Introduction
Coronary artery disease (CAD) is a leading cause of
mortality and morbidity in developed and developing countries
(1). An estimated 16.3 million
Americans aged ≥20 years have been diagnosed with CAD, and the
overall CAD prevalence is 7% in adults in the US (2). Typically, patients with CAD receive
advice for managing the risk factors associated with the
progression of atherosclerosis, as well as pharmacological
treatment. In addition, some patients may undergo coronary
revascularization, which falls into two main categories: Coronary
artery bypass grafting (CABG) and catheter-based percutaneous
coronary intervention (PCI) (3.4). A number of disadvantages are
associated with PCI, including early restenosis and an inability to
relieve the totally occluded arteries or vessels that occur with
extensive atherosclerotic disease (5). CABG has the advantages of a greater
durability (graft patency rates exceed 90% at 10 years with
arterial conduits) and more complete revascularization regardless
of the morphology of the obstructing atherosclerotic lesion
(6).
Numerous surgeons and centers have adopted off-pump
coronary artery bypass (OPCAB). An interest in off-pump techniques
has been driven by an increased awareness of the deleterious
effects of cardiopulmonary bypass and aortic manipulation (7,8). At
present, OPCAB is the first choice for revascularization in
patients with severe coronary artery disease; however, the
mortality rate associated with this type of surgery is relatively
high, since the majority of patients requiring CAB are at a high
risk for cardiac events (9). The
intracoronary shunts are designed to maintain myocardial perfusion
by maintaining blood supply in the distal myocardium (10). However, as the shunt itself does
not increase coronary blood flow, the distal flow remains limited
by stenosis, markedly so in severe cases (11). The present study aimed to establish
porcine models of myocardial ischemia with different levels of
stenosis, and provide myocardial protection using active perfusion
at a site distal to the anastomosis. By comparing this to the
traditional method of shunt perfusion, the present study
investigated the protective effect of this novel perfusion strategy
in pigs with acute myocardial infarction.
Materials and methods
Animals
A total of 30 male pigs (weight, 50–60 kg) were
included in the present study. The animals were provided by the
Animal Center of Binzhou Medical College (Binzhou, China), where
they were maintained under a 12-h light/dark cycle. The present
study was conducted in accordance with the 'Guiding Principles in
the Care and Use of Laboratory Animals', as outlined by the Animal
Center of Binzhou Medical College, and with approval from the
Animal Care Committee of Binzhou Medical College.
Agents
The fentanyl and ketamine hydrochloride injections
were purchased from Yichang Humanwell Pharmaceutical Co., Ltd.
(Yichang, China). The malondialdehyde enzyme-linked immunosorbent
assay (ELISA) kit was purchased from R&D Systems, Inc.
(Minneapolis, MN, USA). The tumor necrosis factor-α (TNF-α),
interleukin (IL)-6 and IL-10 ELISA kits were purchased from
Jiancheng Bioengineering Research Institute (Nanjing, China). The
cardiac troponin (cTnI), creatine kinase and myoglobin (Mb) ELISA
kits were purchased from Beyotime Institute of Biotechnology
(Haimen, China). The terminal deoxynucleotidyl-transferase dUTP
nick end-labeling (TUNEL) kit was purchased from Roche Diagnostics
GmbH (Mannheim, Germany). Atropine, propofol, heparin and lysis
buffer were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Sodium dodecyl sulfate buffer, polyacrylamide gels, nitrocellulose
membranes, Tris-buffered saline containing Tween-20 (TBST),
enhanced chemiluminescence (ECL) solution, 4% paraformaldehyde,
paraffin, Triton X-100, phosphate-buffered saline (PBS) and the
Bicinchoninic Acid Protein Assay kit were purchased from Kangchen
Bio-tech, Inc. (Shanghai, China). Potassium chloride (KCl) was
obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai,
China)
Instruments
The current study used an ALCBio ALC-V10B ventilator
(Shanghai Alcott Biotech, Ltd., Shanghai, China), an invasive
pressure monitor (BeneView T8; Agilent Technologies, Inc., Santa
Clara, CA, USA), an invasive pressure sensor (PT161103; Shenzhen
Mindray Bio-Medical Electronics Co., Ltd., Shenzhen, China), a
blood pressure monitor (MC-6800; Shenzhen Mindray Bio-Medical
Electronics Co., Ltd.), a fluorescence microscope (BX61; Olympus
Corporation, Tokyo, Japan), and the SpectraMax® 190
Microplate Reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
Active perfusion cannula
A scalp acupuncture hose was used to produce the
cannula for active perfusion. The ends of the hose were heated and
stretched to thin the wall, until the diameter of the hose reached
~1.5 mm. A ramp cutoff was made at the end of the hose, which was
then connected to a T-tube. The ends of the T-tube were connected
to a 16G syringe (inner diameter, 1.19 mm), for perfusion, and a
blood pressure monitor.
Grouping of animals
Following 2 days of adaptive feeding, where the pigs
were given free access to food and water and were housed apart and
maintained under a 12-h light/dark cycle, the pigs were randomly
assigned to five groups (n=6), as follows: i) Sham (control); ii)
A1 (shunt; stenosis rate, 55%); iii) A2 (shunt; stenosis rate,
75%); iv) B1 (active perfusion; stenosis rate, 55%); and v) B2
(active perfusion; stenosis rate, 75%).
Establishment of porcine models of acute
stenosis
Sedation was provided by intramuscular injection of
atropine (3 mg). Ketamine (0.1 mg) and fentanyl (0.3 g) were
administered 5 min after atropine injection. The pigs were placed
in the supine position with their limbs fixed on the operating
table, and underwent endotracheal intubation using fentanyl and
propofol (6 mg/kg/h) for anesthesia. Following preparation of the
skin and monitoring by electrocardiogram (ECG), a midline incision
was made in the chest (3 mg/kg heparin for heparinization), and a
pressure sensor was placed in the ascending aorta for measuring
aortic pressure (P0). The proximal left anterior
descending artery (~1 cm) was freed and transiently clipped to
allow measurement of the lumen diameter. In the control group, the
lumen was opened following occlusion, however, for the experimental
group, the suture was positioned using a 7-0 Prolene thread. The
suture was made at a length of one-third and one-half of the lumen
diameter, thus the remaining size of the lumen was four-ninths and
one-quarter of the original, thereby achieving a stenosis rate of
55 and 75%, respectively. A trocar was punctured in the distal
third of the descending branch, and was then connected to the
pressure sensor to monitor the left anterior descending coronary
pressure (P1), in order to calculate the effective
perfusion pressure (P1/P0).
Myocardial perfusion strategy
In the sham group, P0 and left anterior
descending coronary pressure (P1) were monitored at 30
min following opening of the coronary artery. In group A, an
incision was made on the coronary artery to insert a shunt when the
ST segment elevation reached 1 mm. P0 and P1
were monitored following insertion of the shunt, and a blood sample
(5 ml) was taken at 30 min post-perfusion. In group B, an active
perfusion needle was pierced into the ascending aorta when ST
segment elevation reached 1 mm, and P0 was monitored
using the T-tube. An incision was made on the coronary artery to
insert the active perfusion tube distal to the vessel, and active
perfusion was initiated by opening the tube. P1 was
monitored during active perfusion, and a blood sample (5 ml) was
collected at 30 min post-perfusion.
Following blood sampling, the superior and inferior
vena cava and the ascending aorta were clipped, and the heart was
emptied via the aortic root. The pigs were sacrificed by
intravenous injection with 10% KCl immediately following blood
emptying, and the apical myocardium at the left ventricle was
sampled (10 g) for further examination.
Determination of serum TNF-α, cTnI, Mb,
creatine kinase-MB (CK-MB), IL-6 and IL-10 using ELISAs
The ELISA kits were used according to the
manufacturer's protocols. Briefly, blood samples (5 ml) were placed
in an ethylenediaminetetraacetic acid-containing Eppendorf tube
(500 μl) to allow thorough mixing for 20 min. The mixture
was centrifuged at 2,500 × g for 20 min to collect the supernatant.
The concentration/optical density (OD) value standard curve was
generated using the serially diluted standards provided with the
kit. An automated microplate reader (SpectraMax® 190)
was used to measure the OD value of the centrifuged serum, and the
concentration of target proteins was calculated according to their
positions on the standard curve.
Western blot analysis of caspase-3 and
B-cell lymphoma 2 (Bcl-2) in myocardial tissue samples
The collected myocardial tissue samples were
homogenized in a lysis buffer and centrifuged at 12,000 × g for 15
min. The lysates were collected and underwent protein
quantification using the bicinchoninic acid assay. A total of 20 g
protein was boiled with 1X sodium dodecyl sulfate (SDS) buffer for
SDS-polyacrylamide gel electrophoresis (80 v for 30 min), and the
electrophoresed proteins were transferred onto a nitrocellulose
membrane. Western blotting was performed by blocking the membrane
with TBST containing 5 g/l skimmed milk for 1.5 h. The membrane was
incubated with primary antibodies against caspase-3, Bcl-2 and
β-actin (1:1,000; Cell Signaling Technology, Inc., Danvers, MA,
USA) at 4°C overnight. The membrane was washed with TBST three
times (10 min each time), and re-incubated with horseradish
peroxidase-conjugated goat anti-rabbit or goat anti-rat
immunoglobulin G (1:5,000; Zhongshan Bio-tech Co., Ltd., Zhongshan,
China) at room temperature for 2 h, followed by washing with TBST
three times (10 min each time). Blots were developed using the ECL
solution, and Quantity One 4.62 software was used to analyze the
expression level of target proteins (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Measurement of myocardial tissue
apoptosis using the terminal deoxynucleotidyl transferase dUTP nick
end labeling (TUNEL) assay
The myocardial tissue samples were fixed with 4%
paraformaldehyde for 24 h, and dehydrated and embedded in paraffin.
The samples were sliced at a thickness of 0.6 mm, and were
perforated for 5 min with Triton X-100, followed by washing with
PBS three times (5 min each time). TUNEL was performed according to
the manufacturer's protocols, and all surgical procedures were
performed under dark room conditions. The sections were treated
with 50 μl TUNEL solution (containing 2 μl terminal
deoxynucleotidyl transferase (TdT) enzyme and 48 μl
fluorescent label) for 60 min at 37°C and were washed with PBS
(three times for 5 min) and embedded in paraffin. Green
fluorescence was observed under a fluorescence microscope, and 10
fields were randomly selected for cell counting. The rate of
apoptosis was calculated as follows: Apoptotic cell count / total
cell count ×100%. The experiment was repeated three times.
Statistical analysis
Statistical analysis was performed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). Data were presented as the
mean ± standard deviation. Statistical differences between the 55%
and 75% stenosis groups were assessed using a t one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
Successful establishment of the
model
Pigs in all groups survived the experimental period.
Following formation of the stenosis, the ST segment, observed on an
ECG, elevated gradually, and elevation of 1 mm indicated successful
construction of the model. The time for establishment of the model
shortened with the increase in severity of the stenosis (Table I). Groups with 75% stenosis
exhibited a significantly shortened duration for establishment of
the model compared with groups with 55% stenosis (P<0.05).
 | Table ITime required for ST segment elevation
of 1 mm following establishment of the model. |
Table I
Time required for ST segment elevation
of 1 mm following establishment of the model.
| Parameter | Stenosis
|
|---|
| 55% | 75% |
|---|
| T-ST
(min) | 20.12±0.74 | 15.35±0.66a |
Models with stenosis developed myocardial ischemia
due to the significant decrease in P1, and pigs with a
higher rate of stenosis demonstrated lower P1. As
presented in Table II, the
P1 and P1/P0 ratio in all
experimental groups were significantly lower than the control (sham
group; P<0.01). In addition, the absolute P1 value
and the P1/P0 ratio in pigs with 75% stenosis
were significantly lower compared with the 55% stenosis groups
(P<0.01).
 | Table IIP1 and
P1/P0 ratio following establishment of the
stenosis model (n=6; mean ± standard deviation). |
Table II
P1 and
P1/P0 ratio following establishment of the
stenosis model (n=6; mean ± standard deviation).
| Pressure | Control | Stenosis
|
|---|
| 55% | 75% |
|---|
| P0
(mmHg) | 108±6.83 | 96±6.26 | 91±4.83 |
| P1
(mmHg) | 106±5.20 | 70±5.34a | 46±5.11b |
|
P1/P0 | 0.98±0.03 | 0.72±0.04a |
0.51±0.03a,b |
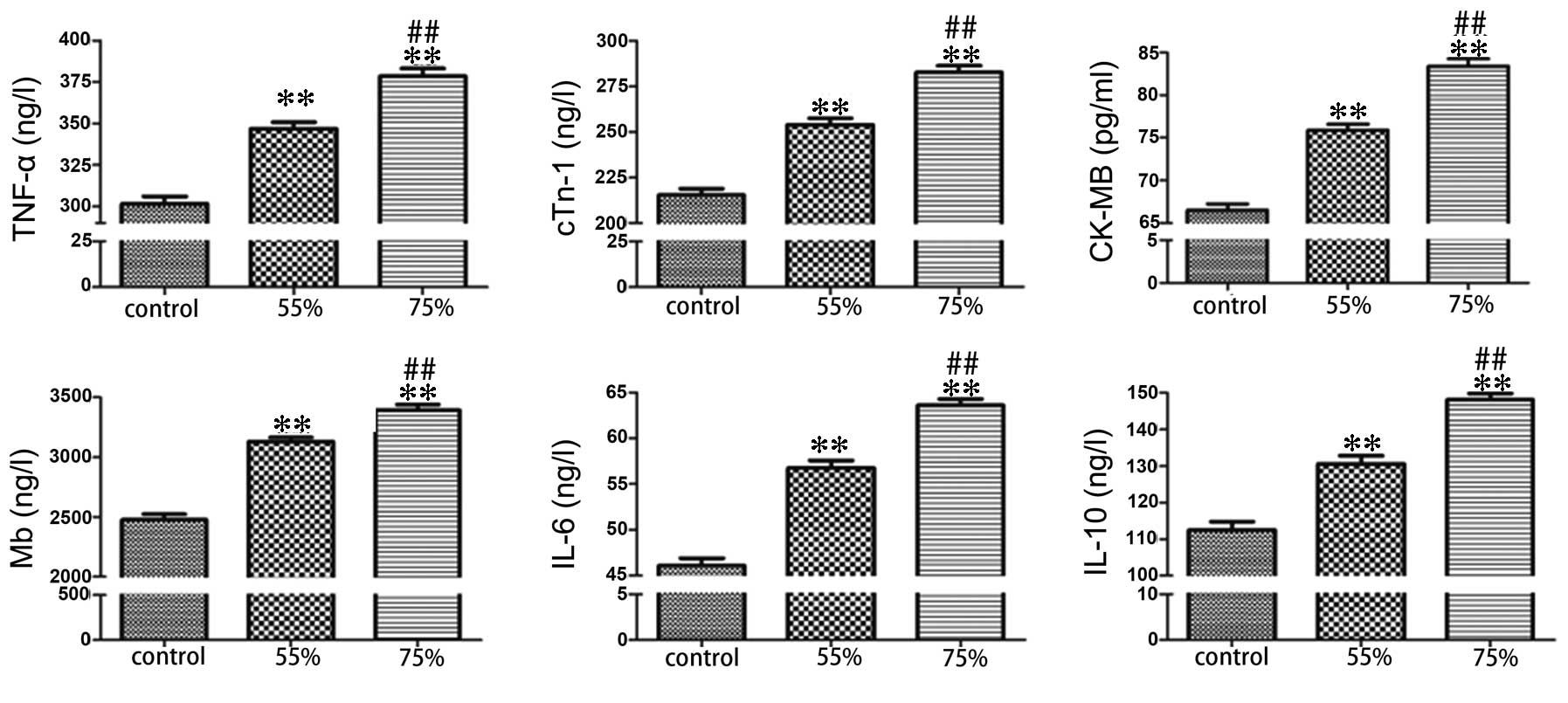
Following formation of the stenosis, ELISA was used
to determine the expression level of serum TNF-α, cTnI, CK-MB, Mb,
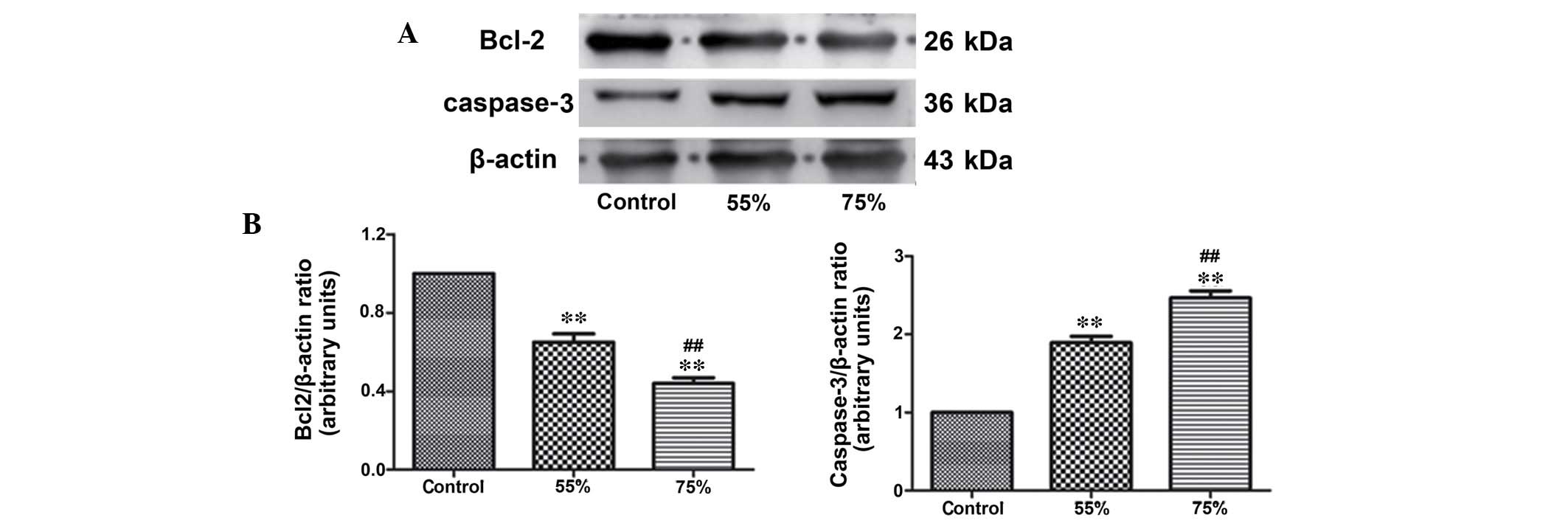
IL-6 and IL-10 (Fig. 1). Western
blotting was used to detect the expression level of the apoptotic
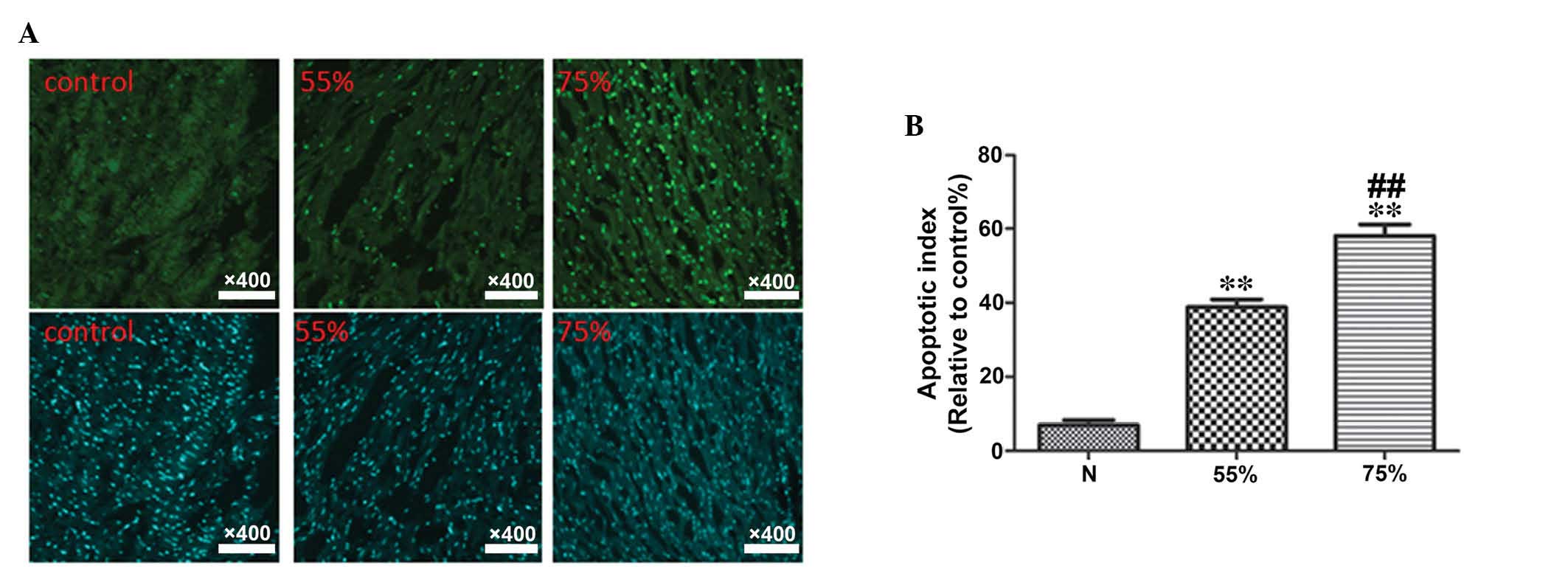
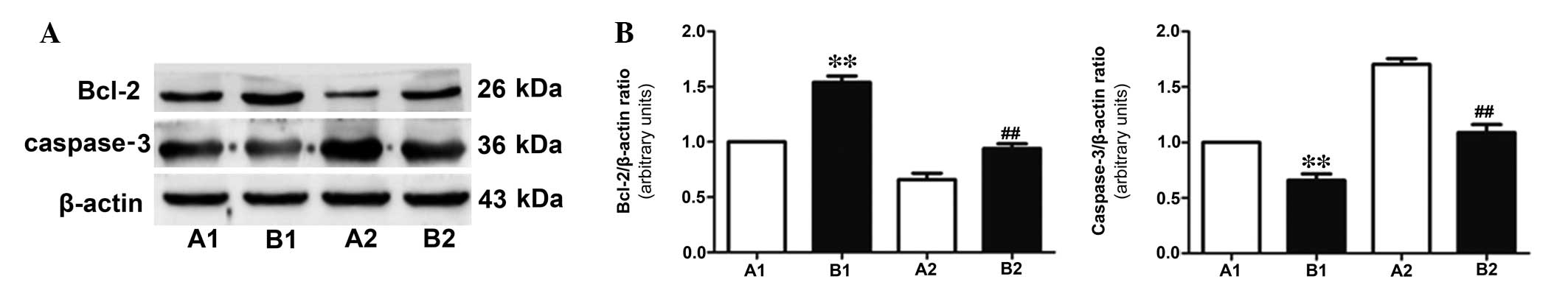
protein, caspase-3 and the anti-apoptotic protein, Bcl-2 (Fig. 2). In addition, TUNEL staining
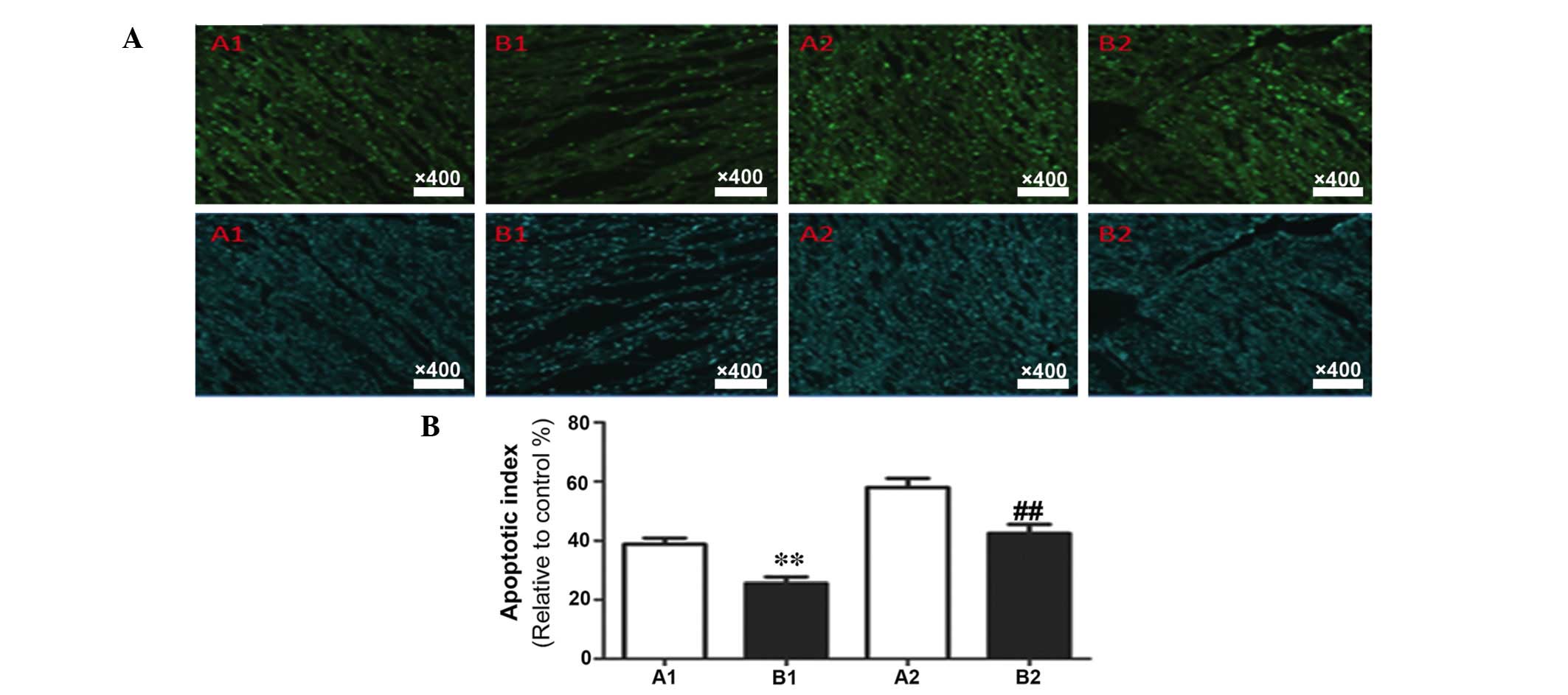
demonstrated the rate of apoptosis in myocardial tissue samples
(Fig. 3). Compared with the sham
group, the stenosis groups exhibited significantly increased levels
of ischemic and apoptotic indicators, while the level of
anti-apoptotic protein, Bcl-2 was decreased (P<0.01). In
addition, the elevation of ischemic and apoptotic indicators, and
the decline of Bcl-2 in the 75% stenosis groups were significant
compared with the 55% stenosis groups (P<0.01; Figs. 1Figure 2–3).
Comparison of the two perfusion
strategies
As the time of ischemia lengthened, the
P1/P0 indicated further decline. Compared
with group A, group B demonstrated significantly elevated
P1 and P1/P0 ratio (P<0.05),
indicating a significant decrease in P1/P0 in
the artery distal to coronary stenosis (P<0.01). The
P1/P0 in the active perfusion groups (B1 and
B2) was higher than in the corresponding shunt groups (A1 and A2;
P<0.05), and the difference was more significant in groups with
the higher stenosis rate. The P1/P0 provided
by shunt decreased significantly as the severity of stenosis
increased (P<0.05), indicating that blood supply at the distal
artery is limited by coronary stenosis. However, as active
perfusion was not affected by coronary stenosis, no significant
difference in blood supply was detected in the models with
different levels of stenosis (P>0.05; Table III).
 | Table IIIP1 and
P1/P0 ratio following shunt or active
perfusion. |
Table III
P1 and
P1/P0 ratio following shunt or active
perfusion.
| Pressure | A1 | A2 | B1 | B2 |
|---|
| P0
(mmHg) | 85±6.26 | 81±4.83 | 87±4.43 | 82±4.88 |
| P1
(mmHg) | 59±5.34 | 40±5.11 | 69±4.81a | 65±5.31b |
|
P1/P0 | 0.70±0.04 | 0.51±0.04 | 0.81±0.04a | 0.80±0.03b |
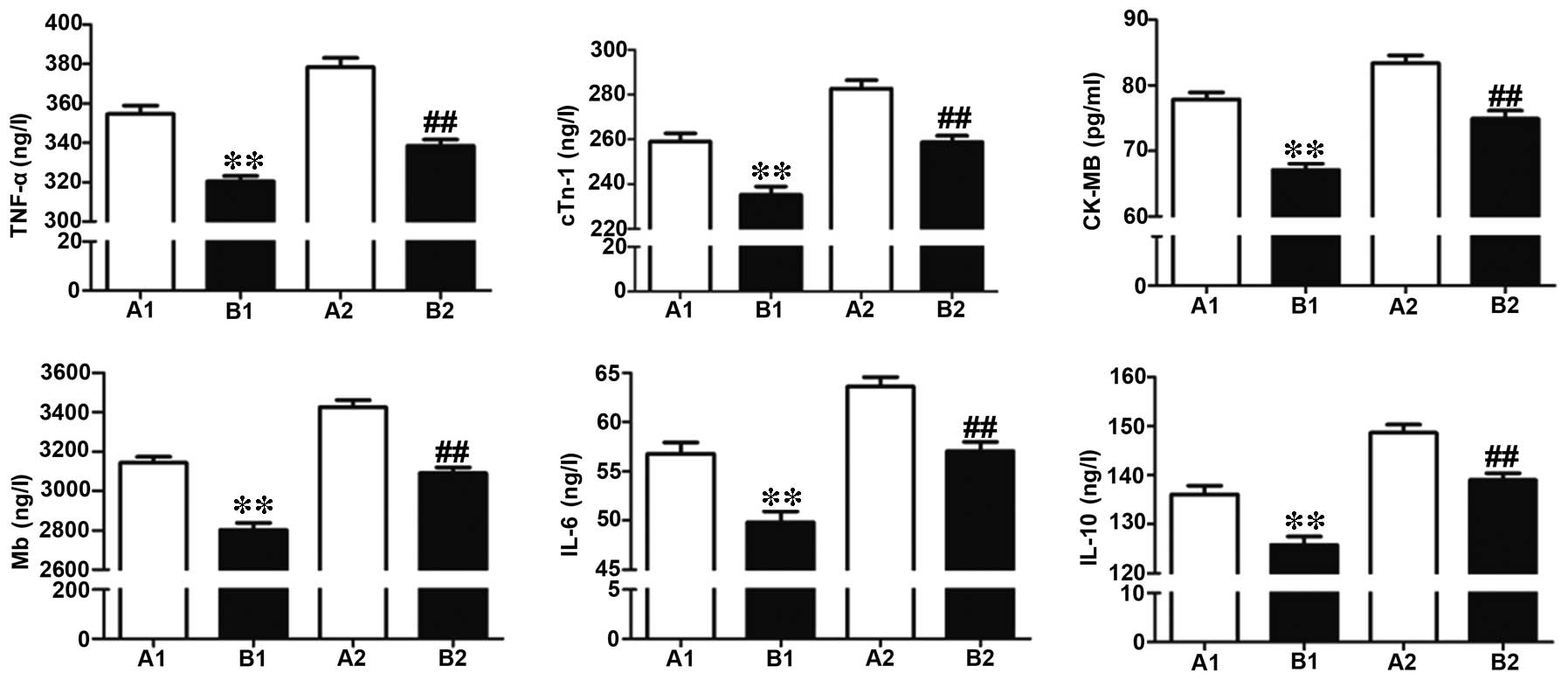
Comparison of myocardial injury
indicators in the two perfusion groups
Coronary perfusion was performed at 30 min following
formation of the stenosis, and ELISA was used to detect the level
of myocardial injury indicators, including TNF-α, cTnI, CK-MB, Mb,
IL-6 and IL-10. Compared to group A, group B demonstrated
significantly decreased expression levels of all myocardial injury
indicators, indicating the myocardial protective function of active
perfusion (Fig. 4).
Comparison of apoptosis-associated
proteins, caspase-3 and Bcl-2 in the two perfusion groups
Following 30 min of coronary perfusion, western
blotting was performed to measure the expression levels of
anti-apoptotic protein, Bcl-2 and apoptotic protein, caspase-3. As
presented in Fig. 5, in group B,
the expression level of caspase-3 was downregulated (P<0.05)
while Bcl-2 was upregulated compared with group A, indicating that
active perfusion alleviated myocardial ischemia more successfully
than conventional shunt perfusion.
Determination of myocardial apoptosis
rate using TUNEL
The results of TUNEL staining and the data
statistics are presented in Fig.
6. Compared with the corresponding subgroups in group A, group
B demonstrated a significantly decreased myocardial apoptosis rate
(P<0.05).
Discussion
Acute myocardial dysfunction predominantly manifests
as ischemic injury (12), and it
is particularly prominent under ischemic, function-inhibitory or
necrotic conditions (4).
Currently, myocardial protections implemented in coronary artery
bypass grafting (CABG) include improvement in myocardial perfusion
and stress response, as well as alleviation of reperfusion injury.
During CABG, the combined use of antegrade, retrograde and bypass
perfusion may improve the uneven distribution of cardioplegic fluid
(13), and during OPCAB, a shunt
alleviates myocardial ischemia by maintaining continuous blood flow
at the anastomosis. Ischemic preconditioning includes
intraoperative and preoperative preconditioning (14). Therapeutic agents, such as
β-1,3/1,6 glucan (15),
levosimendan (16), and diazoxide
are commonly used in preconditioning, while other studies have
suggested that preconditioning with hyperbaric oxygen may also
reduce myocardial injury during ischemia-reperfusion (17,18).
Therapeutic agents, including atrial natriuretic peptide (19), nesiritide, carvedilol,
sodium-hydrogen exchange inhibitor, erythropoietin, reamberin,
L-arginine, large doses of insulin, and C1 esterase inhibitor are
often administered to alleviate reperfusion injury (20,21).
Despite these measures, myocardial protection during CAB is
unsatisfactory, as myocardial blood supply or preservation fluid
perfusion has always been affected and limited by proximal coronary
artery stenosis (22).
In conclusion, in the present study, surgical
methods were used to establish porcine models of myocardial
ischemia with controllable degrees of stenosis at the descending
coronary artery, to more effectively simulate the pathological
anatomy of coronary heart disease. Active perfusion of the coronary
artery was performed distal to stenosis via cannulation across the
anastomosis, thus solving the fundamental problem of ischemic
cardiomyopathy and providing a more effective method for myocardial
protection in clinical CAB. In further studies, hemodynamic methods
are required to investigate the distribution and variation of
pressure at the coronary artery proximal and distal to stenosis,
following formation of the stenosis. On the basis of this, we
expect to modify active perfusion strategies, including altering
the location of active perfusion at the coronary artery distal to
stenosis and the size of the cannula for active perfusion.
Acknowledgments
The present study was supported by grants from the
Academic Promotion Project of Binzhou Medical University Hospital
(grant no. BY2011KJ024). The authors would like to thank Dr
Yongguang Xiao of Renmin Hospital of Wuhan University (Wuhan,
China) for the animals used in the present study.
References
|
1
|
Al Shammeri O, Stafford RS, Alzenaidi A,
Al-Hutaly B and Abdulmonem A: Quality of medical management in
coronary artery disease. Ann Saudi Med. 34:488–493. 2014.
|
|
2
|
Bashinskaya B, Nahed BV, Walcott BP,
Coumans JV and Onuma OK: Socioeconomic status correlates with the
prevalence of advanced coronary artery disease in the United
States. PLoS One. 7:e463142012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Y, Zhou X, Jiang H, Gao M, Wang L, Shi
Y and Gao J: Percutaneous coronary intervention strategies and
prognosis for graft lesions following coronary artery bypass
grafting. Exp Ther Med. 9:1656–1664. 2015.PubMed/NCBI
|
|
4
|
Deja MA and Malinowski M: Conditioning the
heart in cardiac surgery. Kardiol Pol. 69(Suppl 3): S80–S84.
2011.In Polish.
|
|
5
|
Gorenoi V, Dintsios CM, Schonermark MP and
Hagen A: Drug-eluting stents vs. coronary artery bypass-grafting in
coronary heart disease. GMS Health Technol Assess. 4:c132008.
|
|
6
|
Alexander JH and Smith PK: Coronary-artery
bypass grafting. N Engl J Med. 374:1954–1964. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luo T and Ni Y: Short-term and long-term
postoperative safety of off-pump versus on-pump coronary artery
bypass grafting for coronary heart disease: A meta-analysis for
randomized controlled trials. Thorac Cardiovasc Surg. 63:319–327.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu S, Wan F, Zhang Z, Zhao H, Cui ZQ and
Xie JY: Redo coronary artery bypass grafting: On-pump and off-pump
coronary artery bypass grafting revascularization techniques. Chin
Med Sci J. 30:28–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gomez-Lara J, Roura G, Blasco-Lucas A,
Ortiz D, Sbraga F, Romaguera R, Ferreiro JL, Teruel L,
Sanchez-Elvira G, Homs S, et al: Global risk score for choosing the
best revascularization strategy in patients with unprotected left
main stenosis. J Invasive Cardiol. 25:650–658. 2013.PubMed/NCBI
|
|
10
|
Vallely MP and Ross DE: Intracoronary
shunts and off-pump surgery. Ann Thorac Surg. 90:700–701. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guizilini S, Viceconte M, Esperanca GT,
Bolzan DW, Vidotto M, Moreira RS, Câncio AA and Gomes WJ: Pleural
subxyphoid drain confers better pulmonary function and clinical
outcomes in chronic obstructive pulmonary disease after off-pump
coronary artery bypass grafting: A randomized controlled trial. Rev
Bras Cir Cardiovasc. 29:588–594. 2014.
|
|
12
|
Wagner R, Piler P, Gabbasov Z, Maruyama J,
Maruyama K, Nicovsky J and Kruzliak P: Adjuvant cardioprotection in
cardiac surgery: Update. BioMed Res Int. 2014:8080962014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goncu MT, Sezen M, Toktas F, Ari H, Gunes
M, Tiryakioglu O and Yavuz S: Effect of antegrade graft
cardioplegia combined with passive graft perfusion in on-pump
coronary artery bypass grafting. J Int Med Res. 38:1333–1342. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Teoh LK, Grant R, Hulf JA, Pugsley WB and
Yellon DM: The effect of preconditioning (ischemic and
pharmacological) on myocardial necrosis following coronary artery
bypass graft surgery. Cardiovasc Res. 53:175–180. 2002. View Article : Google Scholar
|
|
15
|
Aarsaether E, Rydningen M, Einar Engstad R
and Busund R: Cardioprotective effect of pretreatment with
beta-glucan in coronary artery bypass grafting. Scand Cardiovasc J.
40:298–304. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tritapepe L, De Santis V, Vitale D,
Santulli M, Morelli A, Nofroni I, Puddu PE, Singer M and
Pietropaoli P: Preconditioning effects of levosimendan in coronary
artery bypass grafting-a pilot study. Br J Anaesth. 96:694–700.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yogaratnam JZ, Laden G, Guvendik L, Cowen
M, Cale A and Griffin S: Pharmacological preconditioning with
hyperbaric oxygen: Can this therapy attenuate myocardial ischemic
reper-fusion injury and induce myocardial protection via nitric
oxide? J Surg Res. 149:155–164. 2008. View Article : Google Scholar
|
|
18
|
Jeysen ZY, Gerard L, Levant G, Cowen M,
Cale A and Griffin S: Research report: The effects of hyperbaric
oxygen preconditioning on myocardial biomarkers of cardioprotection
in patients having coronary artery bypass graft surgery. Undersea
Hyperb Med. 38:175–185. 2011.PubMed/NCBI
|
|
19
|
Sezai A, Hata M, Wakui S, Niino T,
Takayama T, Hirayama A, Saito S and Minami K: Efficacy of
continuous low-dose hANP administration in patients undergoing
emergent coronary artery bypass grafting for acute coronary
syndrome. Circ J. 71:1401–1407. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fattouch K, Bianco G, Speziale G,
Sampognaro R, Lavalle C, Guccione F, Dioguardi P and Ruvolo G:
Beneficial effects of C1 esterase inhibitor in ST-elevation
myocardial infarction in patients who underwent surgical
reperfusion: A randomised double-blind study. Eur J Cardiothorac
Surg. 32:326–332. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sidorenko GI, Gelis LG, Medvedeva EA,
Ostrovskiĭ IuP, Lazareva IV, Sevruk TV, Shibeko NA and Petrov IuP:
Pharmacological protection of the myocardium with reamberin in
coronary artery bypass grafting in patients with postinfarction
angina. Ter Arkh. 83:35–40. 2011.In Russian.
|
|
22
|
Michelis KC, Boehm M and Kovacic JC: New
vessel formation in the context of cardiomyocyte regeneration - the
role and importance of an adequate perfusing vasculature. Stem Cell
Res. 13(3 Pt B): 666–682. 2014. View Article : Google Scholar : PubMed/NCBI
|