Introduction
Approximately ten million people worldwide are
bilaterally blind due to corneal involvement (1,2). It
is the second most common cause of reversible blindness, following
cataracts. The majority of cases are connected with corneal
neovascularization (CNV). Various factors can induce CNV, including
aniridia, degeneration, inflammation, infection, extended contact
lens wear (3,4) and chemical or mechanical injuries
(5,6).
Chemical injury of the cornea is an ophthalmic
emergency and a common cause of corneal neovascularization and
blindness in the developing world. Treatment of the chemical
injuries include medical (7) and
surgical approaches (8–10). Numerous cases require surgical
intervention, particularly limbal stem cell transplantation. This
is possible in unilateral disease, when the contralateral cornea is
healthy or less damaged. However, there is a risk of limbal stem
cell deficiency development in the donor eye. Allogeneic limbal
transplantation has been conducted by a number of ophthalmologists,
however, results were not satisfactory and treatment requires
prolonged immunosuppressive therapy. Other sources of cells,
including conjunctival cells (11), buccal mucosal cells, embryonic stem
cells (12), induced pluripotent
stem cells and mesenchymal stem cells (13–16)
have been investigated for reconstruction of the limbal stem cell
niche.
Mesenchymal stem cells (MSCs) are a safe therapeutic
option and may be used for autologous transplantations. These stem
cells were first isolated from bone marrow, but later they were
identified in different tissues and organs, including adipose
tissue, dental pulp, amniotic fluid and the umbilical cord.
Previous studies have demonstrated MSCs have plasticity and can
differentiate into various cell types (17,18).
Furthermore, these cells can regulate immune response by regulation
of T cell (19), B cell and NK
cell activity (20), and can be
safely used for allogeneic transplantation.
MSCs have been used in numerous studies for
reconstitution of the corneal surface (10,14–16,21–30).
Certain previous studies have demonstrated transdifferentiation
ability into corneal cell lines (15,31–34).
Often amniotic membranes were used for stem cell expansion and
further transplantation into the cornea (16,23,25,26,30,35).
However, certain previous studies used supernatants or systemic
administration of stem cells. All these techniques are complicated,
time-consuming and not accessible in developing countries. Simple
subconjunctival injection of stem cells has been proposed by Yao
et al (28), which was
effective in corneal wound healing.
The aim of the present study was to further
investigate the role of MSCs in corneal neovascularization and
wound healing, and also to compare the effectiveness of two
administration routes, subconjunctival injection and
transplantation of amniotic membrane.
Materials and methods
Animals
Female Wistar rats (n=48; age, 6 weeks; weight,
150–180 g) were purchased from the Animal Center of Jilin
University (Changchun, China). All animal procedures were handled
according to the Association for Research in Vision and
Ophthalmology Statements for the Use of Animals in Ophthalmic and
Vision Research and were approved by Jilin University Animal Care
and Use Committee.
The animals were housed 2 per cage at a temperature
of 24–26°C, humidity 55–60% and a 12 h light/dark cycle, with ad
libitum access to food and water. The rats were anesthetized by
injection of 10% chloral hydrate (4 ml/kg). At the end of the study
(week 5), animals were sacrificed using an overdose of the
anesthetic (10 ml/kg).
Isolation, culture and characterization
of BMSCs
BMSCs were isolated and cultured according to the
previously described protocols (36). Briefly, the marrow cavity was
flushed with 1 ml Dulbecco's modified Eagle's medium (DMEM)/F12
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
medium with the bone marrow was transferred into 2 ml Eppendorf
tubes and centrifuged at 350 × g for 5 min at room temperature. The
supernatant was discarded and 2 ml fresh DMEM/F12 and 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), without
antibiotics, was added to the tube. The suspension was transferred
into 25 ml cell culture flasks and incubated at 37°C in a 5%
CO2 incubator. After 48 h, the medium was discarded,
with the non-adherent cells, and 3 ml fresh culture medium was
added to the flask. The medium was changed once every 3 days, until
cells reached 80–90% confluence. Expression of cluster of
differentiation (CD)90, CD45, CD11b and CD44 was detected by flow
cytometry. Briefly, passage 3 cells were trypsinized, centrifuged
at 60 × g for 5 min at room temperature and were washed with three
times with phosphate-buffered saline (PBS). Diluted monoclonal
mouse anti-CD11b (cat. no. CBL1512 CB11; 1:200; EMD Millipore,
Billerica, MA, USA) and monoclonal mouse anti-CD44 (eBioscience,
Inc., San Diego, USA) were added to the cells and incubated at 4°C
for 1 h. PBS was added to the control group. After 1 h, the cells
were washed with three times PBS for 5 min. Fluorescein
isothiocyanate-conjugated goat anti-rabbit secondary antibody (cat.
no. 31635; 1:200; Thermo Fisher Scientific, Inc.) was added to the
cells and incubated at 4°C for 30 min. The cells were washed 3
times with PBS prior to detection by Epics XL flow cytometer
(Beckman Coulter, Inc., Brea, CA, USA).
For CD90 and CD45 direct labeling was conducted with
diluted monoclonal mouse anti-CD90(cat. no. 554897; 1:200; BD
Biosciences, Franklin Lakes, NJ, USA) and monoclonal mouse
anti-CD45 antibodies (cat. no. 11-0461; 1:50; eBioscience, Inc.) to
washed cell suspension, which was incubated at 4°C for 60 min,
washed with PBS and detected by flow cytometer.
Transduction of BMSCs with
lentivirus
BMSCs from passage 2 were transduced with the green
fluorescent protein (GFP) gene using a lentivirus system
(ViraPower™ Lentiviral Packaging mix; Invitrogen; Thermo Fisher
Scientific, Inc.) in order to follow the movement of transplanted
cells.
Optimal multiplicity of infection (MOI) was
determined in preliminary experiments. The toxicity and
transduction efficacy was compared among MOI 1, 5, 10, 50 and 100,
an MOI of 10 was demonstrated to be optimal. To conduct the
transduction, BMSCs from passage 2 were added to 24 well plate
(1×105 cells/well) with 500 μl DMEM/F12 and 10%
FBS, and incubated at 37°C in 5% CO2 overnight.
Subsequently, diluted virus was added into the wells with 5
μl/ml Polybrene (Invitrogen; Thermo Fisher Scientific, Inc.)
with 100 μl DMEM/F12 and 10% FBS and the cells were
incubated overnight. After 24 h, the medium was changed and on day
4, 10 ng/ml puromycin (Invitrogen; Thermo Fisher Scientific, Inc.)
was added to each well to produce a homogenous generation of
transduced cells. On the following day the medium was changed again
and on day 6 the transduction effect was observed using inverted
fluorescence microscopy (Olympus Corporation, Tokyo, Japan). Second
generation transduced cells were used in the following
experiments.
Culture of BMSCs on denuded amniotic
membrane
Following obtaining informed consent, human amniotic
membranes (HAM) were obtained from healthy pregnant women during
their planned caesarean sections. Under sterile conditions, the
chorion was manually separated from the amniotic membrane and all
remaining blood and chorionic membrane pieces were removed. The HAM
was cut into 2×2 mm2 pieces and trypsin/EDTA
(Sigma-Aldrich, St. Louis, MO, USA) was added to each piece and
incubated at 37°C in 5% CO2 for 30 min. The detached
epithelial layer was removed using a cell scrubber in a gentle
manner. The pieces were stained with 4′,6-diamidino-2-phenylindole
(DAPI; Sigma-Aldrich, St. Louis, MO, USA) and examined by confocal
microscopy (Olympus Corporation).
Following confirmation that the HAMs were fully
denuded, second generation transduced BMSCs were seeded onto the
membranes (1×105 cells/piece). The air-lifting culture
technique was used with little culture medium. After 4 days, cells
reached confluence and DAPI was used to visualize the nuclei of the
cells grown on the denuded (d)-HAM, and examination was conducted
under confocal laser scanning microscopy. Following confirmation of
positive results, the membranes seeded with BMSCs were used for
transplantation.
Preparation of animal models and
transplantation
All animal experiments were handled according to the
animal protocols approved by Jilin University Animal Care and Use
Committee. The Wistar rats were anesthetized by injection of 10%
chloral hydrate (4 ml/kg) and anesthetic eye drops were applied for
topical anesthesia. Circular discs (diameter, 3 mm) from filter
paper were soaked in 0.4 ml 1 mol/l sodium hydroxide solution
(Beijing Dingguo Changsheng Biotechnology Co., Ltd., Beijing,
China). The disc was applied to the center of the cornea of the
right eye for 30 sec, following removal of the disc, the surface of
the eye was thoroughly washed by 60 ml normal saline for 1 min. The
eyes were examined daily by penlight. The criteria presented in
Table II were used for
classification of the severity of injury. After 7 days, the rats
were anesthetized and the eyes were examined under a slit-lamp
microscope following the addition of fluorescein dye using Oftan
Flurekain eye drops (Santen Oy, Tampere, Finland). Preliminary data
were recorded and the rats received transplantation. The rats used
in the present study (n=48) were divided into 3 groups as follows:
i) Group 1 (n=16) received subconjunctival injection of BMSCs
suspended in PBS; ii) group 2 (n=16) received amniotic membrane
transplantation seeded with BMSCs; and iii) group 3 (n=16) received
no treatment and served as the control group.
 | Table IIEvaluation score for the corneal
chemical injury. |
Table II
Evaluation score for the corneal
chemical injury.
| Score | 0 | 1 | 2 | 3 | 4 |
|---|
| Corneal
opacity | No opacity,
transparent cornea | Slight opacity,
visible pupil and iris texture | Moderate opacity,
iris texture is unclear, but pupillary margin is visible | Severe opacity,
pupillary margin is visible vaguely | Very severe
opacity, nothing can be visualized under cornea |
| Epithelial
defect | No defect | ≤1/4 of the corneal
surface | 1/4–1/2 of the
corneal surface | 1/2–3/4 of the
corneal surface | ≥3/4 of the corneal
surface |
|
Neovascularization | No vessel | 2 mm within
limbus | ≤1/2 of the corneal
surface | ≥1/2 of the corneal
surface | Total surface of
the corneal surface |
Cells from the second generation of
lentiviral-transduced BMSCs (1×106) were centrifuged and
washed with PBS three times and resuspended in 0.1 ml PBS.
Anesthetized rats from group 1 received subconjunctival injection
of 0.1 ml PBS containing cells by 1 ml insulin syringe under a
ophthalmic surgical microscope. The pieces of d-HAM seeded with
BMSCs were divided into 4 pieces (1×1 mm2) and
anesthetized rats from group 2 received d-HAM transplantation.
Following application of topical anesthetic, the amniotic membrane
was placed on the cornea and sutured by six interrupted 10.0 nylon
sutures with the epithelial side facing up. Thereafter, eyelids
were sutured together by 6.0 nylon sutures. The rats were examined
daily with pen light and weekly with a slit-lamp for 4 weeks.
Eyelids were opened after 5 days, and the majority of the amniotic
membranes were absorbed. After 4 weeks, the animals were sacrificed
by chloral hydrate overdose and the corneas were excised for use in
the experiments.
Preparation of corneal flat mounts
following ink perfusion
This step used five rats from each group. Following
anesthesia, the thoracic cavity was opened and thoracic aorta was
separated from the surrounding tissue using dissecting scissors to
avoid wall disruption. The thoracic aorta was ligated. An
intravenous cannula (or catheter) was inserted into the aorta above
the ligated site. The right atrium was cut to allow blood outflow
and 60 ml normal saline was used to clear the vessels of blood.
When the fluid from the atrium was cleared, 10–20 ml ink was
injected. The injection was stopped when the eyes, ears and upper
limbs of the rat were observed to be staining dark black. The
catheter was then removed and the eyeballs were enucleated and
post-fixed in 4% paraformaldehyde for 3 h. The corneas were
incised, flattened by four incisions and mounted on glass slides.
Images of the slides were captured by a camera attached to the
light microscope (Olympus Corporation) and analyzed using Image-Pro
Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD,
USA).
Cryosections
Cryosectioning was conducted with three eyes from
each group. Fresh, enucleated unfixed eyes were frozen in optimal
cutting temperature compound (Sakura Finetek USA, Inc., Torrance,
CA, USA) on a tissue mold in the cryostat (Leica Microsystems GmbH,
Wetzlar, Germany). Sections were cut at 8-μm at −20°C in
cryostat and divided into three groups, sections were fixed and
dehydrated and stained with hematoxylin and eosin, immediately
examined under fluorescent microscope for labeled BMSC detection
without fixation or dehydration, or used for immunochemical
analyzes to detect vascular endothelial growth factor (VEGF) and
matrix metalloproteinase 9 (MMP-9) levels.
Enzyme-linked immunosorbent assay
(ELISA)
VEGF protein level was detected using an Rat VEGF
Quantikine ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA).
From each group 4 corneas were excised, cut into small pieces and
homogenized using a glass mortar. Subsequently, 400 μl
radioimmunoprecipitation assay buffer containing 1% PMSF
(Sigma-Aldrich) was added to the pieces and incubated for 30 min on
the ice. The mixture was treated with an ultrasonic homogenizer,
centrifuged at 350 × g for 5 min at 4°C, and the supernatant was
collected for further procedures. The ELISA was performed according
to the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from four eyes from each
group using an RNAiso Plus kit (Takara Biotechnology Co., Ltd.,
Dalian, China) according to the manufacturer's protocol. The mRNA
integrity was confirmed by 1.5% agarose gel electrophoresis.
Following extraction of the mRNA, reverse
transcription was performed to synthesize cDNA according to the
manufacturer's protocol using One Step SYBR PrimeScript Plus RT-PCR
kit (Takara Biotechnology Co., Ltd.). PCR was conducted using SYBR
Green and designed primers from Sangon Biotech (Table I). Each reaction required 1
μl of the previously synthesized cDNA, which was mixed with
SGExcelFastSYBR mix, containing ROX (Sangon Company, Shanghai,
China), forward and reverse primers, and RNase-free water. The qPCR
was performed in a LightCycler 480 II (Roche Diagnostics, Basel,
Switzerland) using the following program: 72°C for 5 min; 94°C for
3 min, stage 3: 94°C for 20 sec, 57°C for 20 sec and 72°C for 20
sec for 40 cycles and 72°C for 3 min. Analysis was performed with
LightCycler software, version 1.5.0, which allowed precise analysis
of data. Each sample was examined in triplicate.
 | Table ISequences of the primers. |
Table I
Sequences of the primers.
| Gene | Primer (5′ to 3′)
|
|---|
| Forward | Reverse |
|---|
| VEGF |
CTTGAGTTGGGAGGAGGATG |
TGGCAGGCAAACAGACTTC |
| MMP-9 |
ACCCTGCGTATTTCCATTCA |
ATCTCTCCTGCCGAGTTGC |
| TLR-2 |
AAACTGTGTTCGTGCTTTCTG |
GCGTCATTGTTCTCGTCAAA |
| TLR-4 |
TGGCATCATCTTCATTGTCC |
CAGAGCATTGTCCTCCCACT |
| β-actin |
GCTACAGCTTCACCACCACA |
ATCGTACTCCTGCTTGCTGA |
Statistical analysis
Clinical results were calculated using Welch's
analysis of variance test with Dunnett's T3 post-hoc test. Mean
values and standard deviations were calculated for vessel length
and the Kruskal-Wallis non-parametric test was used to compare mean
values of the three groups. Nonparametric median test was used to
compare qPCR and ELISA test results in three groups. All analyses
were conducted using SPSS 22.0 (IBM SPSS, Armonk, NY, USA) P≤0.05
was considered to indicate a statistically significant
difference.
Results
BMSCs were isolated and
characterized
After 48 h isolation, the cells were washed with PBS
and non-adherent cells were washed out of the flasks. A number of
small colonies of adherent cells were scattered across the bottom
of the flasks. After 2–3 days, the cells began to grow and
elongate, becoming a spindle shape similar to fibroblasts.
Following 2–3 subcultures, cells had typical phenotypic
characteristics of BMSCs, they were attached to the bottom of the
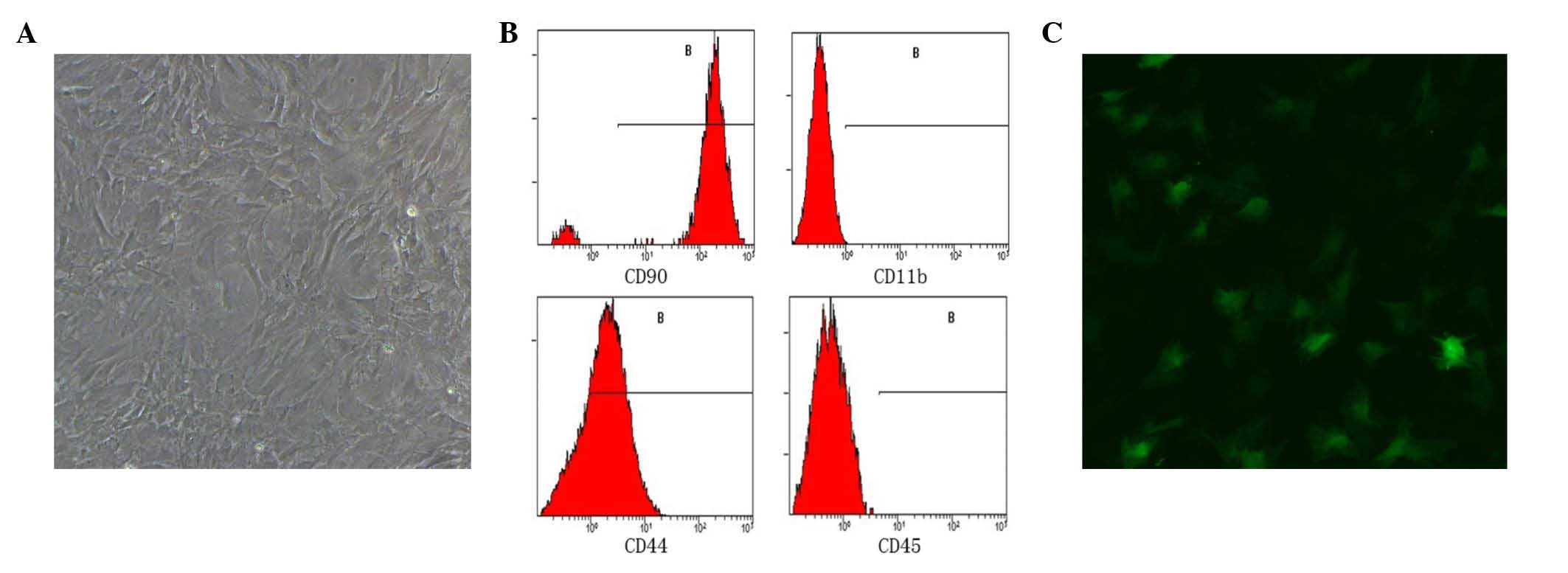
flask with a spindle-like shape and self-renewal ability (Fig. 1A).
The flow cytometry results demonstrated that 95% of
the cells were positive for CD90 expression, 75% were positive for
CD44 expression, and expression of CD11b and CD45 were positive in
only in 0.5 and 0.2% of cells, respectively (Fig. 1B). This pattern of surface marker
expression is consistent with previous descriptions of BMSCs
(17,18,20).
Cell transduction with lentivirus was
successful
After 6 days, transduction cultures were examined
under fluorescence microscopy. Detection of GFP provided evidence
of successful transduction and efficacy was ~50–60%. At MOI 10, the
virus did not result in toxicity and did not affect the
self-renewal ability of the cells (Fig. 1C).
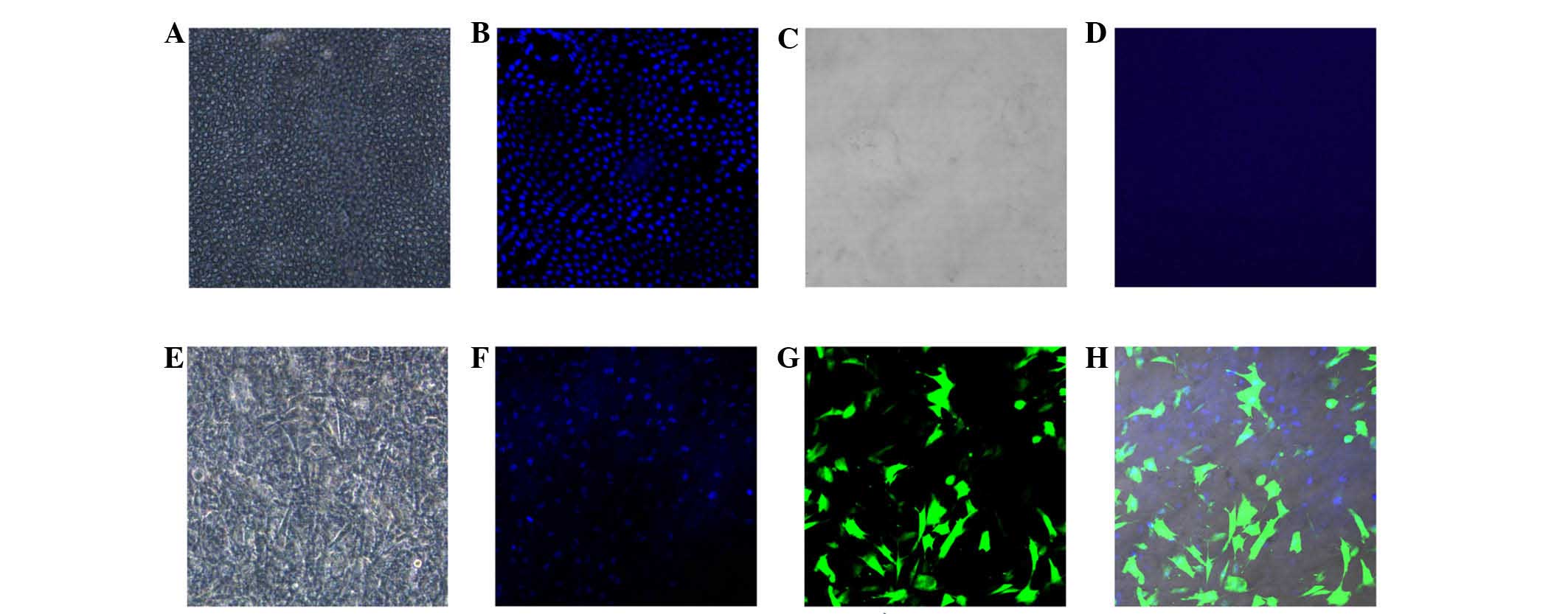
BMSCs were cultured on denuded amniotic
membrane
The epithelial layer of the amniotic membrane was
removed by enzymatic and mechanical processes. The membranes were
stained with DAPI and examined under a confocal laser scanning
microscope. Examination indicated that all epithelial cells were
effectively removed from the membranes as no DAPI stained cells
were observed on the surface of the d-HAM.
Seeded BMSCs exhibited exponential growth on d-HAM
and reached confluence within 4 days. Confocal microscopy was used
to detect mesenchymal stem cells expressing GFP on the amniotic
membranes (Fig. 2).
The rat model was successfully produced
with no significant difference in level of injury
A total 48 rats were used in the present study.
Slit-lamp examination was conducted on the day of injury and then
weekly until week 5 (Fig. 3).
Examination on day 1 demonstrated a total epithelial defect at the
applied disc area, which stained with fluorescein dye. After 7 days
the areas were again stained with the dye in all rats.
Three key characteristics were measured, corneal
opacity, area of the neovascularization, and area of the epithelial
defect stained with fluorescein. A four scale grading system was
used to classify the severity of injury (Table II).
The animals were randomly divided into three groups.
Statistical analysis was conducted on data from all the groups to
compare the level of injury in each group. The result demonstrated
there was no statistically significant difference between groups
[P=0.11, confidence limit (CL) 95%] with all animals developing
middle to severe level injury. Following treatment, the corneas
were examined daily with pen light and weekly with slit-lamp for 4
weeks. At week 4, none of the animals exhibited any adverse
reaction to the treatments. Following the final results were
obtained in week 4, the animals were sacrificed. Statistical
analysis indicated a statistically significant difference in
severity within the 3 groups [P=1.9×10−5, confidence
interval (CI) 95%] and post-hoc tests indicated that the
differences were between group 1 with group 2 (P=0.00016, CI 95%),
and group 1 and group 3 (P=0.00037, CI 95%) were also statistically
significant. Thus, a single subconjunctival injection of BMSCs had
greater impact on corneal wound healing, than BMSCs on amniotic
membrane transplantation (Fig.
3).
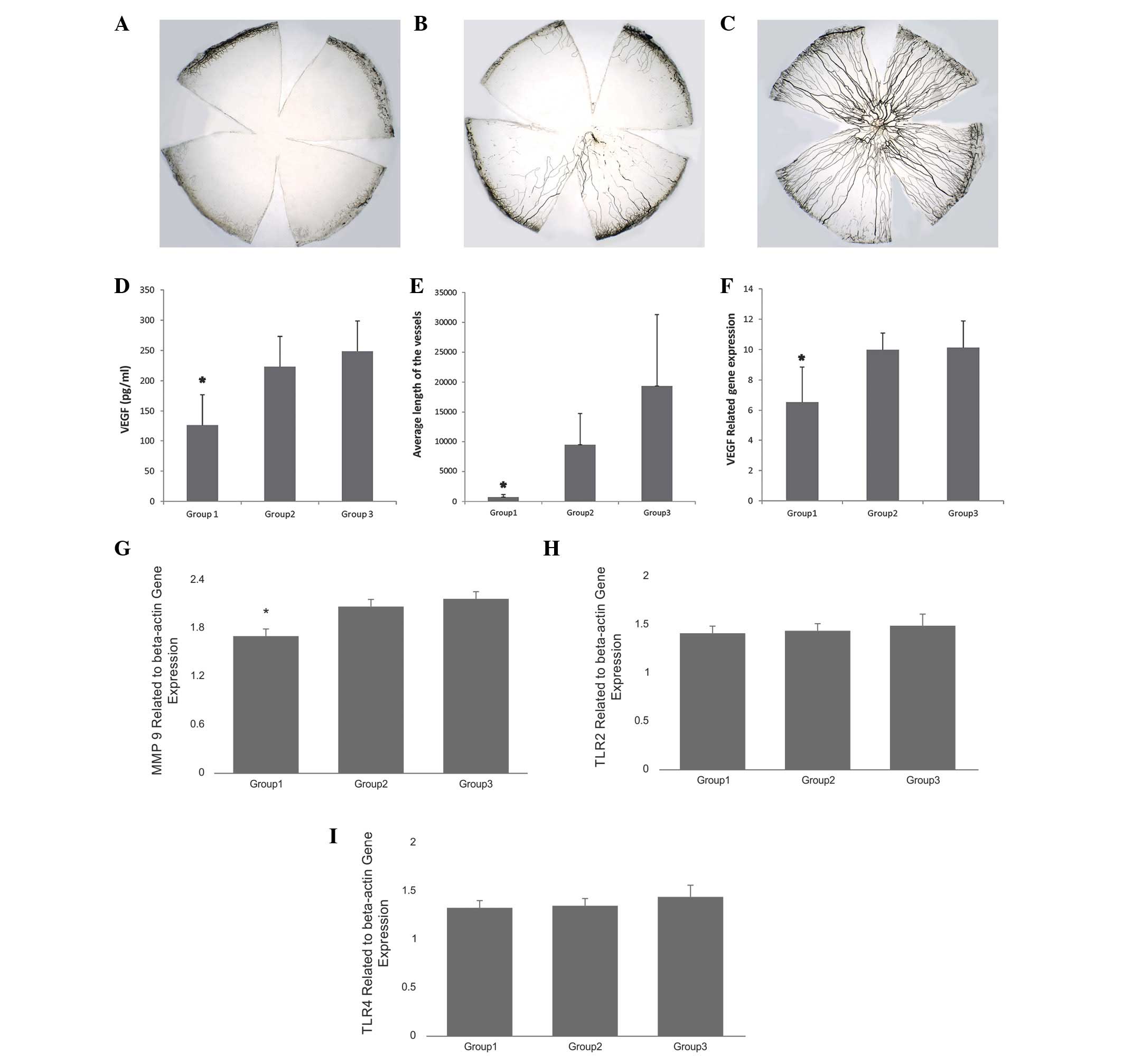
Length of the vessel, level of VEGF and
gene expression of VEGF, MMP-9, Toll-like receptor (TLR)2 and TLR4
were different among the three groups
Ink perfusion was used for improved visualization of
new vessels. In total, images of 15 flat mounts were captured by a
digital camera attached to a light microscope. The images were
processed with Image-Pro Plus 6.0 computer software. Area of
neovascularization and total length for each sample was calculated
and used for statistical analysis. The results demonstrated that
the mean length of the vessels was significantly different among
the 3 groups (χ2 =10.50, P=0.005, CL 95%). The level of
VEGF protein and gene expression also demonstrated similar
differences (Fig. 4). The
difference among the 3 groups was significant with P=0.02, CI 95%
and according to the post-hoc tests difference was observed between
groups 1 and 2 and groups 1 and 3.
VEGF expression significantly decreased
following treatment
Results were determined relative to the reference
gene, actin, and the level of expression of VEGF was demonstrated
to be statistically significant between group 1 and group 2, and
group 1 and group 3 (P=0.03, CI 95%; Fig. 4F). The expression of MMP-9 was
significantly decreased within the 3 groups, and a difference was
observed within group 1 and 2 (P=0.004, CI 95%) and group 1 and 3
(P=0.001, CI 95%; Fig. 4G). No
difference in TLR2 and TLR4 gene expression was observed between
the 3 groups (P=0.06, CI 95%; Fig. 4H
and I).
Histopathology and immunochemistry
Cryosections stained with hematoxylin and eosin
demonstrated that the corneas from the group 1 were thinner, the
stromas were compact with reduced infiltration of inflammatory
cells, and the epithelial layers were fully recovered (Fig. 5). In groups 2 and 3, new vessels
were marked in the sections from the central area of the cornea,
which were absent in group 1.
To be able to track the movement and localization of
the cells, GFP was expressed in the cells. Fresh, non-fixed
cryosections were immediately examined under a confocal laser
scanning microscope. GFP-positive cells were not detected in the
cornea. Thus, the present study suggests stem cells did not migrate
into the cornea or transdifferentiate into limbal or corneal cell
lines.
Discussion
Corneal blindness has a large impact on society.
However, unlike retinal degeneration and glaucoma, it is reversible
and the majority of cases of corneal blindness are treatable and
preventable.
A number triggers affect the cornea and result in
corneal neovascularization, including infection, degeneration, and
injury. People in developing countries are more susceptible to
these triggers, however, extended contact lens wear is also a risk
factor.
The majority of CNV cases require surgical
intervention and transplantation is a common therapeutic strategy.
These transplantation of a whole cornea or its layers, amniotic
membranes, conjunctiva, cultured oral mucosal epithelial cell
sheets and limbal stem cells. Transplantation of the whole cornea
or certain parts is a common treatment method worldwide. It has 95%
success rate, however, for patients with already neovascularized
corneal beds, the success rate decreases to 50%. In addition, there
is a shortage of donor corneal tissues in the majority of
countries, making corneal transplantation an expensive and
difficult method, in addition to the requirement for
immunosuppression. Thus, medical specialists are aiming to develop
novel treatment methods, which can be effective and safe for a
greater number of patients. Mesenchymal stem cells are a promising
source of cells for transplantation, as they do not raise ethical
issues, have low tumorigenesis potential, and they can be used for
autologous and allogeneic transplantation. Transplantation of
mesenchymal stem cells is also a good option for treatment of
corneal injuries. Previous studies have reported the beneficial
effect of MSCs in corneal wound healing and neovascularization
(24,26,28–30).
The majority of the cases used HAM as a bio-scaffold for culture
and transplantation of stem cells. However, this technique can be
complicated, while subconjunctival injection is a simple technique
used in ophthalmic clinics on a daily basis. Thus, direct injection
of stem cells under the conjunctiva near the limbus may be a simple
and safe method of cell transplantation. Yao et al (28) confirmed that simple subconjunctival
injection enhanced corneal wound healing and decreased
neovascularization. This procedure can be repeated a number of
times. Thus, the present study aimed to investigate this treatment
option and to compare the effectiveness and feasibility of two
possible application routes, direct subconjunctival injection of
stem cells and transplantation on d-HAM. To the best of our
knowledge, this is the first paper to discuss the comparison of
these two main routes.
The results of the present study demonstrated that
mesenchymal stem cells enhance corneal wound healing and decrease
neovascularization (P=1.9×10−5, CI 95%). Notably, the
comparison of two application routes demonstrated that the
subconjunctival injection is more effective than transplantation
with the amniotic membrane (P=0.00016, CI 95%). This may be due to
the short survival time of amniotic membranes on the injured
corneas. The difference in clinical outcome was predominantly
significant for neovascularization, rather than for epithelial
defect or opacity. The expression of VEGF was reduced in the
treatment groups, particularly in the subconjunctival injection
group.
VEGF and MMP-9 gene expression was reduced in the
treatment groups compared with the control group. The gene
expression levels of TLR2 and TLR4 were close to equal in the 3
groups. Due to the absence of previous studies on these markers,
the present study may have observed this outcome as a result of the
prolonged observation period. TLR2 and TLR4 are components of
innate immune system and are involved in the initiation of the
inflammatory process. Thus, TLR2 and TLR4 had returned to their
normal level of expression following 5 weeks.
In order to track the movement of the transplanted
cells, they were transduced with the GFP gene using lentiviral
vectors. Following transplantation (after 4 weeks) no GFP-positive
cells were observed in the corneal sections. Thus, the present
study hypothesizes that the underlying mechanism of action was
trophic factor production and regulation of the inflammatory
process and angiogenesis. Histopathology indicated reduced
infiltration of inflammatory cells and neovascularization in the
treatment groups, compared with the control.
None of the animals in the present study exhibited
an adverse reaction to the treatment. Transplantation of allogeneic
cells was successful without episodes of immune rejection.
Corneal blindness is the second leading cause of
blindness worldwide and is an important health burden. A number of
treatment methods have been implemented in ophthalmic clinics for
treatment of CNV, however, for certain patients none of the current
options are acceptable. The majority of modern therapeutic
strategies are expensive and require specific equipment and skilled
professionals. Developing countries, in particular, have a shortage
of those and a high rate of corneal involvement. The development of
inexpensive, feasible and effective treatment methods in these
areas are key. Subconjunctival injection is a simple technique
routinely used in eye centers around the world. Mesenchymal stem
cells can be easily obtained from 'medical wastes', including ex
placenta and amniotic fluid after delivery, adipose tissue after
liposuction and be safely used in autologous and allogeneic
transplantations. They have been used in various animal studies and
numerous clinical trials. All reports on their safety issues have
been satisfactory.
In conclusion, the present study demonstrated that
single subconjunctival injection of stem cells is more effective,
than their transplantation using amniotic membranes. This method
requires further investigation and there are two currently running
clinical trials using mesenchymal stem cells in corneal disease.
However, further clinical trials must be conducted prior to gaining
approval for use in clinical practice. This method may fulfill
requirements for an effective treatment and has potential to be in
clinical use in the near future.
Acknowledgments
The present study was supported by the NSFC Fund,
and Science and Technology Department (grant no. 20130413025GH) and
Health Department of Jilin Province (grant no. 2013Q005) funds.
References
|
1
|
Robaei D and Watson S: Corneal blindness:
A global problem. Clin Experiment Ophthalmol. 42:213–214. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Whitcher JP, Srinivasan M and Upadhyay MP:
Corneal blindness: A global perspective. Bull World Health Organ.
79:214–221. 2001.PubMed/NCBI
|
|
3
|
Alvord LA, Hall WJ, Keyes LD, Morgan CF
and Winterton LC: Corneal oxygen distribution with contact lens
wear. Cornea. 26:654–664. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martins TG, Costa AL, Alves MR and
Gonçalves MM: Contact lens use with intraestromal hemorrhage
secondary to corneal neovascularization. Einstein (Sao Paulo).
10:524–525. 2012.In English, Portuguese. View Article : Google Scholar
|
|
5
|
Cursiefen C, Küchle M and Naumann GO:
Angiogenesis in corneal diseases: Histopathologic evaluation of 254
human corneal buttons with neovascularization. Cornea. 17:611–613.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giacomini C, Ferrari G, Bignami F and Rama
P: Alkali burn versus suture-induced corneal neovascularization in
C57BL/6 mice: An overview of two common animal models of corneal
neovascularization. Exp Eye Res. 121:1–4. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hsu CC, Chang HM, Lin TC, Hung KH, Chien
KH, Chen SY, Chen SN and Chen YT: Corneal neovascularization and
contemporary antiangiogenic therapeutics. J Chin Med Assoc.
78:323–330. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kenyon KR and Tseng SC: Limbal autograft
transplantation for ocular surface disorders. O. phthalmology.
96:709–722; discussion 722–723. 1989. View Article : Google Scholar
|
|
9
|
Kolli S, Ahmad S, Mudhar HS, Meeny A, Lako
M and Figueiredo FC: Successful application of ex vivo expanded
human autologous oral mucosal epithelium for the treatment of total
bilateral limbal stem cell deficiency. Stem Cells. 32:2135–2146.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JY, Jeong HJ, Kim MK and Wee WR: Bone
marrow-derived mesenchymal stem cells affect immunologic profiling
of interleukin-17-secreting cells in a chemical burn mouse model.
Korean J Ophthalmol. 28:246–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kwitko S, Marinho D, Barcaro S, Bocaccio
F, Rymer S, Fernandes S and Neumann J: Allograft conjunctival
transplantation for bilateral ocular surface disorders.
Ophthalmology. 102:1020–1025. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chan AA, Hertsenberg AJ, Funderburgh ML,
Mann MM, Du Y, Davoli KA, Mich-Basso JD, Yang L and Funderburgh JL:
Differentiation of human embryonic stem cells into cells with
corneal keratocyte phenotype. PLoS One. 8:e568312013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arnalich-Montiel F, Pastor S,
Blazquez-Martinez A, Fernandez-Delgado J, Nistal M, Alio JL and De
Miguel MP: Adipose-derived stem cells are a source for cell therapy
of the corneal stroma. Stem Cells. 26:570–579. 2008. View Article : Google Scholar
|
|
14
|
Cejkova J, Trosan P, Cejka C, Lencova A,
Zajicova A, Javorkova E, Kubinova S, Sykova E and Holan V:
Suppression of alkali-induced oxidative injury in the cornea by
mesenchymal stem cells growing on nanofiber scaffolds and
transferred onto the damaged corneal surface. Exp Eye Res.
116:312–323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gu S, Xing C, Han J, Tso MO and Hong J:
Differentiation of rabbit bone marrow mesenchymal stem cells into
corneal epithelial cells in vivo and ex vivo. Mol Vis. 15:99–107.
2009.PubMed/NCBI
|
|
16
|
Jiang TS, Cai L, Ji WY, Hui YN, Wang YS,
Hu D and Zhu J: Reconstruction of the corneal epithelium with
induced marrow mesenchymal stem cells in rats. Mol Vis.
16:1304–1316. 2010.PubMed/NCBI
|
|
17
|
Bianco P, Riminucci M, Gronthos S and
Robey PG: Bone marrow stromal stem cells: Nature, biology, and
potential applications. Stem Cells. 19:180–192. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Herzog EL, Chai L and Krause DS:
Plasticity of marrow-derived stem cells. Blood. 102:3483–3493.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tse WT, Pendleton JD, Beyer WM, Egalka MC
and Guinan EC: Suppression of allogeneic T-cell proliferation by
human marrow stromal cells: Implications in transplantation.
Transplantation. 75:389–397. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ribeiro A, Laranjeira P, Mendes S, Velada
I, Leite C, Andrade P, Santos F, Henriques A, Grãos M, Cardoso CM,
et al: Mesenchymal stem cells from umbilical cord matrix, adipose
tissue and bone marrow exhibit different capability to suppress
peripheral blood B, natural killer and T cells. Stem Cell Res Ther.
4:1252013. View
Article : Google Scholar :
|
|
21
|
Ma Y, Xu Y, Xiao Z, Yang W, Zhang C, Song
E, Du Y and Li L: Reconstruction of chemically burned rat corneal
surface by bone marrow-derived human mesenchymal stem cells. Stem
Cells. 24:315–321. 2006. View Article : Google Scholar
|
|
22
|
Guo T, Wang W, Zhang J, Chen X, Li BZ and
Li LS: Experimental study on repairing damage of corneal surface by
mesenchymal stem cells transplantation. Zhonghua Yan Ke Za Zhi.
42:246–250. 2006.In Chinese. PubMed/NCBI
|
|
23
|
Ye J, Yao K and Kim JC: Mesenchymal stem
cell transplantation in a rabbit corneal alkali burn model:
Engraftment and involvement in wound healing. Eye (Lond).
20:482–490. 2006. View Article : Google Scholar
|
|
24
|
Liu XW and Zhao JL: Transplantation of
autologous bone marrow mesenchymal stem cells for the treatment of
corneal endothelium damages in rabbits. Zhonghua Yan Ke Za Zhi.
43:540–545. 2007.In Chinese. PubMed/NCBI
|
|
25
|
Oh JY, Kim MK, Shin MS, Lee HJ, Ko JH, Wee
WR and Lee JH: The anti-inflammatory and anti-angiogenic role of
mesenchymal stem cells in corneal wound healing following chemical
injury. Stem Cells. 26:1047–1055. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Reinshagen H, Auw-Haedrich C, Sorg RV,
Boehringer D, Eberwein P, Schwartzkopff J, Sundmacher R and
Reinhard T: Corneal surface reconstruction using adult mesenchymal
stem cells in experimental limbal stem cell deficiency in rabbits.
Acta Ophthalmol. 89:741–748. 2011. View Article : Google Scholar
|
|
27
|
Liu H, Zhang J, Liu CY, Hayashi Y and Kao
WW: Bone marrow mesenchymal stem cells can differentiate and assume
corneal keratocyte phenotype. J Cell Mol Med. 16:1114–1124. 2012.
View Article : Google Scholar
|
|
28
|
Yao L, Li ZR, Su WR, Li YP, Lin ML, Zhang
WX, Liu Y, Wan Q and Liang D: Role of mesenchymal stem cells on
cornea wound healing induced by acute alkali burn. PLoS One.
7:e308422012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pinarli FA, Okten G, Beden U, Fışgın T,
Kefeli M, Kara N, Duru F and Tomak L: Keratinocyte growth factor-2
and autologous serum potentiate the regenerative effect of
mesenchymal stem cells in cornea damage in rats. Int J Ophthalmol.
7:211–219. 2014.PubMed/NCBI
|
|
30
|
Rohaina CM, Then KY, Ng AM, Wan Abdul
Halim WH, Zahidin AZ, Saim A and Idrus RB: Reconstruction of limbal
stem cell deficient corneal surface with induced human bone marrow
mesenchymal stem cells on amniotic membrane. Transl Res.
163:200–210. 2014. View Article : Google Scholar
|
|
31
|
Du Y, Roh DS, Funderburgh ML, Mann MM,
Marra KG, Rubin JP, Li X and Funderburgh JL: Adipose-derived stem
cells differentiate to keratocytes in vitro. Mol Vis. 16:2680–2689.
2010.PubMed/NCBI
|
|
32
|
Martinez-Conesa EM, Espel E, Reina M and
Casaroli-Marano RP: Characterization of ocular surface epithelial
and progenitor cell markers in human adipose stromal cells derived
from lipoaspirates. Invest Ophthalmol Vis Sci. 53:513–520. 2012.
View Article : Google Scholar :
|
|
33
|
Monteiro BG, Serafim RC, Melo GB, Silva
MC, Lizier NF, Maranduba CM, Smith RL, Kerkis A, Cerruti H, Gomes
JA and Kerkis I: Human immature dental pulp stem cells share key
characteristic features with limbal stem cells. Cell Prolif.
42:587–594. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nieto-Miguel T, Galindo S, Reinoso R,
Corell A, Martino M, Pérez-Simón JA and Calonge M: In vitro
simulation of corneal epithelium microenvironment induces a corneal
epithelial-like cell phenotype from human adipose tissue
mesenchymal stem cells. Curr Eye Res. 38:933–944. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zeppieri M, Salvetat ML, Beltrami AP,
Cesselli D, Bergamin N, Russo R, Cavaliere F, Varano GP, Alcalde I,
Merayo J, et al: Human adipose-derived stem cells for the treatment
of chemically burned rat cornea: Preliminary results. Curr Eye Res.
38:451–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Soleimani M and Nadri S: A protocol for
isolation and culture of mesenchymal stem cells from mouse bone
marrow. Nat Protoc. 4:102–106. 2009. View Article : Google Scholar : PubMed/NCBI
|