagnosis of
uveitis.
Longdan Xiegan Tang (LXT) is a commonly prescribed
herbal formula in traditional Chinese medicine. It has been widely
used in clinical practice for its anti-inflammatory,
anti-infection, antibacterial, anti-allergy, hepatoprotectant,
cholagogic and immunostimulatory activities (11-14).
Traditionally, LXT is composed of 10 plant extracts: Radix
Gentianae, Radix Scutellariae, Fructus Gardeniae,
Rhizoma Alismatis, Caulis Clematidis Armandii,
Semen Plantaginis, Radix Angelicae Sinensis, Radix
Rehmanniae, Radix Bupleuri and Radix
Glycyrrhizae. Lee and Chang (14) reported that LXT exerts
immunomodulatory effects and regulates the immune function of mice
with systemic autoimmune lupus erythema-tosus. Based on the
theories of traditional Chinese medicine, LXT has been used in
clinical trials for the treatment of uveitis and the syndrome of
burning-heat in the liver and gallbladder (15). However, the detailed mechanisms of
how LXT exerts its beneficial effects remain unclear. To
investigate the effects of LXT on the development of uveitis, the
present study established an EAU model in Lewis rats via
immunization with interphotoreceptor retinoid-binding protein
(IRBP) in complete Freund's adjuvant (CFA) solution supplemented
with Mycobacterium tuberculosis H37Ra strain. Furthermore,
the efficacy of LXT on EAU rats was determined by evaluation of
clinical manifestations and histopathology. Changes to
CD4+ and CD8+ T cell populations, and the
CD4+/CD8+ ratio in EAU and LXT-treated rats
were measured by flow cytometry. Additionally, the expression
levels of IFN-γ, IL-17, TNF-α and IL-10 were determined by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
enzyme-linked immunosorbent assay (ELISA) analysis. The findings of
the present study provide insight into the molecular mechanisms
that mediate the effects of LXT on uveitis.
Materials and methods
Animals
Female Lewis rats (6-8 weeks old; 160-180 g; Grade
II; certificate number of the breeder, SCXK Jing 2012-0001) were
purchased from Beijing Vital River Laboratory Animal, Co., Ltd.
(Beijing, China) and bred at The Eye Institute of Shandong
University of Traditional Chinese Medicine (Jinan, China). Rats
were housed at room temperature (25±1°C) with relative humidity
50±10%. The animal facility was under a 12 h light/dark cycle.
Prior to the experimental procedures, all rats were acclimatized to
the housing room and experimental handling for 1 week. Animal care
and use strictly followed the National Institutes of Health
guidelines (16), and all
experiments were approved by the Laboratory Animal Care and Use
Committee of Shandong University of Traditional Chinese
Medicine.
Reagents
IRBP peptide (amino acids 1177-1191; sequence, ADG
SSW EGV GVV PDV) and primers were synthesized by Shanghai Sangon
Biological Engineering Technology & Services Co., Ltd.
(Shanghai, China). CFA and 2-mercap-toethanol were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Mycobacterium
tuberculosis (strain H37RA) was purchased from Difco; BD
Biosciences (Franklin Lakes, NJ, USA). Recombinant rat IL-2,
fluorescein isothiocyanate (FITC)-conjugated anti-CD4 antibody
(11-0040) and phycoerythrin (PE)-conjugated anti-CD8 antibody
(12-0084) were purchased from eBioscience, Inc. (San Diego, CA,
USA). IFN-γ (DKW12-3000-096), IL-17A (DKW12-3170-096), TNF-α
(DKW12-3720-096) and IL-10 (DKW12-3100-096) ELISA kits were
purchased from Dakewe Biotech Co., Ltd. (Shenzhen, China).
Phosphate-buffered saline (PBS), formaldehyde, paraffin,
hematoxylin and eosin (HE) were purchased from Sinopharm Chemical
Reagent Co., Ltd. (Shanghai, China). RPMI 1640 medium was purchased
from Gibco; Thermo Fisher Scientific, Ltd. (Waltham, MA, USA). All
experimental procedures adhered to the Association for Research in
Vision and Ophthalmology Statement for the use of animals in
ophthalmic and vision research (17).
Induction of EAU and intervention of
LXT
Lewis rats were randomly divided into three groups
(normal control, EAU and LXT group) with 15 rats in each group. On
day 0, each rat in the EAU and LXT groups were subcutaneously
immunized with 300 µl IRBP (100 µg) emulsion
containing 100 µl CFA (2.5 mg/ml) and 100 µg
Mycobacterium tuberculosis, distributed at five sites,
including one footpad, two flanks and the backside. Rats in the
normal control group were treated with 300 µl emulsion
containing 100 µl of CFA (2.5 mg/ml), 100 µg of
Mycobacterium tuberculosis and sterilized PBS.
Rats in the LXT group received LXT decoctions on day
5 following EAU induction. The decoctions included 10 types of
boil-free granules of aforementioned traditional Chinese medicine
extracts. All boil-free granules were purchased from China
Resources Sanjiu Medical & Pharmaceutical Co., Ltd. (Shenzhen,
China). The identification for each granule was determined by thin
layer chromatography (Qingdao Haiyang Chemical Co. Ltd., Qingdao,
China) according to Chinese Pharmacopeia (edition 2010), and the
quality of all products met the requirements of Chinese
Pharmacopeia (18). The
administration of LXT was performed by oral gavage (200 mg/kg/day)
over the experimental period. Rats in normal and EAU groups
received equal volumes of sterilized distilled water.
Clinical and histopathological
assessment
A hand-held retinal camera (Genesis-D; Kowa Company,
Ltd., Aichi, Japan) was used to record the inflammatory response of
the anterior segment of rats each day following immunization until
the end of the experimental protocol. At the desired intervals
following immunization (days 4, 8, 12, 16 and 20), rats were
euthanized using excess phenobarbital and the eyes were extracted.
The harvested eyes were fixed in 4% formaldehyde for 24 h, embedded
in paraffin blocks and serially sectioned (5 µm) in the
transverse plane. All sections were stained with HE and were
observed under a light microscope (Ti; Nikon Corporation, Tokyo,
Japan). The score of eye inflammation was evaluated using
previously described criteria (15) and the severity of EAU was scored on
a scale of 0 (no inflammation) to 4 (maximum inflammation).
Flow cytometry analysis
Briefly, 3 rats in each group were randomly
sacrificed on days 4, 8, 12, 16 and 20 following immunization. T
cells were isolated from lymph node and spleen by passage through a
nylon wool column (Wako Pure Chemical Industries, Ltd., Osaka,
Japan). Following collection, 1×107 cells were cultured
in 6-well plates and stimulated with 10 µl IRBP (10 mg/ml)
and 1 µl recombinant rat IL-2 (10 ng/ml) for 48 h in the
presence of 1×107 irradiated syngeneic spleen antigen
presenting cells in 2 ml RPMI 1640 medium supplemented with
2-mercaptoethanol (final concentration, 5×10−5 mol/l).
Subsequently, the activated T cells were isolated by Ficoll
gradient (GE Healthcare Life Sciences, Uppsala, Sweden) at 600 × g.
T cells were then stained with FITC-CD4 and PE-CD8 antibodies
(1:100) in the dark at 4°C for 25 min. The stained cells were
washed with PBS twice and analyzed using a BD FACSVerse flow
cytometer and BD FACSuite, version 1.0 (BD Biosciences, Franklin
Lakes, NJ, USA).
RT-qPCR
To determine the alterations to IFN-γ, IL-17, TNF-α
and IL-10 mRNA levels, RT-qPCR was performed using samples of
blood, lymph node and spleen from rats at days 4, 8, 12, 16 and 20
post-immunization. The relevant tissues and blood from normal
control rats were used as negative control. Briefly, rats were
humanely euthanized at the indicated intervals and the lymph nodes,
spleen and blood in each group were isolated and stored in liquid
nitrogen. Following homogenization, total RNA was extracted from
these tissues using TRIzol reagent (Thermo Fisher Scientific, Inc.)
and the RNA purity and concentration were determined with a K5600
spectrophotometer (Beijing Kaiao Technology Development Co., Ltd.,
Beijing, China). Synthesis of first-strand cDNA was performed using
1 µg total RNA and Transcriptor First Strand cDNA Synthesis
kit (Roche Diagnostics, Basel, Switzerland) according to the
manufacturer's protocol. The RT-qPCR reaction was performed in a 20
µl reaction using 2X SYBR Green qPCR Mix (Aidlab
Biotechnologies Co., Ltd., Beijing, China). The PCR reaction was
performed using a Realtime PCR System (Agilent Technologies, Inc.,
Santa Clara, CA, USA) with an initial denaturation step of 95°C for
5 min, followed by 45 cycles of 95°C for 20 sec, 58°C for 25 sec
and 72°C for 25 sec. Relative gene expression levels were
quantified using the ΔΔCq method. The ΔΔCq values were calculated
as the fold change in expression over tissues cells in mean ±
standard error following normalization to the β-actin reference
gene (19). The primers used for
RT-qPCR were as follows: β-actin, forward 5′-CAC CCG CGA GTA CAA
CCTTC-3′, reverse 5′-CCC ATA CCC ACC ATC ACACC-3′; IFN-γ, forward
5′-GGA TAT CTG GAG GAA CTG GCA-3′, reverse 5′-GCT AGA TTC TGG TGA
CAG CTG-3′; IL-17, forward 5′-TTG CTG CTA CTG AAC CTG GAG-3′,
reverse 5′-GCA TGG CGG ACA ATA GAG-3′; IL-10, forward 5′-TTC CAT
CCG GGG TGA CAA TAA-3′, reverse 5′-TTC TGG GCC ATG GTT CTC TGC-3′;
TNF-α, forward 5′-TAC TGA ACT TCG GGG TGA TTG GTCC-3′, reverse
5′-CAGCCT TGT CCC TTG AAG AGA ACC-3′.
ELISA
To measure the alterations of cytokines in the blood
on days 4, 8, 12, 16 and 20 post-immunization, 3 rats in each group
were humanely euthanized at the indicated time-point. Blood samples
were then collected and plasma was isolated by centrifugation at
2,000 × g for 10 min at 4°C. The levels of IFN-γ, IL-17A, TNF-α and
IL-10 were measured using the relevant ELISA kit. All procedures
were conducted in accordance with the manufacturer's
instructions.
Statistical analysis
The results are expressed as the mean ± standard
deviation. Each experiment was performed in triplicate and repeated
3 times. SPSS software version 17.0 (SPSS, Inc., Chicago, IL, USA)
was used to perform the statistical analysis. Statistical analysis
was performed using one-way analysis of variance followed by the
least significant difference multiple comparison test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Efficacy of LXT on EAU rats
The inflammatory response of rats in each group was
observed each day until day 20. Additionally, histopathological
examination was performed following euthanization of 3 rats on days
4, 8, 12, 16 and 20. Clinical signs of dilated blood vessels in the
iris of rats were first observed on day 5 post-immunization and
thus, LXT treatment was administered to rats in the LXT group. On
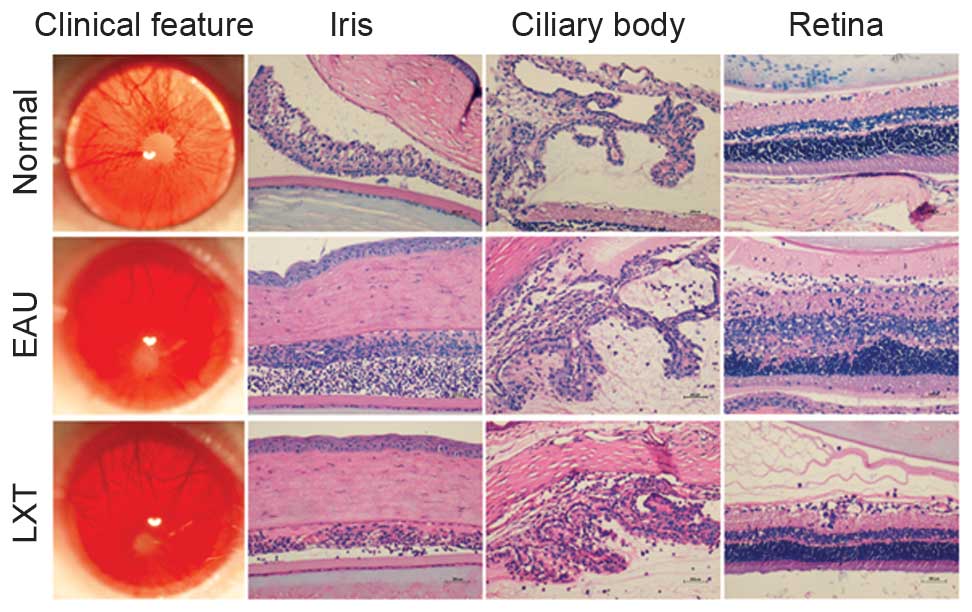
day 12 post-immunization, rats in the EAU group exhibited opaque
anterior chambers with severe fibrin exudation, obscured pupil,
absence of red reflex and proptosis (Fig. 1). The intensity of uveitis was
scored 4. However, the majority of rats in the LXT group exhibited
relatively lighter symptoms, including moderately opaque anterior
chambers and visible pupils (Fig.
1). Furthermore, in the EAU group, histopathological
examination demonstrated a large number of inflammatory cells and
fibrin exudation in anterior chamber, synechia of the iris and
ciliary body structure disorder, and full-thickness retinal damage
on day 12 post-immunization. However, compared with the EAU group,
the LXT group demonstrated decreased inflammatory infiltration and
fibrin exudation in anterior chamber, reduced synechia of the iris
and ciliary body structure disorder, mild to moderate inflammation
of the retina, and photoreceptor outer segment damage or lesions
extending to the outer nuclear layer (Fig. 1).
Although the EAU and LXT groups developed severe
inflammation from day 5 until day 12 post-immunization, rats in the
LXT group exhibited a faster recovery compared with EAU group,
particularly between day 14 until 18 (P<0.001; Fig. 2A). Additionally, the
histopathological scores of LXT mice were significantly reduced
compared with EAU mice at days 12 and 16 (P<0.01), indicating
that LXT has a protective effect on the retina of rats with EAU
(Fig. 2B).
Alterations in the CD4/CD8 ratio in lymph
node and spleen
The results of flow cytometry analysis demonstrated
that in the lymph nodes of normal control rats, the levels of the
CD4+ and CD8+ T cell populations were
77.99±1.03% and 20.85±0.84%, respectively, producing a
CD4+/CD8+ ratio of 3.74±0.20 (Table I). However, following treatment
with IRBP emulsion, the ratio of CD4+/CD8+ in
the lymph nodes of EAU rats was significantly decreased compared
with normal controls at day 4 (P<0.001), and then subsequently
elevated reaching a peak on day 12 (P<0.001). However, following
treatment with LXT, the ratio of CD4+/CD8+
returned to normal levels on day 12, and were significantly reduced
compared with EAU rats at the equivalent time point (P=0.002).
These results demonstrated that LXT regulates the immune response
and maintains the normal physiological functions following EAU
(Table I).
 | Table IAlterations of CD4+ and
CD8+ T cells in lymph nodes. |
Table I
Alterations of CD4+ and
CD8+ T cells in lymph nodes.
| CD4+ T
cell population
| CD8+ T
cell population
|
CD4+/CD8+ ratio
|
|---|
| Lymph node | EAU group | LXT group | EAU group | LXT group | EAU group | LXT group |
|---|
| Normal | 77.99±1.03 | 20.85±0.84 | 3.74±0.20 |
| Day 4 | 71.44±4.47 | 69.14±4.62 | 28.36±2.84 | 29.53±3.13 | 2.55±0.42b | 2.40±0.39b |
| Day 8 | 73.11±4.29 | 60.83±6.16 | 26.80±5.19 | 36.35±5.39 | 2.82±0.75a,c | 1.71±0.39b,c |
| Day 12 | 84.19±1.36 | 77.47±1.68 | 14.20±0.97 | 17.26±1.79 | 5.95±0.51b,d | 4.52±0.54d |
| Day 16 | 81.29±3.43 | 78.66±0.69 | 19.39±2.70 | 18.65±0.42 | 4.26±0.76 | 4.21±0.13 |
| Day 20 | 78.46±3.04 | 77.62±1.65 | 18.06±1.71 | 20.59±0.84 | 4.36±0.50 | 3.78±0.24 |
In the spleen in normal control rats, the percentage
of the CD4+ and CD8+ T cell populations were
52.36±8.40% and 37.95±6.36%, respectively, producing a
CD4+/CD8+ ratio of 1.43±0.45 (Table II). The
CD4+/CD8+ ratio in the spleen was increased
continuously during the development of inflammation reaching a peak
day 12 in the EAU and LXT groups. The ratios differed from those
measured in the lymph nodes. On day 12, the
CD4+/CD8+ ratio in the LXT group (2.98±0.54)
was significantly reduced compared with the EAU group (4.71±1.92;
P=0.016). By contrast, the CD4+/CD8+ ratio in
the LXT group was significantly increased compared with the normal
group at day 12 only (P=0.028; Table
II).
 | Table IIAlterations of CD4+ and
CD8+ T cells in spleens |
Table II
Alterations of CD4+ and
CD8+ T cells in spleens
| CD4+ T
cell population
| CD8+ T
cell population
|
CD4+/CD8+ratio
|
|---|
| Spleen | EAU group | LXT group | EAU group | LXT group | EAU group | LXT group |
|---|
| Normal | 52.36±8.40 | 37.95±6.36 | 1.43±0.45 |
| Day 4 | 59.88±5.88 | 58.77±4.73 | 32.60±3.03 | 30.92±2.67 | 1.86±0.34 | 1.90±0.21 |
| Day 8 | 73.59±4.72 | 62.75±1.63 | 25.13±6.49 | 30.65±2.90 | 3.13±1.15a | 2.06±0.24 |
| Day 12 | 77.17±5.25 | 70.38±3.50 | 17.73±5.02 | 24.02±3.09 | 4.71±1.92b,c | 2.98±0.54b,c |
| Day 16 | 73.59±4.08 | 52.54±2.03 | 23.34±2.03 | 36.15±6.00 | 3.18±0.43b,c | 1.48±0.27c |
| Day 20 | 65.68±6.42 | 52.04±2.74 | 27.99±6.34 | 40.99±3.83 | 2.45±0.71 | 1.28±0.19 |
RT-qPCR
Following different treatments, the levels of IFN-γ,
IL-17, TNF-α and IL-10 were determined by RT-qPCR analysis of rat
blood, lymph node and spleen on days 4, 8, 12, 16 and 20. The
relative expression of the normal rats was set as 1.00, and the
relative gene expression levels were quantified using the ΔΔCq
method.
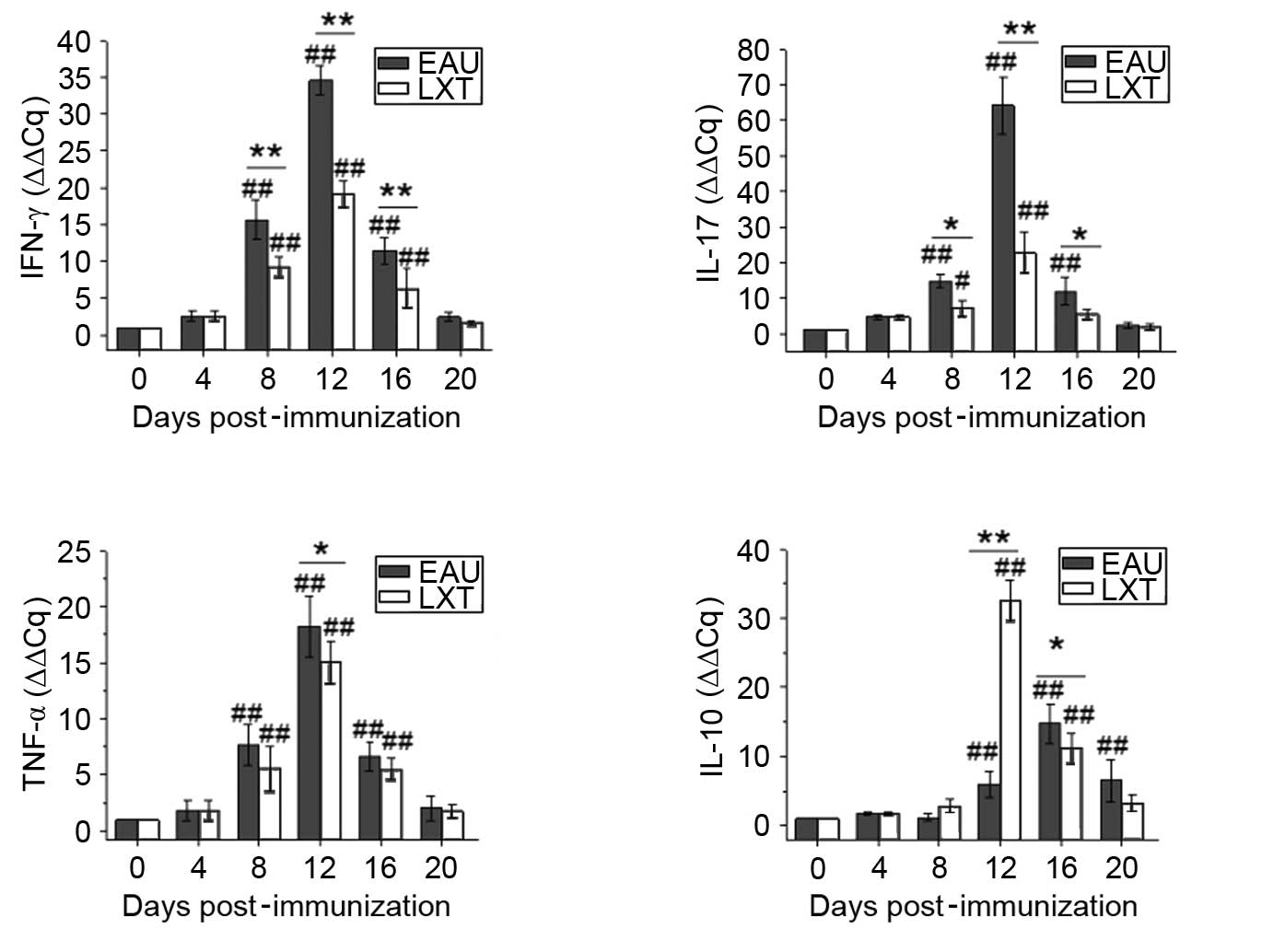
In blood (Fig. 3),
the changes to the mRNA levels of IFN-γ, IL-17 and TNF-α
demonstrated that the alterations in cytokine levels are associated
with the exacerbation and alleviation of inflammation in the EAU
and LXT groups, respectively. The mRNA levels of IFN-γ increased
from day 4 and peaked on day 8 post-immunization, and both IL-17
and TNF-α levels peaked on day 12 in the EAU group. However, the
levels of IL-10 increased from day 12 and peaked on day 16 in the
EAU and LXT groups. Furthermore, the peak mRNA levels of IFN-γ,
IL-17 and TNF-α were significantly decreased (P<0.001), and
IL-10 levels significantly increased (P= 0.042), in the LXT group
compared with those in the EAU group (Fig. 3).
Additionally, following immunization, the mRNA
levels of IFN-γ, IL-17 and TNF-α were elevated from day 8 and
peaked on day 12 in the EAU and LXT groups in lymph node (Fig. 4). By contrast, following LXT
treatment, the mRNA levels of IFN-γ and IL-17 were reduced compared
with the EAU group on days 12 (both P<0.001), 16 (both
P<0.001) and 20 (P=0.032 and P=0.046) post-immunization. The
levels of IL-10 in the EAU group increased on day 4 (P=0.011) and
decreased between day 8 and 12 compared with day 0. Whereas
compared with the levels at day 0, the IL-10 expression levels
increased from day 4 (P=0.098) and peaked on day 12 (P<0.001) in
the LXT group, then were maintained at an increased level
throughout the experiment (Fig.
4).
Correspondingly, compared with the levels at day 0,
the mRNA levels of IFN-γ, IL-17 and TNF-α in the spleen were
increased from day 8 and peaked on day 12 in the EAU and LXT groups
(Fig. 5). However, following LXT
treatment, the mRNA levels of IFN-γ and IL-17 were reduced compared
with the EAU group on days 8 (P<0.001 and P=0.017,
respectively), 12 (both P<0.001) and 16 (P=0.001 and P=0.035,
respectively) post-immunization. By contrast, compared with the
levels at day 0, the mRNA level of IL-10 was significantly
increased from day 12 (P=0.005) and peaked on day 16 (P<0.001)
in the EAU group. Additionally, compared with the EAU group, the
mRNA level of IL-10 was significantly increased on day 12 in LXT
group (P<0.001).
ELISA
Serum samples from EAU at LXT rats were used to
determine the levels of IFN-γ, IL-17 and TNF-α and IL-10. The
results indicated that the levels of IFN-γ, IL-17 and TNF-α in the
EAU group exhibited similar expression patterns associated with the
exacerbation and decrease of inflammation post-immunization
(Fig. 6). The levels of IFN-γ,
IL-17 and TNF-α increased from day 8 and peaked on day 12
post-immunization in the EAU group. However, following LXT
treatment, the peak values of IFN-γ, IL-17 and TNF-α were
significantly reduced compared with the levels of the EAU group
(P=0.028, P<0.001 and P<0.001, respectively). By contrast,
IL-10 expression increased from day 12 and peaked on day 16 in the
EAU and LXT groups, and the levels of IL-10 were significantly
increased in the LXT group compared with the EAU group on days 16
and 20 (P=0.031 and P=0.021; Fig.
6).
Discussion
LXT has been widely used in traditional Chinese
medicine clinical trials to treat patients with anti-inflammatory,
hepatoprotectant and immunoregulatory symptoms (13,14).
Additionally, it has been used to treat the syndrome of
burning-heat in the liver and gallbladder as described in
traditional Chinese medicine theory. In the present study, an EAU
rat model was induced using IRBP emulsion. Notably, in the EAU
model fibrin exudation occurred in the anterior chamber of the eye,
similar to the symptom of rising of yellow fluid in the eyes, which
is a clinical manifestation of the syndrome of burning-heat in the
liver and gallbladder (20).
Therefore, LXT may be useful for the treatment of uveitis. The
present study observed that the majority rats in the LXT group
exhibited reduced fibrin exudation the in anterior chamber compared
with EAU rats, although there was no statistical difference in the
clinical scores of the EAU and LXT groups on day 12. Furthermore,
the results of histopathological examination revealed that LXT
exerts protective effects on various tissues including the iris,
ciliary body and retina in EAU rats. Based on the histopathological
grading of the retina of EAU in Lewis rats (21), mild to moderate inflammatory
infiltration and photoreceptor outer segment damage or lesions
extending to the outer nuclear layer of retina were observed in the
LXT group, which was less severe compared with the changes observed
in the EAU group (Fig. 1).
EAU is initiated by the activation of
CD4+ T cells that respond to ocular antigens located
within or around photoreceptor segments (22). Kerr et al (23) demonstrated that the retinal
infiltration of CD4+ T cells peaked on day 13
post-immunization and indicated EAU in the B10 mouse model. The
induction of EAU in RIII mice induced by IRBP-3 161-180 peptides is
predominantly mediated by CD4+ T cells. Thus, the
characterization of different T-lymphocyte populations
(CD4+ and 8+) during an ocular inflammatory
episode may be useful for the diagnosis of uveitis (8). Kojima et al (9) reported the diagnostic value of
measuring the CD4+/CD8+ ratio in vitreous
fluid in uveitis with ocular sarcoidosis, and further revealed that
a vitreum CD4+/CD8 ratio >3.5 is a useful diagnostic
parameter with 100% sensitivity and 96.3% specificity. The ratio of
CD4+/CD8+ in aqueous humor can also be
measured in patients with sarcoid uveitis (8). The present study demonstrated that
the percentage of CD4+ T cell population and the
CD4+/CD8+ ratio reached maximal values on day
12, which was also the time point of peak inflammation in both
lymph nodes and spleen. However, following LXT treatment, the
percentage of the CD4+ T cell population and
CD4+/CD8+ ratio were reduced in the LXT group
compared with the EAU group at the same time point.
Previous studies have indicated that IFN-γ, which is
highly secreted by CD4+ Th1 cells, is pivotal in the
development of EAU (24,25). Previous studies have demonstrated
that IL-17, which is secreted by CD4+ Th17 cells and γδ
T cells, is important during the pathogenesis of autoimmune
diseases (26-30). Peng et al (31) demonstrated that IL-17+
and IFN-γ+ IRBP-specific T cells are uveitogenic. The
present study observed that alterations of IFN-γ and IL-17 levels
were closely associated with the exacerbation and subsequent
reduction of inflammation in blood, lymph nodes and spleen in EAU
rats, despite variations between levels in the types of sample. A
previous study noted that differentiation of Th1 cells occurred
earlier than Th17 cells in the spleen (32). The present study demonstrated that
the mRNA expression levels of IFN-γ in the blood increased from day
4 post-immunization and peaked on day 8. These findings indicate
that IFN-γ may be associated with the acute onset of inflammation.
However, following LXT treatment, the relative expression of IFN-γ
and IL-17 at the mRNA level were significantly reduced in the
blood, lymph node and spleen. TNF-α is another important pathogenic
cyto-kine and the blockade of TNF-α has been used as a successful
immunotherapy in preclinical models of uveitis and in human disease
(22). The current study observed
that the expression of TNF-α exhibited the same trend as those of
IFN-γ or IL-17 during the development of EAU, and that treatment
with LXT reduced the expression of TNF-α at the peak stage of
inflammation.
IL-10 is predominantly secreted by Th2 and T
regulatory cells. It can alleviate the development of EAU by
suppressing de novo priming of Ag-specific T cells and
inhibiting the recruitment and function of inflammatory leukocytes
(33). Under the negative
regulatory effect of IL-10, the expression of IFN-γ and IL-17
decrease rapidly and exert additional regulatory effects on EAU
(34). The present study observed
that the expression of IL-10 peaked around day 16 post-immunization
in blood, lymph nodes and spleen of the EAU group. However,
following LXT treatment, the IL-10 mRNA expression was elevated in
the LXT group compared with the EAU group at the same time point.
Furthermore, the mRNA expression of IL-10 in the LXT group peaked
on day 12 in the lymph nodes and spleen. These findings indicate
that the elevation of IL-10 expression may mediate the alleviation
of the inflammatory symptoms in EAU rats following LXT
treatment.
In conclusion, LXT effectively alleviates the
clinical manifestation of EAU in rats, suppresses the
differentiation of uveitogenic CD4+ T cells and inhibits
the expression of Th1 and Th17 signature cytokines, including IFN-γ
and IL-17. Furthermore, LXT also promotes the secretion of IL-10,
restores the immune balance, and thus, accelerates the recovery of
autoimmune uveitis, indicating that LXT may be useful for the
treatment of uveitis via regulation of the immune response.
Acknowledgments
The present study was supported by the Development
Project of Science and Technology of Traditional Chinese Medicine
of Shandong Province (grant no. 2013ZDZK-083) and the National
Natural Science Foundation of China (grant nos. 81373826, 81403438
and 81360558).
References
|
1
|
Caspi RR: A look at autoimmunity and
inflammation in the eye. J Clin Invest. 120:3073–3083. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fang CB, Zhou DX, Zhan SX, He Y, Lin Z,
Huang C and Li J: Amelioration of experimental autoimmune uveitis
by leflunomide in Lewis rats. PLoS One. 8:e620712013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo D, Gu P, Liu Z, Tang K, Du Y and Bi H:
Proteomic analysis of rat plasma with experimental autoimmune
uveitis based on label-free liquid chromatography-tandem mass
spectrometry (LC-MS/MS). J Chromatogr B Analyt Technol Biomed Life
Sci. 976–977:84–90. 2015. View Article : Google Scholar
|
|
4
|
Guo D, Li J, Liu Z, Tang K, Song H and Bi
H: Characterization of microRNA expression profiling in peripheral
blood lymphocytes in rats with experimental autoimmune uveitis.
Inflamm Res. 64:683–696. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Trinh L, Brignole-Baudouin F, Pauly A,
Liang H, Houssier M and Baudouin C: Th1- and Th2-related chemokine
and chemokine receptor expression on the ocular surface in
endotoxin-induced uveitis. Mol Vis. 14:2428–2434. 2008.PubMed/NCBI
|
|
6
|
Luger D, Silver PB, Tang J, Cua D, Chen Z,
Iwakura Y, Bowman EP, Sgambellone NM, Chan CC and Caspi RR: Either
a Th17 or a Th1 effector response can drive autoimmunity:
Conditions of disease induction affect dominant effector category.
J Exp Med. 205:799–810. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Horai R and Caspi RR: Cytokines in
autoimmune uveitis. J Interferon Cytokine Res. 31:733–744. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sanz-Marco E, Garces M, Sempere A and
Diaz-Llopis M: CD4/CD8 ratio in aqueous humor in Uveitis. Ocul
Immunol Inflamm. 21:408–409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kojima K, Maruyama K, Inaba T, Nagata K,
Yasuhara T, Yoneda K, Sugita S, Mochizuki M and Kinoshita S: The
CD4/CD8 ratio in vitreous fluid is of high diagnostic value in
sarcoidosis. Ophthalmology. 119:2386–2392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Davis JL, Ruiz P Jr, Shah M and Mandelcorn
ED: Evaluation of the reactive T-cell infiltrate in uveitis and
intraocular lymphoma with flow cytometry of vitreous fluid (an
American Ophthalmological Society thesis). Trans Am Ophthalmol Soc.
110:117–129. 2012.
|
|
11
|
Wang CH, Cheng XM, Bligh SW, White KN,
Branford-White CJ and Wang ZT: Pharmacokinetics and bioavailability
of gentiopicroside from decoctions of Gentianae and Longdan Xiegan
Tang after oral administration in rats - comparison with
gentiopicroside alone. J Pharm Biomed Anal. 44:1113–1117. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng HY, Huang HH, Yang CM, Lin LT and
Lin CC: The in vitro anti-herpes simplex virus type-1 and type-2
activity of Long Dan Xie Gan Tan, a prescription of traditional
Chinese medicine. Chemotherapy. 54:77–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lai SC, Chen KM, Chang YH and Lee HH:
Comparative efficacies of albendazole and the Chinese herbal
medicine long-dan-xie-gan-tan, used alone or in combination, in the
treatment of experimental eosinophilic meningitis induced by
Angiostrongylus cantonensis. Ann Trop Med Parasitol. 102:143–150.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee TY and Chang HH: Longdan Xiegan Tang
has immuno-modulatory effects on CD4+CD25+ T cells and attenuates
pathological signs in MRL/lpr mice. Int J Mol Med. 25:677–685.
2010.PubMed/NCBI
|
|
15
|
Ou YY, Cao SX and Zhang J: Clinical
experience of decoction of gentian for purging liver fire
applicated in ophthalmology. Liao Ning Zhong Yi Yao Da Xue Xue Bao.
10:123–124. 2009.In Chinese.
|
|
16
|
Guide for Care and Use of Laboratory
Animals. (NIH Publication No. 80-23). Washington, D.C: National
Academy Press; 1996
|
|
17
|
The Association for Research in Vision and
Opthamology: Statement for the use of animals in ophthalmic and
visual research. http://www.arvo.org/About_ARVO/Policies/Statement_for_the_Use_of_Animals_in_Ophthalmic_and_Visual_Research/.
Accessed Jun 1, 2016.
|
|
18
|
Chinese Pharmacopeia. China Medical
Science Press; Beijing, China: 2010
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Zaidi AA, Ying GS, Daniel E, Gangaputra S,
Rosenbaum JT, Suhler EB, Thorne JE, Foster CS, Jabs DA, Levy-Clarke
GA, et al: Hypopyon in patients with uveitis. Ophthalmology.
117:366–372. 2010. View Article : Google Scholar :
|
|
21
|
Agarwal RK, Silver PB and Caspi RR: Rodent
models of experimental autoimmune uveitis. Methods Mol Biol.
900:443–469. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Raveney BJ, Copland DA, Dick AD and
Nicholson LB: TNFR1-dependent regulation of myeloid cell function
in experimental autoimmune uveoretinitis. J Immunol. 183:2321–2329.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kerr EC, Raveney BJ, Copland DA, Dick AD
and Nicholson LB: Analysis of retinal cellular infiltrate in
experimental autoimmune uveoretinitis reveals multiple regulatory
cell populations. J Autoimmun. 31:354–361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Caspi RR: Th1 and Th2 responses in
pathogenesis and regulation of experimental autoimmune
uveoretinitis. Int Rev Immunol. 21:197–208. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Crane IJ, Xu H, Wallace C, Manivannan A,
Mack M, Liversidge J, Marquez G, Sharp PF and Forrester JV:
Involvement of CCR5 in the passage of Th1-type cells across the
blood-retina barrier in experimental autoimmune uveitis. J Leukoc
Biol. 79:435–443. 2006. View Article : Google Scholar
|
|
26
|
Park H, Li Z, Yang XO, Chang SH, Nurieva
R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q and Dong C: A distinct
lineage of CD4 T cells regulates tissue inflammation by producing
interleukin 17. Nat Immunol. 6:1133–1141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Komiyama Y, Nakae S, Matsuki T, Nambu A,
Ishigame H, Kakuta S, Sudo K and Iwakura Y: IL-17 plays an
important role in the development of experimental autoimmune
encephalomyelitis. J Immunol. 177:566–573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Langrish CL, Chen Y, Blumenschein WM,
Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA and
Cua DJ: IL-23 drives a pathogenic T cell population that induces
autoimmune inflammation. J Exp Med. 201:233–240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cui Y, Shao H, Lan C, Nian H, O'Brien RL,
Born WK, Kaplan HJ and Sun D: Major role of gamma delta T cells in
the generation of IL-17+ uveitogenic T cells. J Immunol.
183:560–567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zuo A, Liang D, Shao H, Born WK, Kaplan HJ
and Sun D: In vivo priming of IL-17(+) uveitogenic T
cells is enhanced by Toll ligand receptor (TLR)2 and TLR4 agonists
via γδ T cell activation. Mol Immunol. 50:125–133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Peng Y, Han G, Shao H, Wang Y, Kaplan HJ
and Sun D: Characterization of IL-17+ interphotoreceptor
retinoid-binding protein-specific T cells in experimental
autoimmune uveitis. Invest Ophthalmol Vis Sci. 48:4153–4161. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tian Q, Bi H, Cui Y, Guo D, Xie X, Su W
and Wang X: Qing-kailing injection alleviates experimental
autoimmune uveitis in rats via inhibiting Th1 and Th17 effector
cells. Biol Pharm Bull. 35:1991–1996. 2012. View Article : Google Scholar
|
|
33
|
Agarwal RK, Horai R, Viley AM, Silver PB,
Grajewski RS, Su SB, Yazdani AT, Zhu W, Kronenberg M, Murray PJ, et
al: Abrogation of anti-retinal autoimmunity in IL-10 transgenic
mice due to reduced T cell priming and inhibition of disease
effector mechanisms. J Immunol. 180:5423–5429. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shao J, Tian L, Lei B, Wei L, Yang Y,
Kijlstra A and Yang P: AAV2-mediated subretinal gene transfer of
mIL-27p28 attenuates experimental autoimmune uveoretinitis in mice.
PLoS One. 7:e377732012. View Article : Google Scholar : PubMed/NCBI
|