Introduction
The periodontium is composed of gingival tissue,
periodontal ligament, alveolar bone and cementum. During
orthodontic tooth movement, periodontal ligament cells (PDLCs) are
directly subject to mechanical stress, and are critical in
regulating the processes of periodontal tissue repair and
remodeling (1). Previously,
several studies have shown that orthodontic tooth movement is
regulated by PDLCs via modulation of the activity of alkaline
phosphatase (ALP), production of osetocalcin (OCN) and synthesis of
collagen type I (COL-1). In addition, the biological
characteristics of PDLCs can be altered under the action of
mechanical force (2–5). It is generally accepted that ALP is
involved in the process of calcification in various mineralizing
tissues (6). OCN is considered to
be a marker of bone formation (7,8).
Collagen fibers are the predominant component of the periodontal
ligament extracellular matrix (ECM), and collagen types I and III
are important in periodontal tissue remodeling (9). Therefore, the synthesis and
degradation of ECM are key processes in the regulation of bone
remodeling (10).
Hydrogen sulfide (H2S) is an endogenous
gaseous signaling molecule, which has been traditionally classified
as a toxic gas (11). In previous
years, it was reported that low concentrations of H2S
have anti-inflammatory, cytoprotective and chemopreventative
potential (12), and have shown
anticancer effects (13,14). H2S is synthesized
endogenously from L-cysteine in mammals by at least two
pyridoxal-5′-phosphate-dependent enzymes, cystathionine-γ-lyase and
cystathionine β-synthase, in various organs (15,16).
This molecule can permeate the cellular membrane without the
assistance of a specific transporter. There are limited reports
regarding with the effects of H2S on the biological
activity of human PDLCs (hPDLCs), particularly during mechanical
stress. Our previous results showed that H2S upregulated
the expression ratio of OPG/receptor activator of nuclear factor-κB
ligand (RANKL) in hPDLCs, with the maximum effect being observed at
0.5 mM, and tension force enhanced the effect of H2S on
the expression of OPG/RANKL (17).
The present study, investigated the effect of H2S on the
expression levels of ALP, OCN and COL-1 in hPDLCs with and without
tension force in order to further understand the effects of
H2S on periodontal tissue remodeling.
Materials and methods
Cell isolation and culture
The hPDLCs were obtained from 21 patients (10 male
and 11 female) aged between 12 and 16 years who required premolar
extraction during the course of orthodontic treatment between
August 2014 and October 2014 at Affiliated Stomatology Hospital of
Tongji University (Shanghai, China). All patients signed informed
consent and the study was approved by the Ethics Committee
(2013-NSFC002) of Tongji University (Shanghai, China). The teeth,
which were absent of inflammation, were immediately placed into a
tube containing 1% Dulbecco's modified Eagle's medium (DMEM;
Hyclone; GE Healthcare Life Sciences, Logan, UT, USA), 100 U/ml
penicillin and 100 U/ml streptomycin. The cells were scraped from
the middle third of the root and maintained in DMEM supplemented
with 15% charcoal-stripped serum (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), 100 U/ml penicillin and 100 mg/ml
streptomycin at 37°C in a 5% CO2 incubator, with
replacement of the medium every 2 days. On reaching confluence, the
cells were detached with 0.25% trypsin without EDTA and subcultured
at a 1:3 ratio (18). In the
subsequent experiments, cells between the third and eighth passages
were used.
Cell viability assay
To determine cell viability, the hPDLCs were seeded
into two 96-well plates with a cell density of 5×103
cells per well. Each treatment group was evaluated in triplicate.
Sodium hydrosulfide (NaHS), as an exogenous donor (19), has been used to examine various
biological activities of H2S. In the present study, NaHS
(Aladdin, Shanghai, China) was dissolved in phosphate-buffered
saline (PBS) and diluted into four concentrations (0.01, 0.05, 0.1
and 0.5 mM) with DMEM supplemented with 2% charcoal-stripped serum.
The cells were then treated with H2S (0.01–0.5 mM) for
1–5 days and incubated at 37°C in 5% CO2. A Cell
Counting Kit 8 (CCK 8) was then used to determine the viability of
the cells. The optical density (OD) values of the media were
measured at λ=450 nm using a microplate reader (Infinite™ 200;
Tecan Austria GmbH, Grödig, Austria).
Flow cytometric analysis
The hPDLCs (1×106 cells/ml) were seeded
in six-well plates and treated with H2S (0.01, 0.05, 0.1
and 0.5 mM) for 1–5 days at 37°C in 5% CO2. An Annexin
V-fluorescein isothiocyanate (FITC) kit (BioVison, Inc., Mountain
View, CA, USA) was used to assess the apoptosis of cells following
treatment with H2S for different durations.
Tension force stimulation
The cells (1×106 cells/ml) were seeded
into four flexible plates and pre-treated with H2S (0,
0.01, 0.05, 0.1 and 0.5 mM) in DMEM containing 2% charcoal-stripped
serum for 24 h at 37°C in 5% CO2. The four flexible
plates were subjected to a wave of 5% elongation, 0.5Hz (2 sec)
(20) for 1, 3 and 6 h every time.
The cells were collected for western blot and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analyses.
RT-qPCR analysis
Total cellular mRNA was obtained using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. Complementary DNA was synthesized
using a PrimeScript 1st Strand cDNA Synthesis kit (Takara Bio,
Inc., Tokyo, Japan). The qPCR was performed using a SYBR Premix Ex
Taq II (Tli RNase H Plus) kit (Takara Bio, Inc.) in an ABI Prism
7500 Detection System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The primer sequences are listed in Table I. The thermocycling conditions were
as follows: 37°C for 15 min and 85°C for 5 sec (for the reverse
transcription); followed by 40 cycles of 95°C for 5 sec and 60°C
for 34 sec. Relative fold changes were calculated using the
2−∆∆Cq method (21) and
standard curves were produced. The Cq values of the samples were
normalized to the appropriate endogenous housekeeping gene, GAPDH.
Each measurement was performed in triplicate.
 | Table IPrimer sequences for ALP, OCN, COL-1
and GAPDH. |
Table I
Primer sequences for ALP, OCN, COL-1
and GAPDH.
| Gene | Primer
sequence
(5′–3′) | Length
(bp) |
|---|
| ALP | F:
CTACACGGTCCTCCTATAC | 19 |
| R:
CTCGCTCTCGGTAACATC | 18 |
| OCN | F:
CAGAGTCCAGCAAAGGTG | 18 |
| R:
CCAGCCATTGATACAGGTA | 19 |
| COL-1 | F:
GCTGTCTTATGGCTATGATGAGAA | 24 |
| R:
GACCACGAGGACCAGAGG | 18 |
| GAPDH | F:
AGAAGGCTGGGGCTCATTTG | 20 |
| R:
AGGGGCCATCCACAGTCTTC | 20 |
Western blot analysis
The cells were lysed using radioimmunoprecipitation
assay buffer (Beyotime Institute of Biotechnology, Haimen, China)
and stored at −80°C. The cell lysates were separated on 10% (ALP
and COL-1) and 15% (OCN) SDS-polyacrylamide electrophoresis gels
and transferred onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA) using a semi-dry transfer cell
(Tannon Science & Technology Co., Ltd., Shanghai, China). The
membranes were blocked for 1 h in 5% dry milk, rinsed and incubated
with rabbit polyclonal anti-ALP antibody (cat. no. ab95462), rabbit
monoclonal anti-OCN antibody (cat. no. ab133612), rabbit monoclonal
anti-COL 1 antibody (cat. no. ab138492; all obtained from Abcam,
Cambridge, MA, USA) at 1:1,000 dilutions in Tris-buffered saline
(TBS) overnight at 4°C. The primary antibodies were then removed by
washing the membranes three times in TBS. The primary antibodies
were labeled by incubation with 0.1 mg/ml horseradish
peroxidase-labeled goat anti-rabbit secondary antibodies (1:2,000;
cat. no. KGAA35; Nanjing KeyGen Biotech Co., Ltd., Nanjing, China)
at room temperature for 1 h. Following three washes in TBS, the
antibody-bound proteins were detected using an enhanced
chemiluminescence system (EMD Millipore).
Statistical analysis
All data in the present study are presented as the
mean ± standard deviation from three independent experiments. Data
was analyzed using Student's t-test with SAS 8.2 (SAS
Institute, Cary, NC, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of H2S on cell
proliferation
The cells were treated with different concentrations
of H2S for 1–5 days. H2S had no significant
effect on cell proliferation when the hPDLCs were treated for 3
days. However, H2S reduced the proliferation of the
hPDLCs in a concentration-dependent manner following 4 and 5 days
of incubation (Fig. 1).
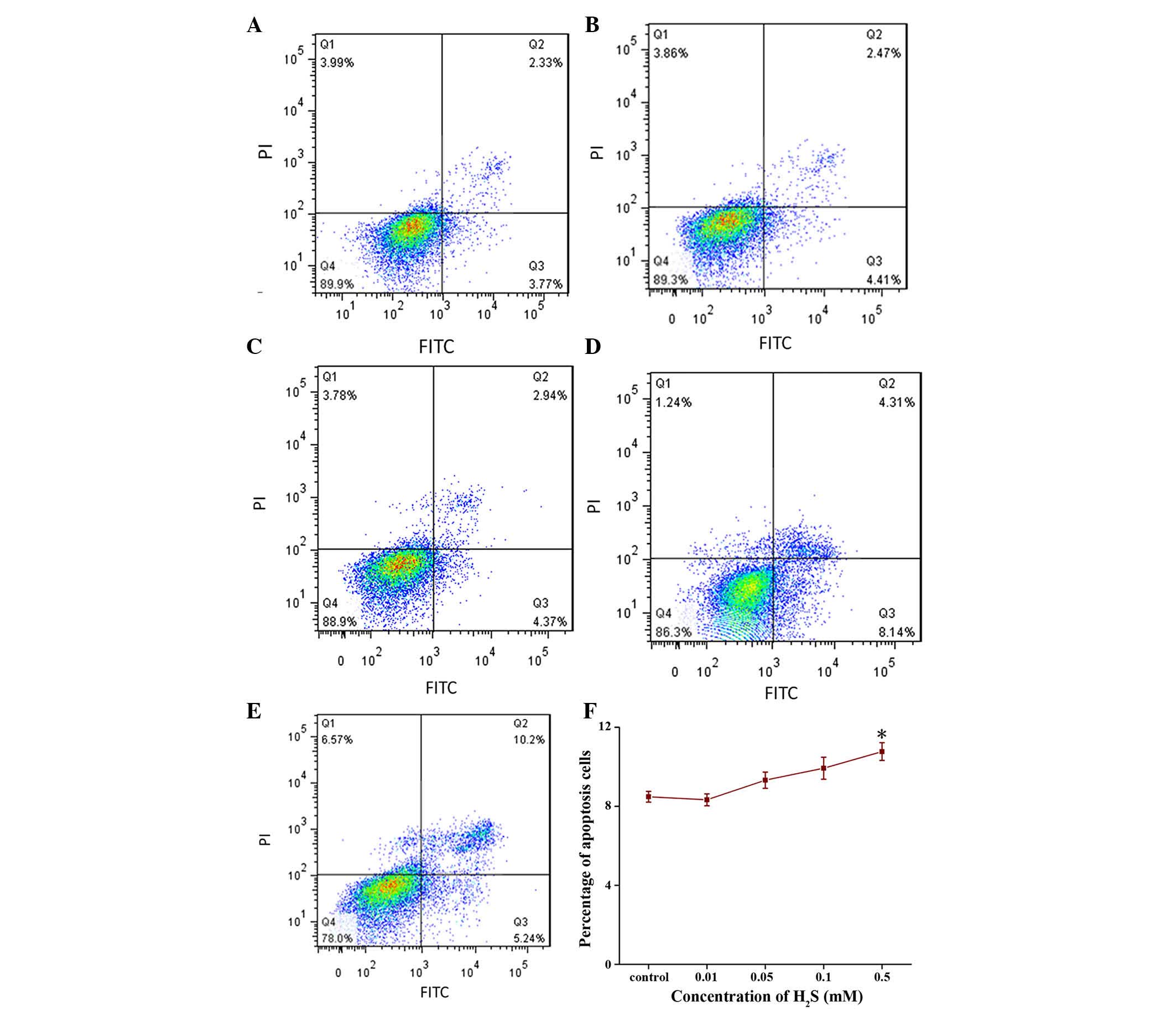
Effect of H2S on cell
apoptosis
No effects of H2S on cell apoptosis were
detected using flow cytometry when the cells were treated up to 4
days. However, H2S significantly induced apoptosis at
the concentration of 0.5 mM following 5 days of treatment (Fig. 2A–E).
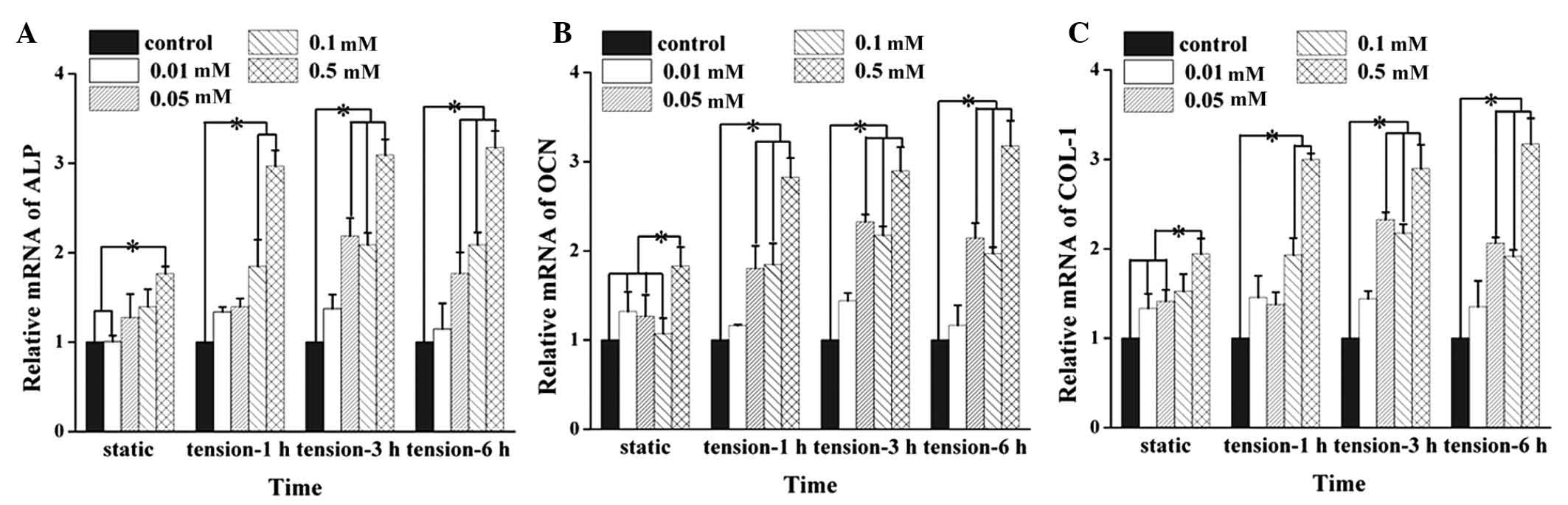
Effect of H2S on mRNA
expression levels of ALP, OCN and COL-1
Data on the mRNA expression levels were obtained
using RT-qPCR analysis, and the results showed that H2S
significantly upregulated the mRNA expression levels of ALP, OCN
and COL-1 in the hPDLCs at the concentration of 0.5 mM (Fig. 3A–C). Treatment with 0.1 and 0.5 mM
H2S increased the mRNA expression levels of ALP and
COL-1 induced by 1 h tension force stimulation (Fig. 3A and C). Treatment with 0.05–0.5 mM
H2S upregulated the mRNA expression levels of ALP and
COL-1 following tension force application for 3 and 6 h (Fig. 3A and C). The mRNA expression levels
of OCN induced by tension force stimulation for 1, 3 and 6 h
significantly increased following pretreatment with 0.05, 0.1 and
0.5 mM H2S (Fig.
3B).
Effect of H2S on protein
expression levels of ALP, OCN and COL-1
To investigate the role of H2S in hPDLCs,
the protein expression levels of ALP, OCN and COL-1 were determined
using western blot analysis following H2S pretreatment
with or without subsequent tension force application (Fig. 4A–D). The results showed that
treatment with 0.05–0.5 mM H2S significantly upregulated
the protein expression levels of ALP, OCN and COL-1 (Fig. 4E–G). Treatment with 0.01–0.5 mM
H2S significantly upregulated the protein expression
levels of ALP and OCN in the cells subjected to 1 and 3 h of
tension force application, and the protein expression levels of ALP
and OCN induced by 6h tension force application significantly
increased in the cells pre-exposed to H2S 0.05–0.5 mM;
Fig. 4E and F). Treatment with
0.01–0.5 mM H2S significantly upregulated the protein
expression of COL-1 in the cells induced by tension force
application for 1 h, and treatment with 0.05–0.5 mM H2S
significantly upregulated the protein expression of COL-1 in the
cells subjected to tension force for 3 and 6 h. However, protein
expression levels of OCN and COL-1 induced by 3 h of tension force
was more marked, compared with those subjected to 1 and 6 h tension
force application following pretreatment with 0.5 mM H2S
(Fig. 4F and G).
 | Figure 4Protein expression of ALP, OCN and
COL-1 in hPDLCs following H2S pretreatment. The hPDLCs
were treated with H2S (0–0.5 mM) and subjected to
tension force application for (A) 0, (B) 1, (C) 3 or (D) 6 h. The
protein expression levels of (E) ALP, (F) OCN and (G) COL-1 in the
cells were examined using western blot analysis. Prior to
application of tension force, the protein expression of ALP and
COL-1 was significantly increased at concentrations of 0.05–0.5 mM.
The protein expression of OCN was significantly increased at 0.05
and 0.5mM. Following tension force application, 0.01–0.5 mM
H2S significantly upregulated the protein expression
levels of ALP and OCN in response to 1 and 3 h tension force, and
0.05–0.5 mM H2S H2S significantly increased
the protein expression levels of ALP and OCN induced by 6 h tension
force. Pretreatment with 0.01–0.5 mM H2S significantly
upregulated the protein expression of COL-1 following tension force
stimulation for 1 h. Treatment with 0.05–0.5 mM H2S
significantly increased the protein expression of COL-1 in response
to 3 and 6 h tension force. Protein expression levels of OCN and
COL-1 were higher in response to 3 h tension, compared with 1 and 6
h tension following incubation with 0.5 mM H2S. Data are
expressed as the mean ± standard deviation. (*P<0.01
and **P<0.01). H2S, hydrogen sulfide; ALP,
alkaline phosphatase; OCN, osteocalcin; COL-1, collagen type I. |
Discussion
H2S has been recognized as a
gasotransmitter, which has multiple physiological and
pathophysiological functions in various mammalian systems (22,23).
It has been reported that H2S has potential
anti-inflammatory, anti-apoptotic, anticancer and neuroprotective
effects (24,25). Previous studies have shown that the
exogenous donor of NaHS significantly protects PC12 cells against
formaldehyde-induced cytotoxicity and apoptosis through attenuating
the accumulation of reactive oxygen species (ROS), upregulating
levels of B cell lymphoma-2 (Bcl-2) and downregulating the
expression of Bcl-2-associated X protein (26,27).
In the present study, cytotoxicity was observed in response to
H2S at a high concentration (Fig. 2). A high concentration of
H2S may increase ROS formation and mitochondrial
depolarization (28), decreasing
the concentration of oxygen and leading to hypoxia and cell death.
By contrast, low concentrations of H2S were protective
and relatively safe to hPDLCs, suggesting <0.5 mM H2S
is useful in hPDLCs.
hPDLCs produce ECM components, including collagen,
which build up the periodontal ligament to secure attachment of the
root cementum to the surrounding alveolar bone and is important in
the restoration of mineralized tissue (29–31).
In the present study, hPDLCs were isolated and characterized for
their mesenchymal origin, with confirmation of their
fibroblast-like morphology, as in our previous report (17). hPDLCs are capable of producing ALP,
OCN and COL-1, suggesting osteoblast- and fibroblast-like features,
which is consistent with previous studies (32,33).
hPDLCs can differentiate into either osteoblasts or cementoblasts
in response to mechanical force (34–38).
In the present study, in order to investigate the effect of
H2S on hPDLCs during orthodontic tooth movement, an
appropriate tension force was applied to the hPDLCs following
H2S treatment. As mentioned previously, our previous
study demonstrated that H2S had a regulatory role within
the periodontal remodeling process by promoting osteogenic
differentiation via upregulating the expression ratio of OPG/RANKL
in the hPDLCs. This promoting effect of H2S on OPG/RANKL
was enhanced by tension force application (17). In the present study, it was
observed that H2S upregulated the expression levels of
ALP, OCN and COL-1 in a concentration-dependent manner, and
pre-treatment with H2S enhanced the expression levels of
ALP, OCN and COL-1 induced by tension force application.
ALP is a marker for the calcification and
osteoblastic differentiation of PDLCs (39,40).
OCN is a vitamin K-dependent Ca2+-binding protein of the
bone matrix, which is synthesized by osteoblast-like cells and is
considered to be a marker for bone formation (7,8). The
collagen molecules in the periodontal ligament are known to respond
to mechanical stimuli (41,42).
COL-1 is one of the major fibrous elements of the periodontal
ligament. It forms solid fibers anchored to the cementum and
alveolar bone, and protects the periodontal ligament from tensile
stress and masticatory loading (34,35).
Therefore, the present study hypothesized that H2S
regulates hPDLC differentiation, tissue mineralization and collagen
synthesis through modulation of the mRNA and protein levels of ALP,
OCN and COL-1.
The data obtained in the present study showed that
the H2S-induced mRNA and protein expression levels of
ALP, OCN and COL-1 were upregulated following tension force
application (Fig. 4E). The
mechanism underlying this H2S-induced expression of ALP,
OCN and COL-1 in response to tension-force was not determined in
the present study. It has been demonstrated that light mechanical
strain induces biological changes in hPDLCs, including the release
of various types of adhesion molecules and osteogenic factors,
including ALP, OCN and COL-1 (2–4).
Studies have shown that static mechanical deformation of PDLCs
activates c-Jun and c-Fos, and increases the binding of activator
protein-1 to the promoter of ALP, which is a major effector of
osteoblastic differentiation (43). The gene expression of ALP in hPDLCs
as a response to tension stimulation is associated with the
intensity of the mechanical force and the culture conditions used
(44–47). These findings indicate a potential
synergetic effect between H2S and tension force
application.
In conclusion, the present study demonstrated that
exposure of hPDLCs to a high concentration and prolonged duration
with H2S reduced the viability of the hPDLCs. It was
observed that H2S significantly upregulated the
expression levels of ALP, OCN and COL-1 in the hPDLCs, and
pretreatment of the cells with H2S enhanced the
expression levels of the proteins induced by tension force
stimulation. These results suggested that H2S may be
important in remodeling and functional regulation of periodontal
tissue. Further investigations are required to elucidate the
underlying cellular mechanism.
Acknowledgments
This study was supported by the National Science
Foundation of China (grant no. 81371177).
References
|
1
|
McCulloch CA, Lekic P and McKee MD: Role
of physical forces in regulating the form and function of the
periodontal ligament. Periodontol. 24:56–72. 2000. View Article : Google Scholar
|
|
2
|
Matsuda N, Morita N, Matsuda K and
Watanabe M: Proliferation and differentiation of human osteoblastic
cells associated with differential activation of MAP kinases in
response to epidermal growth factor, hypoxia, and mechanical stress
in vitro. Biochem Biophys Res Commun. 249:350–354. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shimizu N, Ozawa Y, Yamaguchi M, Goseki T,
Ohzeki K and Abiko Y: Induction of COX-2 expression by mechanical
tension force in human periodontal ligament cells. J Periodontol.
69:670–677. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Myokai F, Oyama M, Nishimura F, Ohira T,
Yamamoto T, Arai H, Takashiba S and Murayama Y: Unique genes
induced by mechanical stress in periodontal ligament cells. J
Periodontal Res. 38:255–261. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu M, Dai J, Lin Y, Yang L, Dong H, Li Y,
Ding Y and Duan Y: Effect of the cyclic stretch on the expression
of osteogenesis genes in human periodontal ligament cells. Gene.
491:187–193. 2012. View Article : Google Scholar
|
|
6
|
De Bernard B: Glycoproteins in the local
mechanism of calcification. Clin Orthop Relat Res. 233–244.
1982.PubMed/NCBI
|
|
7
|
Hauschka PV: Osteocalcin: The vitamin
K-dependent Ca2+-binding protein of bone matrix. Haemostasis.
16:258–272. 1986.PubMed/NCBI
|
|
8
|
Neugebauer BM, Moore MA, Broess M,
Gerstenfeld LC and Hauschka PV: Characterization of structural
sequences in the chicken osteocalcin gene: Expression of
osteocalcin by maturing osteoblasts and by hypertrophic
chondrocytes in vitro. J Bone Miner Res. 10:157–163. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang YH, Ohsaki Y and Kurisu K:
Distribution of type I and type III collagen in the developing
periodontal ligament of mice. Matrix. 11:25–35. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mariotti A: The extracellular matrix of
the periodontium: Dynamic and interactive tissues. Periodontol
2000. 3:39–63. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beauchamp RO Jr, Bus JS, Popp JA, Boreiko
CJ and Andjelkovich DA: A critical-review of the literature on
hydrogen-sulfide toxicity. Crit Rev Toxicol. 13:25–97. 1984.
View Article : Google Scholar
|
|
12
|
Zanardo RC, Brancaleone V, Distrutti E,
Fiorucci S, Cirino G and Wallace JL: Hydrogen sulfide is an
endogenous modulator of leukocyte-mediated inflammation. FASEB J.
20:2118–2120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma HB, Huang S, Yin XR, Zhang Y and Di ZL:
Apoptotic pathway induced by diallyl trisulfide in pancreatic
cancer cells. World J Gastroenterol. 20:193–203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee ZW, Teo XY, Tay EY, Tan CH, Hagen T,
Moore PK and Deng LW: Utilizing hydrogen sulfide as a novel
anti-cancer agent by targeting cancer glycolysis and pH imbalance.
Br J Pharmacol. 171:4322–4336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tay AS, Hu LF, Lu M, Wong PT and Bian JS:
Hydrogen sulfide protects neurons against hypoxic injury via
stimulation of ATP-sensitive potassium channel/protein kinase
C/extracellular signal-regulated kinase/heat shock protein 90
pathway. J Neuroscience. 167:277–286. 2010. View Article : Google Scholar
|
|
16
|
Baskar R and Bian J: Hydrogen sulfide gas
has cell growth regulatory role. Eur J Pharmacol. 656:5–9. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liao C and Hua Y: Effect of hydrogen
sulphide on the expression of osteoprotegerin and receptor
activator of NF-kB ligand in human periodontal ligament cells
induced by tension-force stimulation. Arch Oral Biol. 58:1784–1790.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Y, Liu F, Zhang X and Shu L: Insulin
modulates cytokines expression in human periodontal ligament cells.
Arch Oral Biol. 59:1301–1306. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hughes MN, Centelles MN and Moore KP:
Making and working with hydrogen sulfide: The chemistry and
generation of hydrogen sulfide in vitro and its measurement in
vivo: A review. Free Radical Bio Med. 47:1346–1353. 2009.
View Article : Google Scholar
|
|
20
|
Dong-Xu L, Hong-Ning W, Chun-Ling W, Hong
L, Ping S and Xiao Y: Modulus of elasticity of human periodontal
ligament by optical measurement and numerical simulation. Angle
Orthod. 81:229–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ito M, Suga T, Akiyoshi K, Nukuzuma S,
Kon-no M, Umegaki Y, Kohdera U and Ihara T: Detection of measles
virus RNA on SYBR green real-time reverse transcription-polymerase
chain reaction. Pediatr Int. 52:611–615. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kimura H, Nagai Y, Umemura K and Kimura Y:
Physiological roles of hydrogen sulfide: Synaptic modulation,
neuroprotection, and smooth muscle relaxation. Antioxid Redox
Signal. 7:795–803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Piper HM, Meuter K and Schäfer C: Cellular
mechanisms of ischemia-reperfusion injury. Ann Thorac Surg.
75:S644–S648. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abe K and Kimura H: The possible role of
hydrogen sulfide as an endogenous neuromodulator. J Neurosci.
16:1066–1071. 1996.PubMed/NCBI
|
|
25
|
Wei HJ, Li X and Tang XQ: Therapeutic
benefits of H2S in Alzheimer's disease. J Clin Neurosci.
21:1665–1669. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang XQ, Yang CT, Chen J, Yin WL, Tian SW,
Hu B, Feng JQ and Li YJ: Effect of hydrogen sulphide on
beta-amyloid-induced damage in PC12 cells. Clin Exp Pharmacol
Physiol. 35:180–186. 2008.
|
|
27
|
Tang XQ, Ren YK, Zhou CF, Yang CT, Gu HF,
He JQ, Chen RQ, Zhuang YY, Fang HR and Wang CY: Hydrogen sulfide
prevents formaldehyde-induced neurotoxicity to PC12 cells by
attenuation of mitochondrial dysfunction and pro-apoptotic
potential. Neurochem Int. 61:16–24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eghbal MA, Pennefather PS and O'Brien PJ:
H2S cytotoxicity mechanism involves reactive oxygen
species formation and mitochondrial depolarization. Toxicology.
203:69–76. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nyman S, Gottlow J, Karring T and Lindhe
J: The regenerative potential of the periodontal ligament. An
experimental study in the monkey. J Clin Periodontol. 9:257–265.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nyman S, Lindhe J, Karring T and Rylander
H: New attachment following surgical treatment of human periodontal
disease. J Clin Periodontol. 9:290–296. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nyman S, Gottlow J, Lindhe J, Karring T
and Wennstrom J: New attachment formation by guided tissue
regeneration. J Periodont Res. 22:252–254. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Somerman MJ, Archer SY, Imm GR and Foster
RA: A comparative study of human periodontal ligament cells and
gingival fibroblasts in vitro. J Dent Res. 67:66–70. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liang L, Yu JF, Wang Y, Wang G and Ding Y:
Effect of estrogen receptor beta on the osteoblastic
differentiation function of human periodontal ligament cells. Arch
Oral Biol. 53:553–557. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kawarizadeh A, Bourauel C, Götz W and
Jäger A: Early responses of periodontal ligament cells to
mechanical stimulus in vivo. J Dent Res. 84:902–906. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roberts WE, Mozsary PG and Klingler E:
Nuclear size as a cell-kinetic marker for osteoblast
differentiation. Am J Anat. 165:373–384. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wada N, Maeda H, Tanabe K, Tsuda E, Yano
K, Nakamuta H and Akamine A: Periodontal ligament cells secrete the
factor that inhibits osteoclastic differentiation and function: The
factor is osteoprotegerin/osteoclastogenesis inhibitory factor. J
Periodontal Res. 36:56–63. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kobawashi M, Takiguchi T, Suzuki R,
Yamaguchi A, Deguchi K, Shionome M, Miyazawa Y, Nishihara T, Nagumo
M and Hasegawa K: Recombinant human bone morphogenic protein-2
stimulates osteoblastic differentiation in cells isolated from
human periodontal ligament. J Dent Res. 78:1624–1633. 1999.
View Article : Google Scholar
|
|
38
|
Verna C, Zaffe D and Siciliani G:
Histomorphometric study of bone reactions during orthodontic tooth
movement in rats. Bone. 24:371–379. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Groeneveld MC, Everts V and Beertsen W:
Alkaline phosphatase activity in the periodontal ligament and
gingiva of the rat molar: Its relation to cementum formation. J
Dent Res. 74:1374–1381. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Okamoto T, Yatsuyzuka N, Tanaka Y, Kan M,
Yamanaka T, Sakamoto A, Takata T, Akagawa Y, Sato GH, Sato JD and
Takada K: Growth and differentiation of periodontal
ligament-derived cells in serum-free defined culture. In Vitro Cell
Dev Biol Anim. 33:302–309. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Von den Hoff JW: Effects of mechanical
tension on matrix degradation by human periodontal ligament cells
cultured in collagen gels. J Periodont Res. 38:449–457. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wescott DC, Pinkerton MN, Gaffey BJ, Beggs
KT, Milne TJ and Meikle MC: Osteogenic gene expression by human
periodontal ligament cells under cyclic tension. J Dent Res.
86:1212–1216. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Peverali FA, Basdra EK and Papavassiliou
AG: Stretch-mediated activation of selective MAPK subtypes and
potentiation of AP-1 binding in human osteoblastic cells. Mol Med.
7:68–78. 2001.PubMed/NCBI
|
|
44
|
Chiba M and Mitani H: Cytoskeletal changes
and the system of regulation of alkaline phosphatase activity in
human periodontal ligament cells induced by mechanical stress. Cell
Biochem Funct. 22:249–256. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jacobs C, Grimm S, Ziebart T, Walter C and
Wehrbein H: Osteogenic differentiation of periodontal fibroblasts
is dependent on the strength of mechanical strain. Arch Oral Biol.
58:896–904. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yamaguchi N, Chiba M and Mitani H: The
induction of c-fos mRNA expression by mechanical stress in human
periodontal ligament cells. Arch Oral Biol. 47:465–471. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Molina T, Kabsch K, Alonso A, Kohl A,
Komposch G and Tomakidi P: Topographic changes of focal adhesion
components and modulation of p125FAK activation in stretched human
periodontal ligament fibroblasts. J Dent Res. 80:1984–1989. 2001.
View Article : Google Scholar
|