Introduction
Rheumatoid arthritis (RA) is an autoimmune disease
characterized by abnormal immune responses, chronic inflammation of
peripheral joints and progressive bone destruction (1,2).
Under the pathological conditions of RA, bone homeostasis is in
persistent disequilibrium, resulting in uncoordinated bone
formation and degradation, and is mediated by abnormal osteoclast
formation (3). Osteoclasts are
derived from osteoclast precursor cells (OPCs), which originate
from the monocyte/macrophage lineage. Previous studies have
demonstrated that the osteoblast-associated synthesis and secretion
of receptor activator of nuclear factor κB ligand (RANKL) and
osteoclast-associated RANK are essential in osteoclastogenesis, and
that RANK on monocytes binds to RANKL, initiating osteoclast
differentiation (4,5). Furthermore, macrophage-derived
proinflammatory mediators in RA directly contribute to the
degradation of articular cartilage and subchondral bone, and the
activation of RA monocytes/macrophages occurs locally and in the
peripheral circulation (6,7). Therefore, the present study
investigated the osteoclastogenic potential of peripheral blood
mononuclear cells (PBMCs) from rheumatoid arthritis patients.
Curcumin is the primary active ingredient of
turmeric (Curcuma longa) and has been demonstrated to
possess anti-inflammatory and anti-arthritic properties (8). Previous studies have revealed its
underlying mechanisms of action as the induction of apoptosis in
human fibroblast-like synoviocytes and protection against
collagen-induced arthritis (9,10). A
randomized pilot study in patients with RA indicated that curcumin
treatment is safe and is not associated with any adverse events
(11). Accumulating evidence
suggests that signaling pathways malfunction in RA (12). Curcumin has been demonstrated to
mediate the suppression of mitogen-activated protein kinases
(MAPKs)/RANK and extracellular signal-regulated kinase 1 and 2
(ERK1/2) signaling pathways and thus promotes chondrogenic
differentiation (13,14). Furthermore, curcumin inhibits
osteoclast differentiation and function via the inhibition of the
signalosome-associated kinase inhibitor of κB in a dose-dependent
manner (15). Therefore, the
present study aimed to investigate the effect of curcumin on
osteoclastogenesis of PBMCs from patients with RA via the
suppression of the MAPK/RANK/c-Fos/nuclear factor of activated T
cells, cytoplasmic 1 (NFATc1) signaling pathways.
Materials and methods
Patients and PBMC culture
A total of 12 patients and 10 healthy controls were
recruited from the Department of Integrated Traditional Chinese and
Western Medicine, Jinling Hospital (Nanjing, China), and written
informed consent obtained. The study was approved by the Ethics
Committee of the Department of Integrated Traditional Chinese and
Western Medicine, Jinling Hospital. Human blood samples from the
patients with RA and healthy controls were collected between
January and December 2014.
PBMCs were separated from erythrocytes by density
centrifugation at 650 × g, 18°C for 20 min, using
Ficoll® PM 400 Histopaque®-1077
(Sigma-Aldrich, St. Louis, MO, USA) of blood samples, and were
maintained in α-minimal essential medium (α-MEM; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C
in a humidified 5% CO2 incubator (Thermo Fisher
Scientific, Inc.). The medium was replenished every second day.
Osteoclast differentiation
Isolated PBMCs were seeded onto plates. Non-adherent
cells were harvested after 24 h and were seeded (3.5×105
cells/well in 96-well plates) onto glass coverslips in the presence
of 50 ng/ml recombinant human macrophage colony-stimulating factor
(rhM-CSF; R&D Systems China Co., Ltd., Shanghai, China) and 100
ng/ml rhRANKL (R&D Systems China Co., Ltd.) for 14 days.
Osteoclasts with ≥3 nuclei/cell were identified as
tartrate-resistant acid phosphatase (TRAP) -positive cells.
Osteoclast differentiation was additionally confirmed by the
expression of RANK using reverse transcription-quantitative
polymerase chain reaction (RT-qPCR). To investigate the effect of
curcumin treatment, 0–10 µM curcumin was added to wells for
the 14 days of osteoclast differentiation.
Cell viability detection by Cell Counting
kit-8 (CCK-8)
PBMCs (1×105/well) were seeded in 96-well
plates (three wells per group) and treated with with curcumin (0–40
µM) for 48 h. Subsequently, 10 µl CCK-8 solution
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added
to each well, and cell viability was measured at 490 nm using an
enzyme-linked immunosorbent assay reader (BioTek Instruments, Inc.,
Winooski, VT, USA), according to the manufacturer's
instructions.
TRAP staining and bone resorption pit
assay
Cells were washed with phosphate-buffered saline,
fixed with 4% paraformaldehyde for 15 min and permeabilized with
0.1% Triton X-100. Fixed cells were subjected to an assay for TRAP
activity using an Acid Phosphatase, Leukocyte (TRAP) kit
(Sigma-Aldrich) according to the manufacturer's instructions.
Images were captured with a digital camera attached to the
microscope (Leica DM 2500; Leica Microsystems GmbH, Wetzlar,
Germany). TRAP positive multinucleated cells (≥3 nuclei) were
identified as osteoclasts, and the number of osteoclasts was
counted in 10 fields per sample.
PBMCs were seeded onto 100-µm thick bovine
bone slices and incubated with α-MEM containing 50 ng/ml M-CSF
and100 ng/ml RANKL. After 21 days, cells were removed by sonication
and the bovine bone slices were stained with 0.25% toluidine blue
(Sigma-Aldrich) to identify resorption pits. In addition,
resorption lacunae were visualized using a Hitachi S-3400N scanning
electron microscope (Hitachi High-Technologies Corporation, Tokyo,
Japan).
RT-qPCR
RNA was extracted from the PBMCs using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Synthesis of cDNA was
performed on 2 µg total RNA using Moloney Murine Leukemia
Virus reverse transcriptase (Promega Corporation, Madison, WI, USA)
and oligo dT 15 primers (Thermo Fisher Scientific, Inc.) according
to the manufacturer's instructions. qPCR was performed using the
Applied Biosystems 7300 Real-Time PCR System (Thermo Fisher
Scientific, Inc.). Reaction mixtures (20 µl) were prepared
using the TaqMan® Universal PCR Master Mix (Thermo
Fisher Scientific, Inc.), according to the manufacturer's
instructions. Following initial denaturation at 95°C for 3 min, 40
cycles were performed of denaturation at 95°C for 15 sec, annealing
at 56°C for 20 sec and extension at 72°C for 20 sec. The Cq
(quantification cycle fluorescence value) was calculated using SDS
software, version 2.1 (Applied Biosystems; Thermo Fisher
Scientific, Inc.), and the relative expression levels of RANK mRNA
were calculated using the 2−ΔΔCq method (16) and normalized to the internal
control, glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The
following primer sequences were used: Forward,
5′-CCTCGGGGTcTGGGAGTTCG-3′ and reverse,
5′-CGTACACCACGATGATGTCACCCT-3′ for RANK; and forward,
5′-ACAGGGGAGGTGATAGCATT-3′ and reverse,
5′-GACCAAAAGCCTTCATACATCTC-3′ for GAPDH.
Western blotting
PBMCs were homogenized and lysed in NP-40 buffer
(Beyotime Institute of Biotechnology, Haimen, China). Following
5–10 min boiling, cells were centrifuged at 10,000 × g, 4°C for 10
min to obtain the supernatant. Protein samples (50 µg) were
separated by 10% sodium dodecyl sulfate-polyacrylimide gel
electrophoresis and transferred to polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA). Membranes were
blocked with 5% (w/v) non-fat milk powder in Tris-buffered saline
and 0.1% (w/v) Tween 20 (TBST), and incubated with the following
primary antibodies: Rabbit anti-RANK (1:500; sc-9072), mouse
anti-c-Fos (1:1,000; sc-271243), mouse anti-NFATc1 (1:1,000;
sc-17834), mouse anti-phosphorylated (p)-ERK1/2 (1:1,000;
sc-136521), mouse anti-ERK1/2 (1:1,000; sc-514302), rabbit
anti-p-p38 (1:1,000; sc-17852-R), mouse anti-p38 (1:1,000;
sc-81621), goat anti-p-c-Jun N-terminal kinase (JNK; 1:1,000;
sc-12882) and mouse anti-JNK (1:1,000; sc-137019) all from Santa
Cruz Biotechnoogy, Inc. (Dallas, TX, USA), and rabbit anti-β-actin
antibody (1:2,000; catalog no. AP0060; Bioworld Technology, Inc.,
St. Louis Park, MN, USA), at 4°C overnight. Following three washes
with TBST, membranes were incubated with the following secondary
antibodies conjugated to horseradish peroxidase: Donkey anti-goat
IgG (1:10,000; sc-2020), donkey anti-mouse IgG (1:10,000; sc-2096)
and goat anti-rabbit IgG (1:10,000; catalog no. sc-2004) from Santa
Cruz Biotechnoogy, Inc. at a dilution of 1:10,000–1:20,000.
Following a 1-h incubation at 37°C, membranes were washed three
times with TBST. Blots were visualized using an enhanced
chemiluminescence system (Amersham; GE Healthcare Life Sciences,
Chalfont, UK). Signals were densitometrically assessed using
Quantity One® software version 4.5 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and normalized to the
β-actin signals to correct for unequal loading.
Clinical assessments
The Sharp score of patients with RA was calculated
using Multix Select DR X-ray (Siemens AG, Munich, Germany), as
described previously (17), from
the measurements of arthritis (scale, 0–5) and joint space
abnormality (scale, 0–4).
The T-score of Patients with RA was calculated as
described previously (18), from
the measurement of the bone mineral density of lumbar vertebra
L1-L4 by dual-energy X-ray absorptiometry.
Statistical analysis
Data are expressed as the mean ± standard deviation.
All statistical analyses were performed using GraphPad Prism
software, version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Correlations were assessed by Spearman's coefficient rho (ρ) with a
95% confidence interval. Groups were compared using one-way
analysis of variance, followed by Tukey's multiple comparison test
as a post hoc test to compare the mean values of each group.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Enhanced osteoclastogenic potential in
patients with RA
The osteoclastogenic potential of PBMCs from
patients with RA was induced in the presence of 50 ng/ml rhM-CSF
and 100 ng/ml rhRANKL. A total of three days later, PBMCs were
adhesive and had narrowly elongated morphologies. Following six
days of culture, fibroblast-like cells were significantly
increased, and PBMCs were in cell condensation following nine days
of culture. Notably, a large number of large polynuclear giant
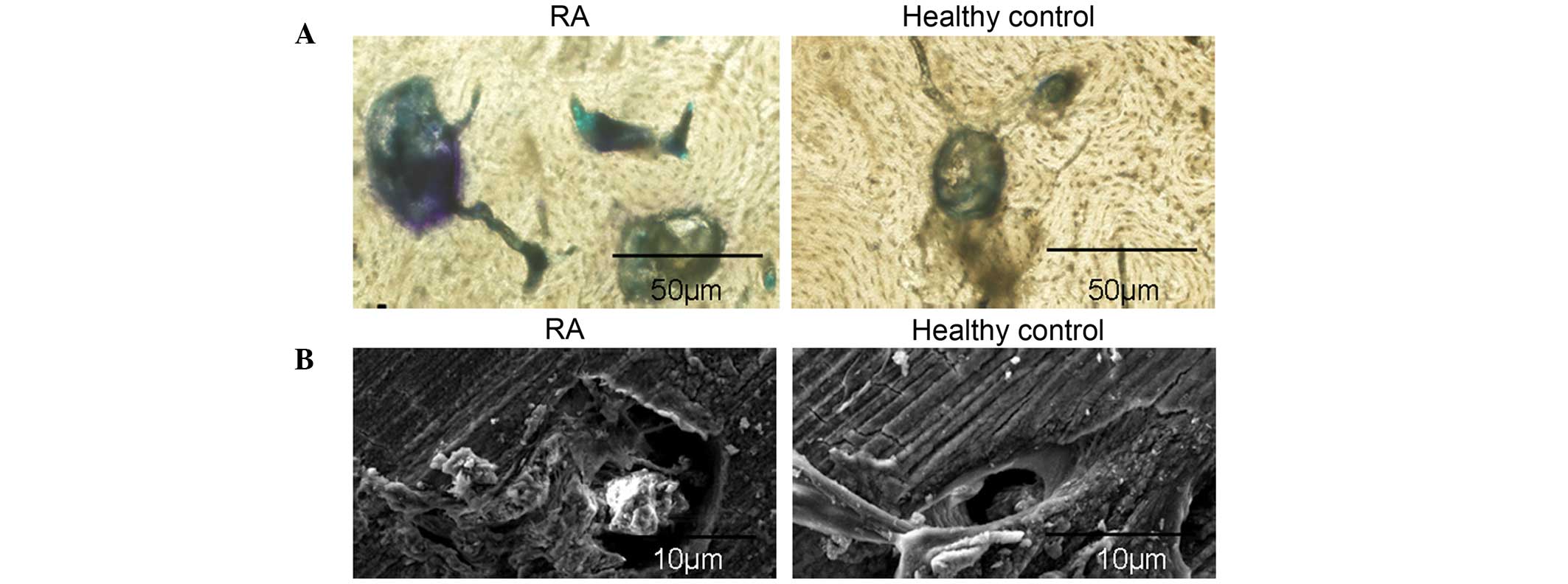
cells were observed at day 14 (Fig.
1A). Osteoclasts with ≥3 nuclei/cell were identified as
TRAP-positive cells, confirming that osteoclast differentiation was
induced by rhM-CSF and rhRANKL (Fig.
1B). Furthermore, the number of TRAP-positive osteoclasts
differentiated from PBMCs from patients with RA was significantly
increased compared with healthy controls (P=0.002; Fig. 1C). The resorption area was measured
as a separate indicator of osteoclast formation. The bone
resorption pits on bone slices were stained with toluidine blue,
and resorption areas were analyzed by light and electron microcopy.
The results demonstrated that the lacunar number and area of bone
resorption were increased in the RA group compared with the healthy
control group (Fig. 2A and B).
These results confirmed the increased osteoclastogenic potential of
PBMCs isolated from patients with RA.
 | Figure 1Enhanced osteoclastogenic potential of
PBMCs from patients with RA. (A) The morphology of PBMCs following
culture with 50 ng/ml M-CSF and 100 ng/ml RANKL. Following three
days in culture, PBMCs were adhesive and narrowly elongated,
following six days fibroblast-like cells were significantly
increased, and PBMCs were in cell condensation following nine days
of culture. Polynuclear giant cells were observed at day 14. (B)
Observation of osteoclast morphology in PBMCs from RA patients
using TRAP staining following 14 days in culture. Cells were fixed
in 4% formalin, permeabilized with 0.1% Triton X-100 and stained
with TRAP solution. (C) PBMCs isolated from RA patients and healthy
controls were cultured with 50 ng/ml M-CSF and 100 ng/ml RANKL for
14 days and TRAP staining performed. The number of osteoclasts
(TRAP-positive cells containing ≥3 nuclei/cell counted in 10 fields
of each sample) differentiated from PBMCs from patients with RA was
significantly increased compared with healthy controls. Data are
expressed as the mean ± standard deviation, n=10 per group. PBMCs,
peripheral blood mononuclear cells; M-CSF, macrophage
colony-stimulating factor; RANKL, receptor activator of nuclear
factor κB ligand; TRAP, tartrate-resistant acid phosphatase; RA,
rheumatoid arthritis; OC, osteoclasts. |
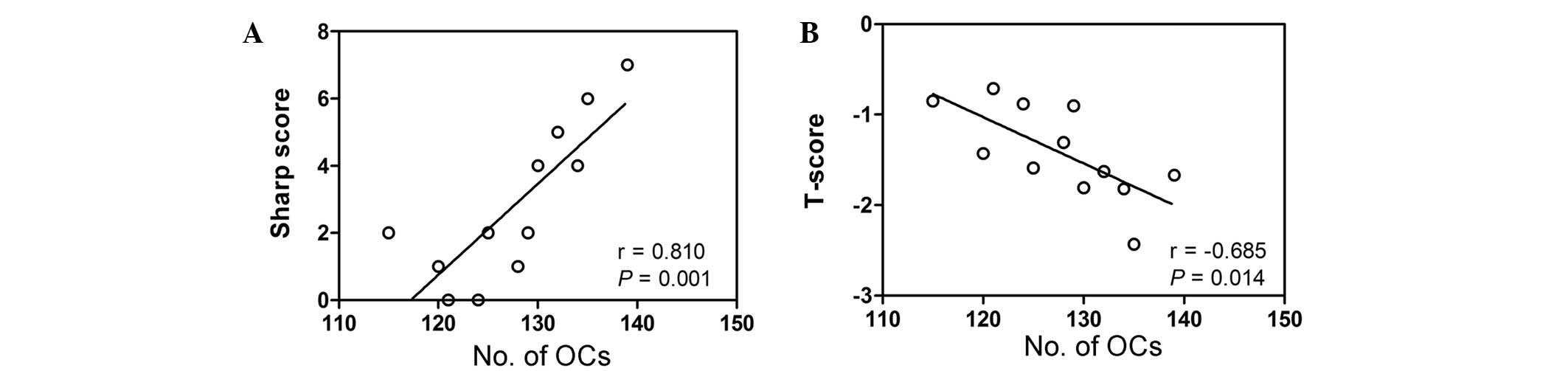
Correlation between osteoclastogenic
potential and clinical indicators
To determine whether there was a correlation between
the number of osteoclasts and a clinical indicator of disease, the
Sharp score was calculated by assessing articulation in the two
hands of patients with RA. This Sharp score was correlated with the
number of osteoclasts counted in TRAP-stained PBMCs isolated from
patients with RA and healthy controls. As presented in Fig. 3A, the Sharp score was significantly
positively correlated with the number of osteoclasts in patients
with RA (r=0.810; P=0.001). In addition, a significant negative
correlation was detected between the osteoclast number and the
lumbar T-score in patients with RA (r=−0.685; P=0.014; Fig. 3B).
Curcumin inhibits osteoclastogenic
potential of PBMCs from patients with RA
To investigate the cytotoxicity of curcumin, PBMCs
isolated from healthy controls were incubated with various
concentrations of curcumin for 48 h. The CCK-8 assay revealed that
PBMCs had similar viability following treatment with 0–10 µM
curcumin for 48 h. However, viability was significantly reduced at
concentrations of ≥20 µM (20 µM, P= 0.031; 40
µM, P= 0.006; Fig. 4A). To
evaluate the inhibitory effects of curcumin on the
osteoclastogenesis of PBMCs isolated from RA patients, M-CSF and
RANKL-treated cells were exposed to concentrations of curcumin
<20 µM. The results demonstrated that curcumin treatment
inhibited the number of osteoclasts generated in a dose-dependent
manner (2.5 µM, P=0.012; 5 µM, P=0.003; 10 µM,
P<0.001; Fig. 4B). This
reduction in osteoclast differentiation following curcumin
treatment was confirmed by the dose-dependent reduction in RANK
mRNA (2.5 µM, P=0.018; 5 µM, P=0.008; 10 µM,
P<0.001; Fig. 4C) and protein
(2.5 µM, P=0.035; 5 µM, P=0.009; 10 µM,
P<0.001; Fig. 4D) expression
levels. c-Fos and NFATc1 are crucial for osteoclast differentiation
(5,19). Therefore, it was examined whether
curcumin inhibited the osteoclastogenic potential of PBMCs from
patients with RA through regulation of the expression of c-Fos and
NFATc1 in response to M-CSF and RANKL stimulation. The western
blotting results demonstrated that the protein expression levels of
c-Fos and NFATc1 were significantly suppressed in PBMCs from RA
patients by curcumin in a dose-dependent manner (P<0.001 at all
curcumin concentrations; Fig. 4E).
To determine the involvement of signaling pathways and the
molecular mechanisms underlying the effects of curcumin on M-CSF
and RANKL-stimulated osteoclast differentiation of PBMCs from
patients with RA, the activation of MAPKs in PBMCs from RA patients
was evaluated. The results indicated that curcumin inhibited the
protein expression levels of p-ERK1/2, p-p38 and p-JNK in PBMCs
from RA patients in a dose-dependent manner (P<0.001 at all
curcumin concentrations; Fig. 4F).
These results suggest that curcumin inhibited M-CSF and
RANKL-stimulated osteoclast differentiation via intracellular MAPK
signaling pathways.
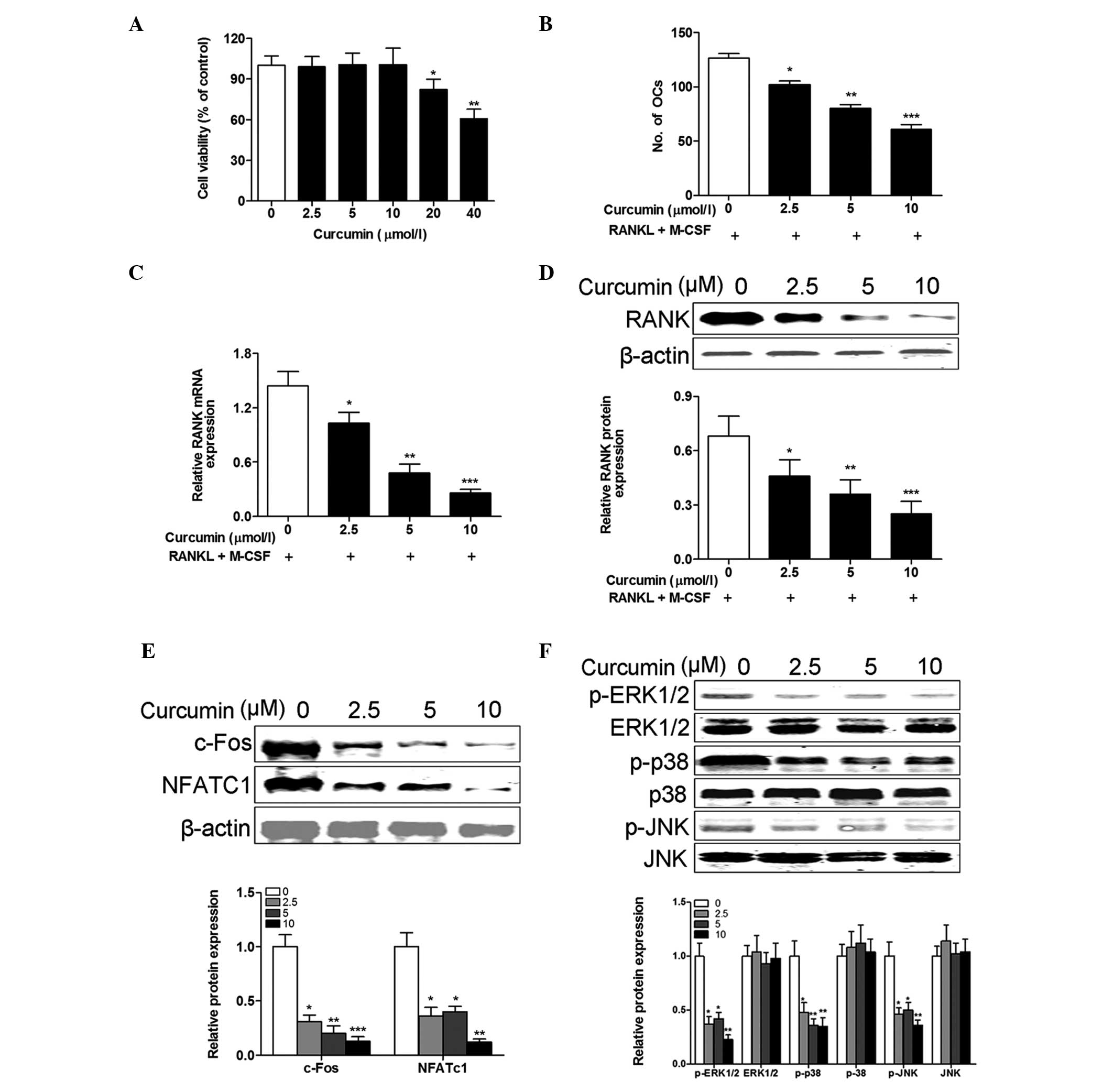 | Figure 4Curcumin inhibits the osteoclastogenic
potential of PBMCs from patients with RA. (A) PBMCs from healthy
controls were incubated with various concentrations of curcumin
(0–40 µM) in the presence of M-CSF (50 ng/ml) and RANKL (100
ng/ml) for 48 h, and the cell viability was examined using the Cell
Counting kit-8 assay. Cell viability was not affected by <20
µM curcumin. PBMCs from patients with RA were incubated with
various concentrations of curcumin (0–10 µM) in the presence
of 50 ng/ml M-CSF and 100 ng/ml RANKL for 14 days. (B) The
osteoclast differentiation was measured by TRAP staining and the
number of osteoclasts (TRAP-positive cells containing ≥3
nuclei/cell) was counted in 10 fields of each sample. Curcumin
treatment inhibited the number of osteoclasts generated in a
dose-dependent manner. The (C) mRNA and (D) protein expression
levels of RANK were measured by reverse transcription-quantitative
polymerase chain reaction and western blotting, respectively, and
were reduced by curcumin treatment. (E) The protein expression
levels of (E) c-Fos and NFATc1, and (F) p-ERK1/2, ERK1/2, p-p38,
P-38, p-JNK and JNK were measured and normalized to β-actin.
Curcumin treatment decreased the protein expression levels of
c-Fos, NFATc1, p-ERK1/2, p-p38 and p-JNK in a dose-dependent
manner. Data are expressed as the mean ± standard deviation, n=3
per group. *P<0.05, **P<0.01 and
***P<0.001 vs. 0 µmol/l curcumin treatment.
PBMCs, peripheral blood mononuclear cells; M-CSF, macrophage
colony-stimulating factor; RANKL, receptor activator of nuclear
factor κB ligand; RA, rheumatoid arthritis; RANK, receptor
activator of nuclear factor κB; TRAP, tartrate-resistant acid
phosphatase; NFATc1, nuclear factor of activated T cells,
cytoplasmic 1; p, phosphorylated; ERK1/2, extracellular
signal-regulated kinases 1 and 2; JNK, c-Jun N-terminal kinase. |
Discussion
Curcumin has been demonstrated to possess
anti-inflammatory activities in interleukin (IL)-1β-stimulated
human chondrocytes (14) and
murine macrophages (3). In RA,
proinflammatory cytokines, including IL-1, -6, -8 and -11 and tumor
necrosis factor α, have been reported to be osteoclastogenic
(3). However, the role of curcumin
in RA and its contribution to the inhibition of the
osteoclastogenic potential of PBMCs remains unclear. In the present
study, the anti-osteoclastogenic effect of curcumin and its
underlying mechanisms were investigated.
Monocytes migrate out of the peripheral blood and
into inflammatory tissue where they differentiate into resident
macrophages and dendritic cells, which secrete a variety of
inflammatory cytokines involved in the pathogenesis of RA (20,21).
In the present study, PBMCs from patients with RA were isolated to
investigate the systemic enhancement of osteoclastogenesis in RA.
The results confirmed the increased osteoclastogenic potential of
PBMCs isolated from patients with RA compared with healthy
controls. Furthermore, increased numbers of mature osteoclasts were
observed in PBMC cultures from patients with RA compared with
healthy controls. These results suggest that PBMCs may contribute
to osteoclast formation in the presence of osteoclastogenic
cytokines or RANKL in RA patients. Therefore, RA may have direct
effects on bone metabolism. Ikic et al (7) demonstrated that PBMC-derived OPCs
trans-differentiate into osteoclasts in the presence of M-CSF and
RANKL in vitro, suggesting that inflammatory factors may
directly contribute to osteoclastogenesis. The results of the
present study confirm a number of previous studies (22,23),
simultaneously, the Sharp score was positively correlated with
osteoclast number, and lumbar T-score was negatively correlated
with osteoclast number.
Previous studies indicate that inflammatory
cytokines enhance osteoclastogenesis via a RANKL-RANK dependent
mechanism, upregulating the expression levels of RANK on osteoclast
precursors and increasing their sensitivity to RANKL, which may
result in bone erosion in RA (3,24,25).
In the present study, curcumin inhibited the number of osteoclasts
generated from PBMCs in a dose-dependent manner, and reduced the
RANK mRNA and protein expression levels in PBMCs from patients with
RA. It has been previously demonstrated that RANKL activates
multiple signaling pathways in osteoclast precursors via RANK and
stimulates critical transcription factors for osteoclast
differentiation (26,27). NFATc1 is a crucial transcription
factor that is expressed in osteoclast precursors through
Ca2+ oscillation, MAPKs and c-Fos or RANK in response to
RANKL (28). In the present study,
curcumin inhibited the osteoclastogenic potential of PBMCs from
patients with RA, potentially via the suppression of ERK1/2, p38
and JNK activation, and the inhibition of c-Fos and NFATc1
expression. In conclusion, the results of the present study suggest
that curcumin inhibits osteoclast formation via preventing the
phosphorylation of components of the MAPK signaling pathways, and
that it may be a potential novel therapeutic agent for managing
osteoporosis or bone deterioration in inflammatory diseases
including RA. However, future studies are required to demonstrate
the anti-osteoclastogenic potential of curcumin in animal models of
osteoporosis and RA.
References
|
1
|
Wendling D, Abbas W, Godfrin-Valnet M,
Kumar A, Guillot X, Khan KA, Vidon C, Coquard L, Toussirot E, Prati
C and Herbein G: Dysregulated serum IL-23 and SIRT1 activity in
peripheral blood mononuclear cells of patients with rheumatoid
arthritis. PloS One. 10:e01199812015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Han M, Sung YK, Cho SK, Kim D, Won S, Choi
CB, Bang SY, Cha HS, Choe JY, Chung WT, et al: Factors associated
with the use of complementary and alternative medicine for Korean
patients with rheumatoid arthritis. J Rheumatol. 42:2075–2081.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jung SM, Kim KW, Yang CW, Park SH and Ju
JH: Cytokine-mediated bone destruction in rheumatoid arthritis. J
Immunol Res. 2014:2636252014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jules J, Wang S, Shi Z, Liu J, Wei S and
Feng X: The IVVY motif and tumor necrosis factor receptor
associated factor (TRAF) sites in the cytoplasmic domain of the
receptor activator of nuclear factor κB (RANK) cooperate to induce
osteoclastogenesis. J Biol Chem. 290:23738–23750. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kong X, Yang Y, Wu W, Wan H, Li X, Zhong
M, Su X, Jia S and Lin N: Triterpenoid Saponin W3 from Anemone
flaccida suppresses osteoclast differentiation through inhibiting
activation of MAPKs and NF-κB pathways. Int J Biol Sci.
11:1204–1214. 2015. View Article : Google Scholar :
|
|
6
|
Schulze-Koops H, Davis LS, Kavanaugh AF
and Lipsky PE: Elevated cytokine messenger RNA levels in the
peripheral blood of patients with rheumatoid arthritis suggest
different degrees of myeloid cell activation. Arthritis Rheum.
40:639–647. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ikić M, Jajić Z, Lazić E, Ivčević S,
Grubišić F, Marušić A, Kovačić N and Grčević D: Association of
systemic and intra-articular osteoclastogenic potential,
proinflammatory mediators and disease activity with the form of
inflammatory arthritis. Int Orthop. 38:183–192. 2014. View Article : Google Scholar
|
|
8
|
Yang Y, Wu X, Wei Z, Dou Y, Zhao D, Wang
T, Bian D, Tong B, Xia Y, Xia Y and Dai Y: Oral curcumin has
anti-arthritic efficacy through somatostatin generation via
cAMP/PKA and Ca(2+)/CaMKII signaling pathways in the small
intestine. Pharmacol Res. 95–96:71–81. 2015. View Article : Google Scholar
|
|
9
|
Kloesch B, Becker T, Dietersdorfer E,
Kiener H and Steiner G: Anti-inflammatory and apoptotic effects of
the polyphenol curcumin on human fibroblast-like synoviocytes. Int
Immunopharmacol. 15:400–405. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang G, Xu Z, Huang Y, Duan X, Gong W,
Zhang Y, Fan J and He F: Curcumin protects against collagen-induced
arthritis via suppression of BAFF production. J Clin Immunol.
33:550–557. 2013. View Article : Google Scholar
|
|
11
|
Chandran B and Goel A: A randomized, pilot
study to assess the efficacy and safety of curcumin in patients
with active rheumatoid arthritis. Phytother Res. 26:1719–1725.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Niu X, Lu C, Xiao C, Zhang Z, Jiang M, He
D, Bian Y, Zhang G, Bian Z and Lu A: The shared crosstalk of
multiple pathways involved in the inflammation between rheumatoid
arthritis and coronary artery disease based on a digital gene
expression profile. PloS One. 9:e1136592014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Buhrmann C, Mobasheri A, Matis U and
Shakibaei M: Curcumin mediated suppression of nuclear factor-κB
promotes chondrogenic differentiation of mesenchymal stem cells in
a high-density co-culture microenvironment. Arthritis Res Ther.
12:R1272010. View
Article : Google Scholar
|
|
14
|
Shakibaei M, Mobasheri A and Buhrmann C:
Curcumin synergizes with resveratrol to stimulate the MAPK
signaling pathway in human articular chondrocytes in vitro. Genes
Nutr. 6:171–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
von Metzler I, Krebbel H, Kuckelkorn U,
Heider U, Jakob C, Kaiser M, Fleissner C, Terpos E and Sezer O:
Curcumin diminishes human osteoclastogenesis by inhibition of the
signalosome-associated I kappaB kinase. J Cancer Res Clin Oncol.
135:173–179. 2009. View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Ravindran J, Cavill C, Balakrishnan C,
Jones SM, Korendowych E and McHugh NJ: A modified Sharp score
demonstrates disease progression in established psoriatic
arthritis. Arthritis Care Res (Hoboken). 62:86–91. 2010. View Article : Google Scholar
|
|
18
|
Chen P, Miller PD, Binkley NC, Kendler DL,
Wong M and Krohn K: Use of lowest single lumbar spine vertebra bone
mineral density T-score and other T-score approaches for diagnosing
osteoporosis and relationships with vertebral fracture status. J
Clin Densitom. 11:525–531. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim JY, Min JY, Baek JM, Ahn SJ, Jun HY,
Yoon KH, Choi MK, Lee MS and Oh J: CTRP3 acts as a negative
regulator of osteoclastogenesis through AMPK-c-Fos-NFATc1 signaling
in vitro and RANKL-induced calvarial bone destruction in vivo.
Bone. 79:242–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi C and Pamer EG: Monocyte recruitment
during infection and inflammation. Nat Rev Immunol. 11:762–774.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
McInnes IB and Schett G: The pathogenesis
of rheumatoid arthritis. N Engl J Med. 365:2205–2219. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pathak JL, Bravenboer N, Verschueren P,
Lems WF, Luyten FP, Klein-Nulend J and Bakker AD: Inflammatory
factors in the circulation of patients with active rheumatoid
arthritis stimulate osteoclastogenesis via endogenous cytokine
production by osteoblasts. Osteoporos Int. 25:2453–2463. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Izawa T, Mori H, Shinohara T, Mino-Oka A,
Hutami IR, Iwasa A and Tanaka E: Rebamipide attenuates mandibular
condylar degeneration in a murine model of TMJ-OA by mediating a
chondroprotective effect and by downregulating RANKL-mediated
osteoclastogenesis. PloS One. 11:e01541072016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adamopoulos IE, Chao CC, Geissler R,
Laface D, Blumenschein W, Iwakura Y, McClanahan T and Bowman EP:
Interleukin-17A upregulates receptor activator of NF-kappaB on
osteoclast precursors. Arthritis Res Ther. 12:R292010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gravallese EM, Harada Y, Wang JT, Gorn AH,
Thornhill TS and Goldring SR: Identification of cell types
responsible for bone resorption in rheumatoid arthritis and
juvenile rheumatoid arthritis. Am J Pathol. 152:943–951.
1998.PubMed/NCBI
|
|
26
|
Lee EG, Yun HJ, Lee SI and Yoo WH: Ethyl
acetate fraction from Cudrania tricuspidata inhibits
IL-1beta-stimulated osteoclast differentiation through
downregulation of MAPKs, c-Fos and NFATc1. Korean J Intern Med.
25:93–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee ZH and Kim HH: Signal transduction by
receptor activator of nuclear factor kappaB in osteoclasts. Biochem
Biophys Res Commun. 305:211–214. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takayanagi H: Mechanistic insight into
osteoclast differentiation in osteoimmunology. J Mol Med (Berl).
83:170–179. 2005. View Article : Google Scholar
|