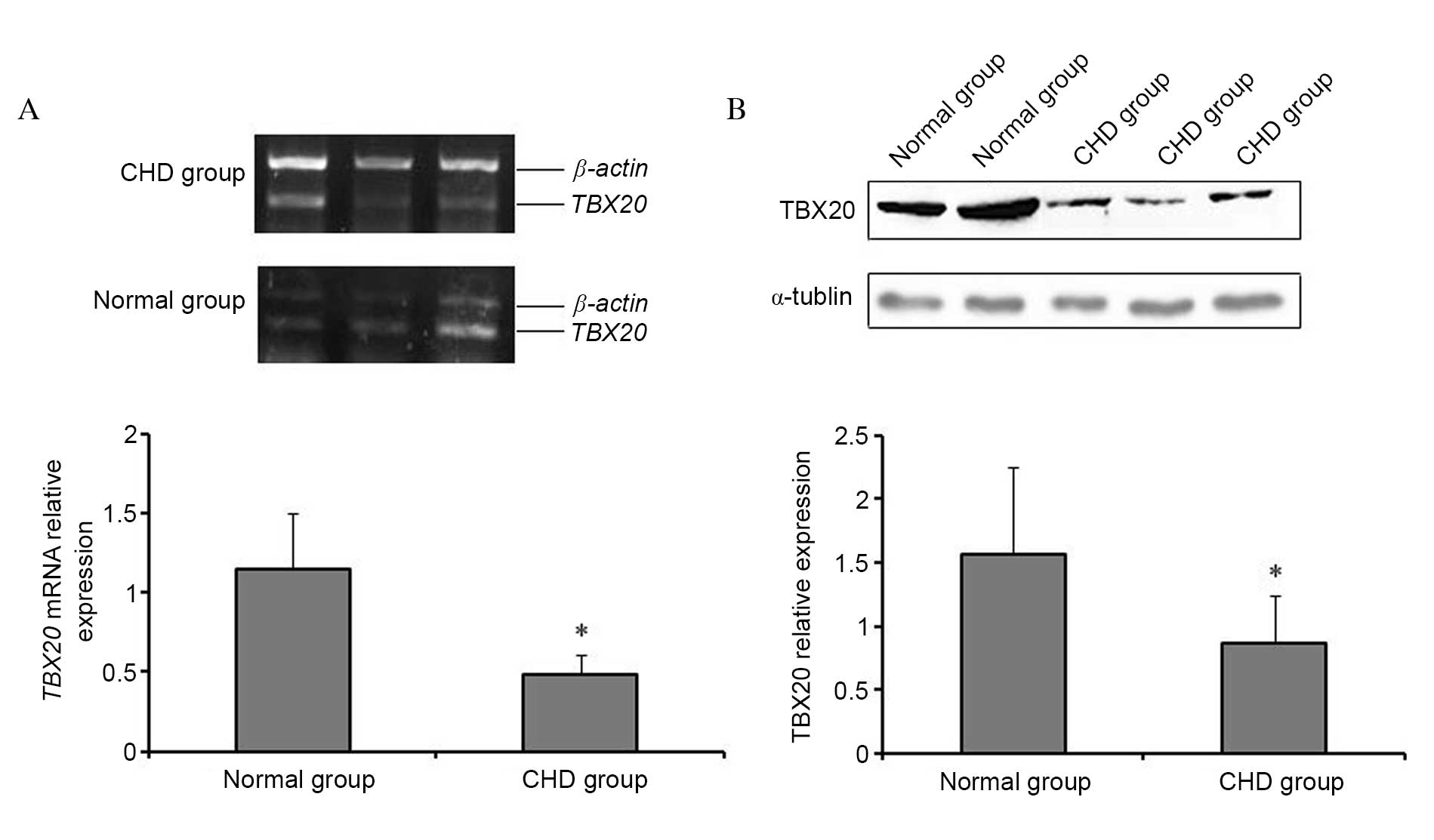
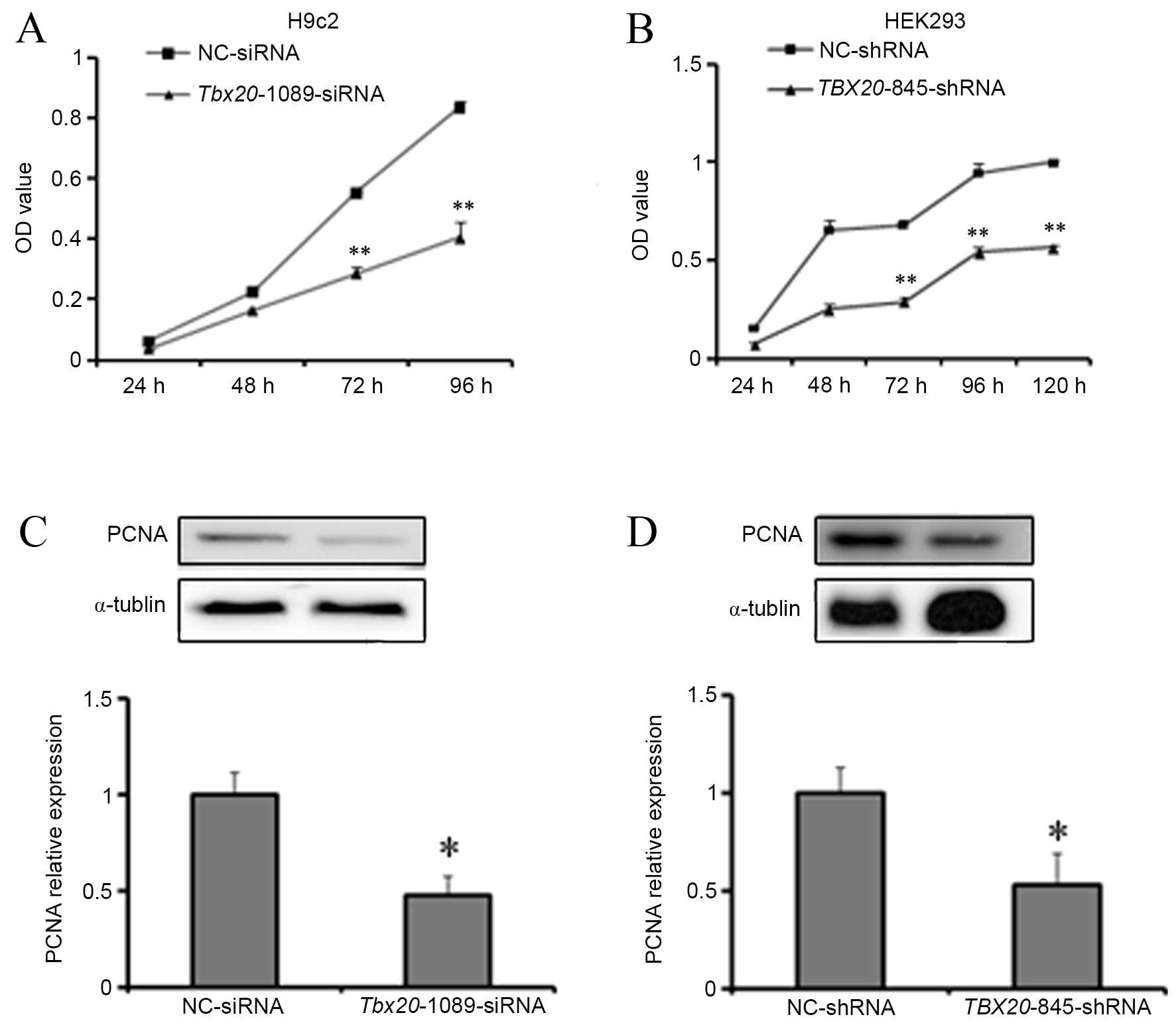
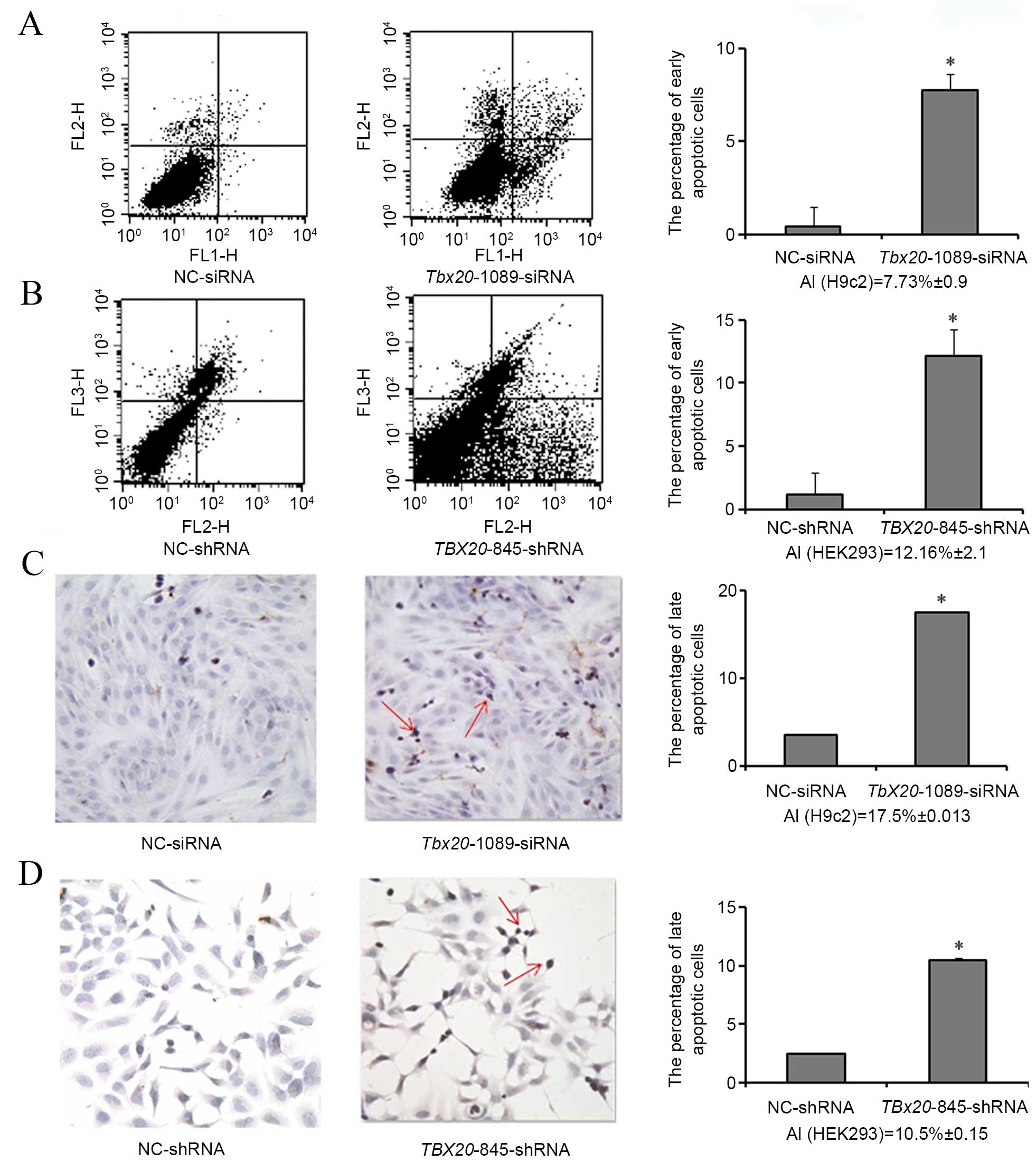
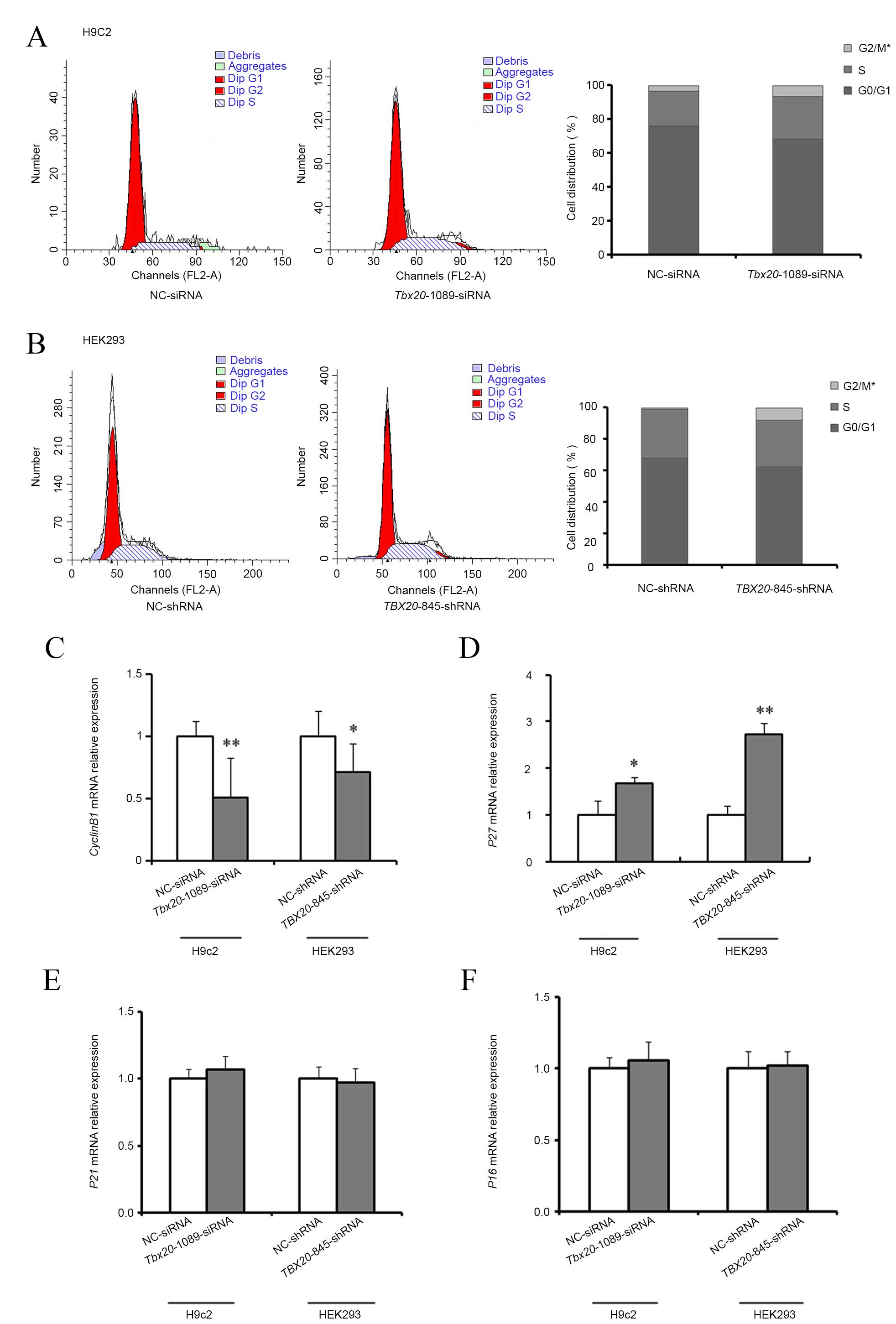
|
1
|
Hoffman JI and Kaplan S: The incidence of
congenital heart disease. J Am Coll Cardiol. 39:1890–1900. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van der Linde D, Konings EE, Slager MA,
Witsenburg M, Helbing WA, Takkenberg JJ and Roos-Hesselink JW:
Birth prevalence of congenital heart disease worldwide: A
systematic review and meta-analysis. J Am Coll Cardiol.
58:2241–2247. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thienpont B, Mertens L, de Ravel T,
Eyskens B, Boshoff D, Maas N, Fryns JP, Gewillig M, Vermeesch JR
and Devriendt K: Submicroscopic chromosomal imbalances detected by
array-CGH are a frequent cause of congenital heart defects in
selected patients. Eur Heart J. 28:2778–2784. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Corrigan N, Brazil DP and McAuliffe F:
Fetal cardiac effects of maternal hyperglycemia during pregnancy.
Birth Defects Res A Clin Mol Teratol. 85:523–530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gong LG, Qiu GR, Jiang H, Xu XY, Zhu HY
and Sun KL: Analysis of single nucleotide polymorphisms and
haplotypes in HOXC gene cluster within susceptible region 12q13 of
simple congenital heart disease. Zhonghua Yi Xue Yi Chuan Xue Za
Zhi. 22:497–501. 2005.PubMed/NCBI
|
|
6
|
Srivastava D: Making or breaking the
heart: From lineage determination to morphogenesis. Cell.
126:1037–1048. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cai CL, Zhou W, Yang L, Bu L, Qyang Y,
Zhang X, Li X, Rosenfeld MG, Chen J and Evans S: T-box genes
coordinate regional rates of proliferation and regional
specification during cardiogenesis. Development. 132:2475–2487.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singh MK, Christoffels VM, Dias JM, Trowe
MO, Petry M, Schuster-Gossler K, Bürger A, Ericson J and Kispert A:
Tbx20 is essential for cardiac chamber differentiation and
repression of Tbx2. Development. 132:2697–2707. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stennard FA, Costa MW, Lai D, Biben C,
Furtado MB, Solloway MJ, McCulley DJ, Leimena C, Preis JI,
Dunwoodie SL, et al: Murine T-box transcription factor Tbx20 acts
as a repressor during heart development, and is essential for adult
heart integrity, function and adaptation. Development.
132:2451–2462. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takeuchi JK, Mileikovskaia M,
Koshiba-Takeuchi K, Heidt AB, Mori AD, Arruda EP, Gertsenstein M,
Georges R, Davidson L, Mo R, et al: Tbx20 dose-dependently
regulates transcription factor networks required for mouse heart
and motoneuron development. Development. 132:2463–2474. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qian L, Mohapatra B, Akasaka T, Liu J,
Ocorr K, Towbin JA and Bodmer R: Transcription factor
neuromancer/TBX20 is required for cardiac function in Drosophila
with implications for human heart disease. Proc Natl Acad Sci USA.
105:19833–19838. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kirk EP, Sunde M, Costa MW, Rankin SA,
Wolstein O, Castro ML, Butler TL, Hyun C, Guo G, Otway R, et al:
Mutations in cardiac T-box factor gene TBX20 are associated with
diverse cardiac pathologies, including defects of septation and
valvulogenesis and cardiomyopathy. Am J Hum Genet. 81:280–291.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu C, Shen A, Li X, Jiao W, Zhang X and
Li Z: T-box transcription factor TBX20 mutations in Chinese
patients with congenital heart disease. Eur J Med Genet.
51:580–587. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Posch MG, Gramlich M, Sunde M, Schmitt KR,
Lee SH, Richter S, Kersten A, Perrot A, Panek AN, Al Khatib IH, et
al: A gain-of-function TBX20 mutation causes congenital atrial
septal defects, patent foramen ovale and cardiac valve defects. J
Med Genet. 47:230–235. 2010. View Article : Google Scholar :
|
|
15
|
Pan Y, Geng R, Zhou N, Zheng GF, Zhao H,
Wang J, Zhao CM, Qiu XB, Yang YQ and Liu XY: TBX20 loss-of-function
mutation contributes to double outlet right ventricle. Int J Mol
Med. 35:1058–1066. 2015.PubMed/NCBI
|
|
16
|
Chen J, Sun F, Fu J and Zhang H:
Association of TBX20 gene polymorphism with congenital heart
disease in Han Chinese neonates. Pediatr Cardiol. 36:737–742. 2015.
View Article : Google Scholar
|
|
17
|
Shen T, Aneas I, Sakabe N, Dirschinger RJ,
Wang G, Smemo S, Westlund JM, Cheng H, Dalton N, Gu Y, et al: Tbx20
regulates a genetic program essential to adult mouse cardiomyocyte
function. J Clin Invest. 121:4640–4654. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (-Delta Delta C (T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Hammer S, Toenjes M, Lange M, Fischer JJ,
Dunkel I, Mebus S, Grimm CH, Hetzer R, Berger F and Sperling S:
Characterization of TBX20 in human hearts and its regulation by
TFAP2. J Cell Biochem. 104:1022–1033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kubben FJ, Peeters-Haesevoets A, Engels
LG, Baeten CG, Schutte B, Arends JW, Stockbrügger RW and Blijham
GH: Proliferating cell nuclear antigen (PCNA): A new marker to
study human colonic cell proliferation. Gut. 35:530–535. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stennard FA and Harvey RP: T-box
transcription factors and their roles in regulatory hierarchies in
the developing heart. Development. 132:4897–4910. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chakraborty S and Yutzey KE: Tbx20
regulation of cardiac cell proliferation and lineage specialization
during embryonic and fetal development in vivo. Dev Biol.
363:234–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shelton EL and Yutzey KE: Tbx20 regulation
of endocardial cushion cell proliferation and extracellular matrix
gene expression. Dev Biol. 302:376–388. 2007. View Article : Google Scholar
|
|
24
|
Tang L, Zhang Y, Pan H, Luo Q, Zhu XM,
Dong MY, Leung PC, Sheng JZ and Huang HF: Involvement of cyclin B1
in progesterone-mediated cell growth inhibition, G2/M cell cycle
arrest and apoptosis in human endometrial cell. Reprod Biol
Endocrinol. 7:1442009. View Article : Google Scholar
|
|
25
|
Paruthiyil S, Cvoro A, Tagliaferri M,
Cohen I, Shtivelman E and Leitman DC: Estrogen receptor β causes a
G2 cell cycle arrest by inhibiting CDK1 activity through the
regulation of cyclin B1, GADD45A and BTG2. Breast Cancer Res Treat.
129:777–784. 2011. View Article : Google Scholar
|
|
26
|
Yadav V, Sultana S, Yadav J and Saini N:
Gatifloxacin induces S and G2-phase cell cycle arrest in pancreatic
cancer cells via p21/p27/p53. PloS One. 7:e477962012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mitrea DM, Yoon MK, Ou L and Kriwacki RW:
Disorder-function relationships for the cell cycle regulatory
proteins p21 and p27. Biol Chem. 393:259–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee Y, Shioi T, Kasahara H, Jobe SM, Wiese
RJ, Markham BE and Izumo S: The cardiac tissue-restricted homeobox
protein Csx/Nkx2.5 physically associates with the zinc finger
protein GATA4 and cooperatively activates atrial natriuretic factor
gene expression. Mol Cell Biol. 18:3120–3129. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sepulveda JL, Vlahopoulos S, Iyer D,
Belaguli N and Schwartz RJ: Combinatorial expression of GATA4,
Nkx2-5, and serum response factor directs early cardiac gene
activity. J Biol Chem. 277:25775–25782. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vincentz JW, Barnes RM, Firulli BA, Conway
SJ and Firulli AB: Cooperative interaction of Nkx2.5 and Mef2c
transcription factors during heart development. Dev Dyn.
237:3809–3819. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Snyder M, Huang XY and Zhang JJ: Stat3
directly controls the expression of Tbx5, Nkx2.5, and GATA4 and is
essential for cardiomyocyte differentiation of P19CL6 cells. J Biol
Chem. 285:23639–23646. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schlesinger J, Schueler M, Grunert M,
Fischer JJ, Zhang Q, Krueger T, Lange M, Tönjes M, Dunkel I and
Sperling SR: The cardiac transcription network modulated by Gata4,
Mef2a, Nkx2.5, Srf, histone modifications and microRNAs. PLoS
Genet. 7:e10013132011. View Article : Google Scholar
|
|
33
|
Chen Y and Cao X: NFAT directly regulates
Nkx2-5 transcription during cardiac cell differentiation. Biol
Cell. 101:335–349. 2009. View Article : Google Scholar
|
|
34
|
Zhao R, Watt AJ, Battle MA, Li J, Bondow
BJ and Duncan SA: Loss of both GATA4 and GATA6 blocks cardiac
myocyte differentiation and results in acardia in mice. Dev Biol.
317:614–619. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pu WT, Ishiwata T, Juraszek AL, Ma Q and
Izumo S: GATA4 is a dosage-sensitive regulator of cardiac
morphogenesis. Dev Biol. 275:235–244. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oka T, Maillet M, Watt AJ, Schwartz RJ,
Aronow BJ, Duncan SA and Molkentin JD: Cardiac-specific deletion of
Gata4 reveals its requirement for hypertrophy, compensation, and
myocyte viability. Circ Res. 98:837–845. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guner-Ataman B, Paffett-Lugassy N, Adams
MS, Nevis KR, Jahangiri L, Obregon P, Kikuchi K, Poss KD, Burns CE
and Burns CG: Zebrafish second heart field development relies on
progenitor specification in anterior lateral plate mesoderm and
nkx2.5 function. Development. 140:1353–1363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao L, Ju D, Gao Q, Zheng X and Yang G:
Over-expression of Nkx2.5 and/or cardiac α-actin inhibit the
contraction ability of ADSCs-derived cardiomyocytes. Mol Biol Rep.
39:2585–2595. 2012. View Article : Google Scholar
|
|
39
|
Liu H, Harris TM, Kim HH and Childs G:
Cardiac myocyte differentiation: The Nkx2.5 and Cripto target genes
in P19 clone 6 cells. Funct Integr Genomics. 5:218–239. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yamak A, Temsah R, Maharsy W, Caron S,
Paradis P, Aries A and Nemer M: Cyclin D2 rescues size and function
of GATA4 haplo-insufficient hearts. Am J Physiol Heart Circ
Physiol. 303:H1057–H1066. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Laforest B and Nemer M: GATA5 interacts
with GATA4 and GATA6 in outflow tract development. Dev Biol.
358:368–378. 2011. View Article : Google Scholar : PubMed/NCBI
|