Introduction
Traditional plant-based medicines have served an
important role in health care, and numerous drugs are known to
originate from these medicines (1). Citrus unshiu (C.
unshiu) Marcov, which belongs to the Rutaceae family is a
seedless and easy-to-peel Korean citrus fruit, and constitutes 30%
of the total volume of fruit produced in Korea (2). Its dried peel has been used to
improve bronchial and asthmatic conditions, and blood circulation
in Korea, China, and Japan for thousands of years (3,4).
The C. unshiu peel (also known as Jin-pee) is
the primary waste product of citrus fruits and has been used as a
source of molasses, pectin, cold-pressed oils and limonene
(5). The peel has been studied
extensively, as it contains numerous biologically active compounds,
such as natural antioxidants (phenolic acids and flavonoids)
(6,7). In addition, the C. unshiu peel
is reported to possess anti-allergy (8,9),
antibacterial, anti-fungal (10,11),
anticancer (12), antidiabetic
(13,14), anti-inflammatory (15,16),
antioxidant (17–19), antiviral (20) and lipid-lowering activities
(2,4). C. unshiu peel has been used in
Korea to treat a variety of digestive disorders, including
tympanites, nausea, vomiting and dyspepsia (16,21).
Despite reports that the peel functions as a prokinetic agent to
prevent or alleviate gastrointestinal (GI) motility dysfunctions
(22), little is known about its
effects on GI motility or its mechanisms of action.
Interstitial cells of Cajal (ICCs) are the pacemaker
cells of the GI muscles that generate rhythmic oscillations in
membrane potentials (termed 'slow waves') (23,24),
and mediate or transduce inputs from the enteric nervous system
(25). Research into the biology
of ICCs has provided exciting and novel opportunities to understand
the etiology of GI diseases (26).
Therefore, the aim of the present study was to investigate the
effect of C. unshiu peel extracts (CPE) on the pacemaker
potentials of cultured ICCs from the murine small intestine.
Materials and methods
Preparation of samples and
high-performance liquid chromatography (HPLC) analysis
The dried peel of C. unshiu was purchased
from Kapdang Co. (Seoul, Korea). The sample was identified by Dr
Yun Tai Kim (Korea Food Research Institute, Seongnam, Korea)
according to the 'Illustrated Guide to Clinical Medical Herbs'
(27) and a voucher specimen
(reference no. NP-1505) was deposited with the Research Group of
Innovative Special Food (Korea Food Research Institute). C.
unshiu dried peel (600 g) was incubated with 70% ethanol (6,000
ml) for 2 h at 20°C. This process was repeated with fresh 70%
ethanol, and the extract solution was combined and filtered through
a 0.45-µm membrane filter (EMD Millipore, Billerica, MA,
USA). The solvents were removed by rotary evaporation and the
remaining extracts were freeze-dried, which yielded ~21.1% of the
dried peel weight (w/w).
The freeze-dried extract powder (100 mg) was
dissolved in 5 ml methanol/dimethyl sulfoxide (DMSO; 1:1, v/v),
before it was filtered through a 0.45-µm regenerated
cellulose-membrane filter (Sartorius AG, Goettingen, Germany), and
diluted in methanol/DMSO (1:1, v/v) to a final concentration of 10
mg/ml prior to injection of 10 µl of the solution into the
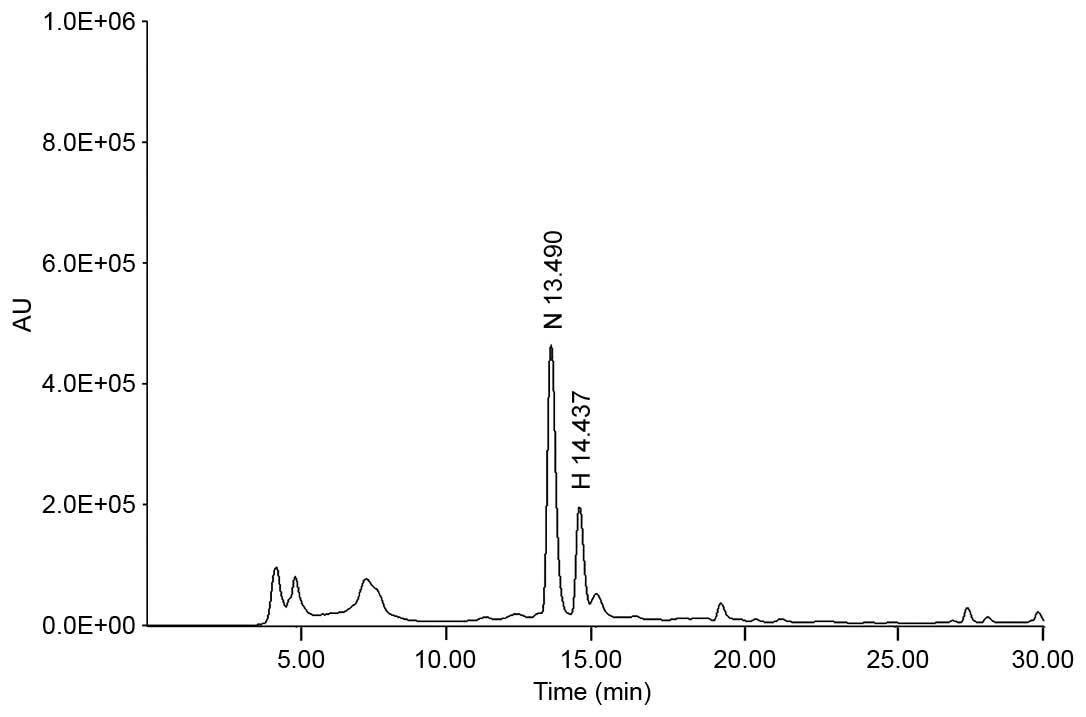
HPLC. Analytical HPLC was performed using a Jasco HPLC system
(Jasco, Inc., Tokyo, Japan), which comprised a PU-980 pump, an
AS-950-10 autosampler and an MD-2010 Plus multi-wavelength
detector.
The chromatographic separation was conducted at 30°C
using a Symmetry® C18 column (4.6×250 mm, particle size
5 µm; Waters Corporation, Milford, MA, USA) with gradient
elution using a mobile phase composed of 40% methanol (mobile phase
A) and 100% methanol (mobile phase B). Alterations in the mobile
phase was achieved using a linear gradient system from 100% mobile
phase A to 100% mobile phase B over 30 min and with a 0.5 ml/min
flow rate, before the samples were detected at 284 nm. Quantitative
analysis was performed in triplicate. The regression equation and
correlation coefficient (r2) of each standard
curve were automatically calculated by the Jasco HPLC system. The
regression equations for narirutin and hesperidin were
y=35,103.0278x−55,481.6311 (r2,
0.99994) and y=39,824.0428x−72,092.8906
(r2, 0.99973), respectively, indicating that a
high linear correlation was achieved for all standard curves. The
concentration of narirutin and hesperidin were determined to be
21.72±0.716 and 8.51±0.296 mg/g, respectively using the peak area
in the chromatogram and the regression equation (Fig. 1).
Ethical approval
Animal care and experiments were conducted in
accordance with the guidelines issued by the ethics committee of
Pusan National University (Busan, Korea; approval no.
PNU-2014-0725) and the Guide for the Care and Use of Laboratory
Animals published by the US National Institute of Health (NIH
Publication No. 85–23, revised 2011).
Preparation of cells and culture
conditions
A total of 82 male (52%) and female (48%) BALB/c
mice (age, 3–7 days; weight, 1.9–2.2 g; Samtako Bio Korea Inc.,
Osan-si, Korea) were anesthetized with ether and euthanized by
cervical dislocation. They were maintained under controlled
conditions (temperature, 21±3°C; humidity 50±6%; 12 h light/dark
cycles) and were allowed free access to food and water. Mice were
fed a diet comprised of crude protein (≥18%), crude fat (≥5%),
crude fiber (≤4.5%), crude ash (≤8%), calcium (≥0.7%) and
phosphorus (≤1.2%) (Samtako Bio Korea Inc.). The small intestines
from 1 cm below the pyloric ring to the cecum were removed, opened
along the mesenteric border, and the luminal contents were removed
by washing with a Krebs-Ringer bicarbonate solution. Tissues were
pinned to the base of a Sylgard dish and the mucosae were removed
by sharp dissection. Small tissue strips of intestinal muscle,
consisting of circular and longitudinal muscles, were equilibrated
in a Ca2+-free Hank's Balanced Salt Solution (containing
5.36 mmol/l KCl, 125 mmol/l NaCl, 0.34 mmol/l NaOH, 0.44 mmol/l
Na2HCO3, 10 mmol/l glucose, 2.9 mmol/l
sucrose and 11 mmol/l HEPES) for 30 min. Cells were then dispersed
using an enzyme solution containing 1.3 mg/ml collagenase
(Worthington Biochemical Corporation, Lakewood, NJ, USA), 2 mg/ml
bovine serum albumin (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany), 2 mg/ml trypsin inhibitor (Sigma-Aldrich; Merck
Millipore) and 0.27 mg/ml adenosine triphosphate (ATP;
Sigma-Aldrich; Merck Millipore). Cells were subsequently plated
onto Falcon sterile glass coverslips coated with murine collagen
(2.5 µg/ml; BD Biosciences, Franklin Lakes, NJ, USA) in a
35-mm culture dish, and maintained in smooth muscle growth medium
(Clonetics Corporation, San Diego, CA, USA) supplemented with 2%
Penicillin-Streptomycin solution (Gibco; Thermo Fisher Scientific,
Waltham, MA, USA) and 5 ng/ml murine stem cell factor
(Sigma-Aldrich; Merck Millipore) at 37°C in an O2
(95%)/CO2 (5%) incubator. ICCs were identified
immunocytochemically by incubating cells with a
phycoerythrin-conjugated rat anti-mouse monoclonal anti-c-Kit
antibody (cat. no. 12–1172; dilution, 1:50; eBioscience, Inc., San
Diego, CA, USA) for 20 min as described previously (28). ICCs were morphologically distinct
from other cell types in culture, and it was therefore possible to
identify these cells by phase contrast microscopy after they had
been stained with the anti-c-Kit antibody.
Patch-clamp experiments
The whole-cell patch-clamp configuration was used to
record membrane potentials (in current clamp mode) in cultured
ICCs. An Axopatch 1D (Molecular Devices, LLC, Sunnyvale, CA, USA)
was used to amplify membrane currents and potentials. The command
pulse was applied using pCLAMP software (version 6.1; Molecular
Devices, LLC). Data were obtained by filtering at 5 kHz and were
displayed on an oscilloscope, a computer monitor, and detected
using a Gould 2200 Series Analog Recorder (Gould Instrument
Systems, Inc., Valley View, OH, USA). Results were analyzed using
pCLAMP and Origin software (version 6.0; MicroCal, Northampton, MA,
USA). All experiments were performed at 30–32°C.
Solutions and drugs
The physiological salt solution used to bathe cells
(Na+-containing Tyrode's Solution) consisted of 5 mmol/l
KCl, 135 mmol/l NaCl, 2 mmol/l CaCl2, 10 mmol/l glucose,
1.2 mmol/l MgCl2 and 10 mmol/l HEPES, adjusted to pH 7.4
with NaOH. CPE (1–10 mg/ml) was added to ICC bath solutions for 2
min. The pipette solution consisted of 140 mmol/l KCl, 5 mmol/l
MgCl2, 2.7 mmol/l K2ATP, 0.1 mmol/l NaGTP,
2.5 mmol/l creatine phosphate disodium, 5 mmol/l HEPES and 0.1
mmol/l EGTA adjusted to pH 7.2 with KOH. All drugs including,
methoctramine, diphenylacetoxypiperidinium iodide (4-DAMP),
guanosine 5′-(β-thio) diphosphate trilithium salt (GDP-β-S),
U-73312, U-73343, PD98059, SB203580 and the JNK II inhibitor
SP600125, were obtained from Sigma-Aldrich (Merck Millipore). Drugs
were dissolved in distilled water and added to the physiological
salt solution at the desired concentrations immediately prior to
use. The addition of these drugs to the solution for 5 min did not
alter the pH. 4-DAMP was dissolved in DMSO to produce a 50 mmol/l
stock solution, which was subsequently added to the bathing
solution at a final concentration of 10 µM on the day of the
experiment for 5 min. The final concentration of DMSO in the
culture solution was <0.1% and preliminary experiments confirmed
that this concentration of DMSO did not affect results. In
addition, 25 µl methoctramine was dissolved in distilled
water to produce a 50 mmol/l stock solution, which was added to the
culture solution at a final concentration of 10 µM on the
day of the experiment for 5 min. GDP-β-S was dissolved in DMSO to
produce a 1 mol/l stock solution, which was added to the pipette
solution at a final concentration of 1 mM on the day of the
experiment. Both U-73312 and U-73343 were dissolved in DMSO to
produce a 5 mmol/l stock solution, which was added to the culture
solution at a final concentration of 5 µM on the day of the
experiment for 5 min. PD98059, SB203580 and the JNK II inhibitor
were dissolved in DMSO to produce 10 mmol/l stock solutions, which
were added to the culture solution at a final concentration of 10
µM on the day of the experiment for 5 min.
Statistical analysis
Results are expressed as the mean ± standard error.
The Student's t-test and one-way analysis of variance with
Bonferroni's post-hoc tests were used to test for significance
among groups. P<0.05 was considered to indicate a statistically
significant difference. The n values refer to the number of
cells used in patch-clamp experiments.
Results
Effect of CPE on pacemaker potentials in
cultured ICCs
The initial aim of the current study was to
investigate the effects of CPE on ICC pacemaker potentials.
Recordings from cultured ICCs under current clamp mode (I=0)
demonstrated the occurrence of spontaneous pacemaker potentials,
with a resting membrane potential of −58.2±1.2 mV and an amplitude
of 25.3±1.7 mV. In the presence of CPE (1–10 mg/ml), membrane
potentials were significantly depolarized compared with the control
group to 6.8±1.0 mV at 1 mg/ml (P=0.0012), 24.8±1.3 mV at 5 mg/ml
(P<0.0001) and 28.8±0.9 mV at 10 mg/ml (P<0.0001) CPE, with
corresponding significantly reduced amplitudes of 20.2±1.5 mV
(P=0.012), 2.3±0.5 mV (P<0.0001) and 2.2±0.6 mV (P<0.0001),
respectively (Fig. 2A–D). A
summary of values, together with a bar graph demonstrating the
effects of CPE on pacemaker potentials are provided in Fig. 2E and F (n=7).
Identification of CPE-target receptor
subtypes in cultured ICCs
Muscarinic receptors are known to mediate membrane
depolarization and excitatory junction potentials in the GI tract
(29,30). In addition, it has been reported
that isolated ICCs express M2 and M3
muscarinic receptor subtypes in the GI tract (31). Therefore, in order to determine
whether CPE-induced membrane depolarization involves muscarinic
receptors, the effect of CPE on M2 and M3
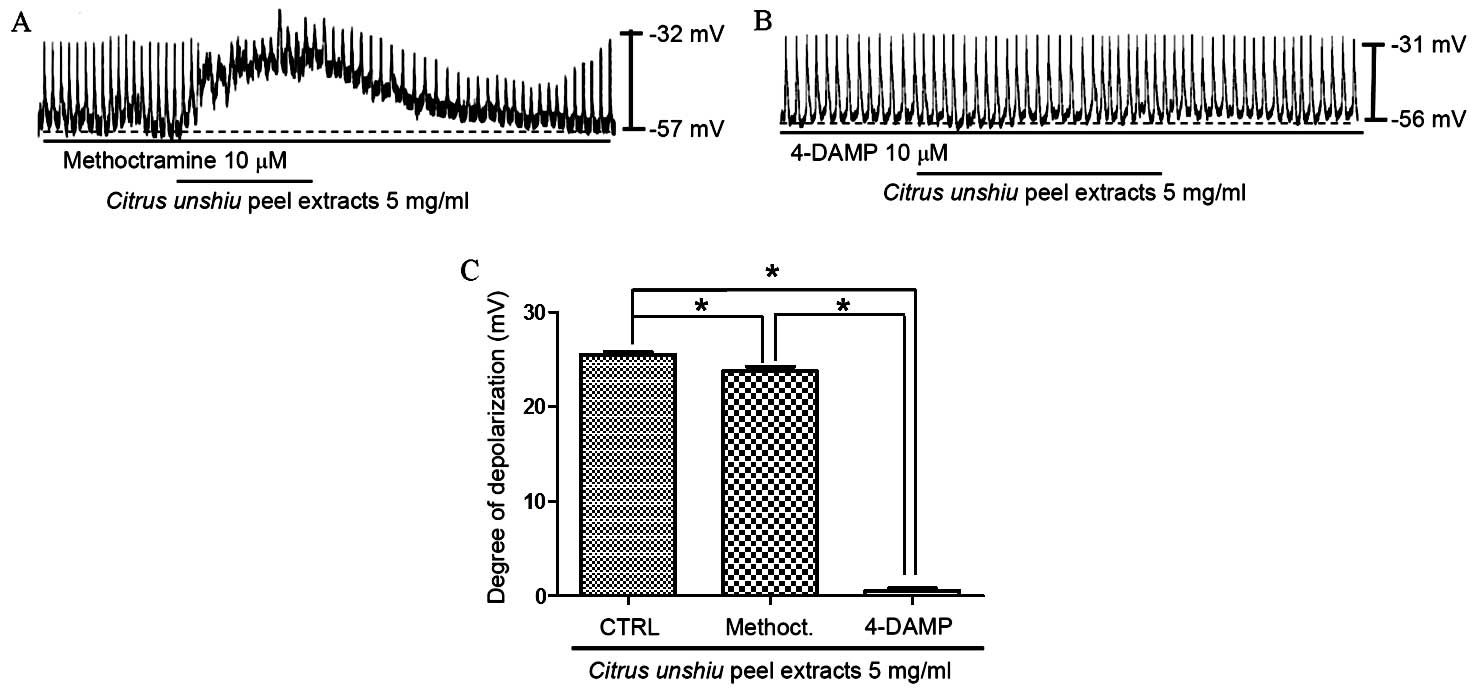
muscarinic receptors was investigated. ICCs were pretreated with
muscarinic receptor antagonists prior to treatment with CPE. To
achieve this, ICCs were first exposed to the muscarinic
M2 receptor antagonist, methoctramine, and the
muscarinic M3 receptor antagonist, 4-DAMP, at a
concentration of 10 µM for 5 min, before 5 mg/ml CPE was
added. Treatment with methoctramine or 4-DAMP alone did not affect
pacemaker potentials (data not shown), and pretreatment with
methoctramine did not significantly inhibit the effect of CPE on
the pacemaker potential compared with CPE treatment alone (Fig. 3A). Membrane depolarization in the
presence of methoctramine by CPE was 23.7±1.1 mV (n=6), however,
following the pretreatment of ICCs with 4-DAMP, membrane
depolarization was inhibited compared with CPE treatment alone
(P<0.0001; Fig. 3B and C). The
membrane depolarization signal produced in the presence of 4-DAMP
was 0.6±0.6 mV (n=6; Fig. 3C).
These results suggest that CPE may affect ICC membrane potential
through the M3 receptor.
Involvement of G-proteins in CPE-induced
depolarization of pacemaker potentials in cultured ICCs
The effect of CPE-induced pacemaker potential
depolarization in ICCs following treatment with GDP-β-S, a
non-hydrolysable guano-sine 5′-diphosphate analogue that
permanently inactivates G-protein binding proteins (32,33),
was examined in order to determine the role of G proteins in
mediating this effect. As demonstrated in Fig. 2C, CPE (5 mg/ml) induced ICC
membrane depolarization. However, upon exposure to 1 mM GDP-β-S,
CPE membrane depolarization was only partially induced compared
with CPE alone (Fig. 4A). As a
result, the membrane depolarization induced by CPE was
significantly reduced in the presence of GDP-β-S (P=0.0009; n=6;
Fig. 4B). These results suggest
that G proteins may be involved in mediating CPE-induced pacemaker
depolarization in ICCs.
Effect of phospholipase C (PLC)
inhibition on CPE-induced pacemaker potential depolarization
A previous study demonstrated that membrane
depolarization in ICCs may be associated with intracellular
Ca2+ mobilization (28). Therefore, the current study aimed
to determine whether the CPE-induced effects on the pacemaker
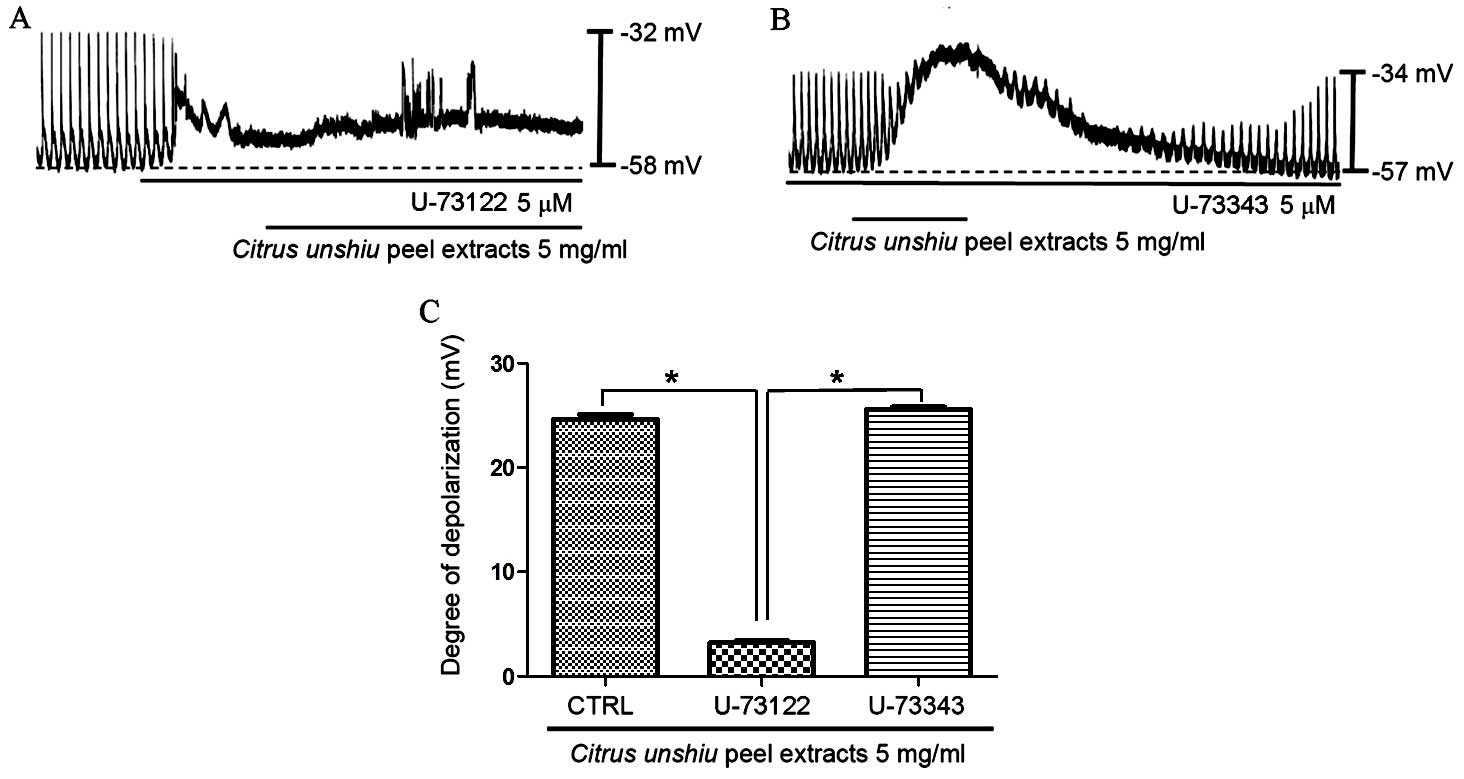
potential of ICCs require PLC. To investigate this, CPE (5
mg/ml)-induced membrane depolarization in the absence and presence
of the active PLC inhibitor U-73122 (5 µM) was examined
(34). As demonstrated in Fig. 5A, CPE-induced pacemaker membrane
depolarization was eliminated upon exposure of cells to U-73122.
Under these conditions, CPE induced minor membrane depolarization
(n=5; Fig. 5A). In the presence of
U-73122, the membrane depolarization produced by CPE was 3.2±0.5
mV, and the membrane depolarization signal generated by exposure to
CPE in the presence of U-73122 was significantly lower compared
with CPE-only treated controls (P<0.0001). By contrast,
pretreatment of ICCs with an inactive analog of U-73122 (U-73343; 5
µM) did not significantly alter the pacemaker potential,
thus, CPE-induced membrane depolarization was not suppressed by
U-73343 (n=5; Fig. 5B). These
results suggest that the PLC pathway may be involved in CPE-induced
pacemaker depolarization in ICCs.
Involvement of mitogen-activated protein
kinases (MAPKs) on CPE-induced ICC pacemaker potential
depolarization
Stimulation of muscarinic receptors has been
demonstrated to activate MAPKs in a variety of cellular systems
(35). Therefore, the role of
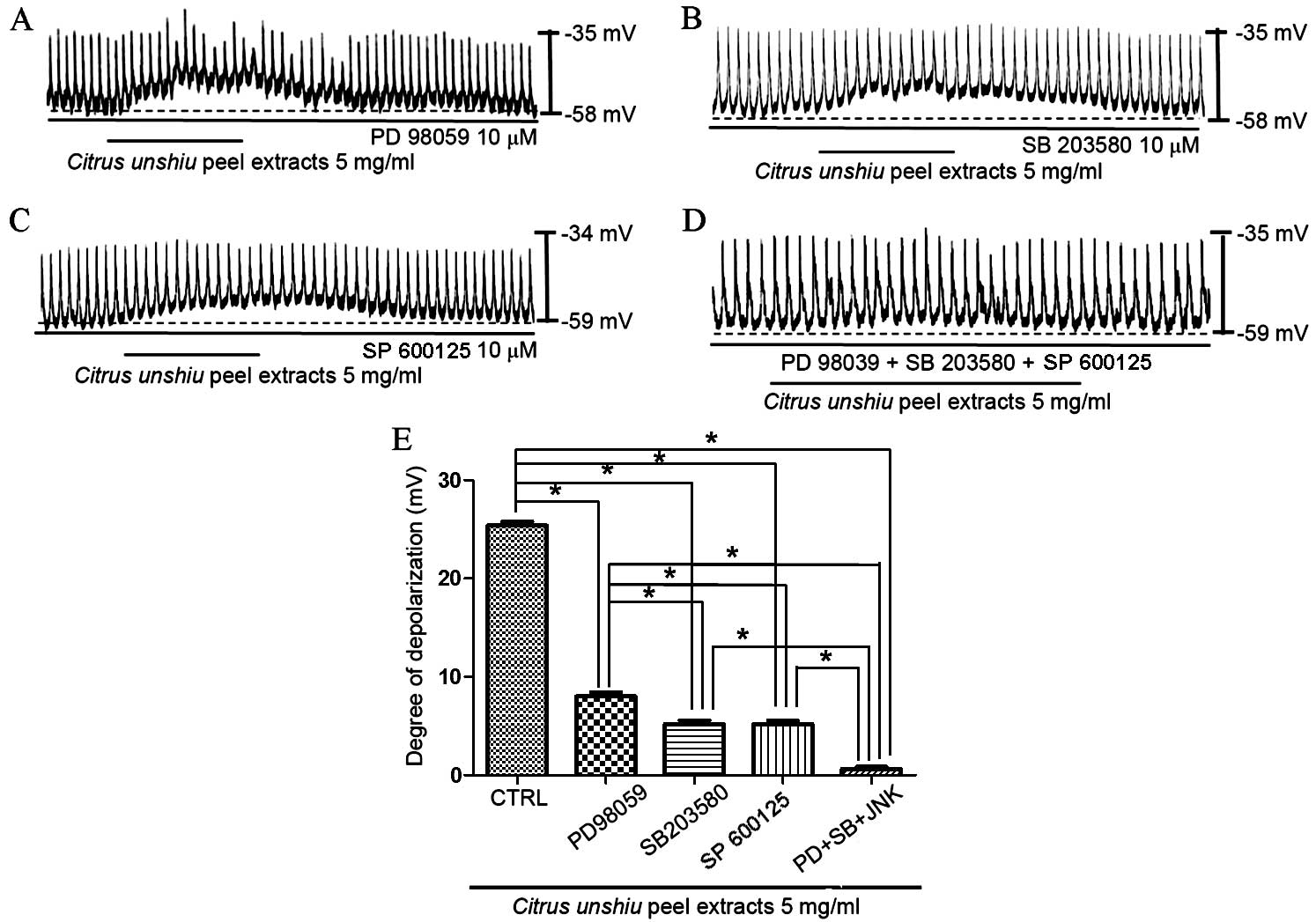
MAPKs in the effects of CPE on membrane depolarization in cultured
ICCs was investigated using a p42/44 MAPK inhibitor, PD98059, a p38
MAPK inhibitor, SB203580, and a c-jun NH2-terminal kinase (JNK) II
inhibitor, SP600125. In the presence of PD98059 (10 µM), CPE
generated partial membrane depolarization signal (n=5; Fig. 6A), which indicates that p42/44 may
affect CPE-induced membrane depolarization. In addition, exposure
to SB203580 (Fig. 6B) or SP600125
(Fig. 6C) partially inhibited the
pacemaker potential depolarization induced by CPE (n=5).
Depolarization was significantly reduced in the presence of the
MAPK inhibitors compared with CPE treatment alone (P<0.0001,
PD98059; P<0.0001, SB203580; P<0.0001, SP600125). Membrane
depolarization was inhibited to the greatest degree upon exposure
to PD98059, SB203580 and SP600125 (n=5; Fig. 6D and E). These results suggest that
MAPKs are important in modulating CPE-induced ICC pacemaker
potential depolarization.
Discussion
Citrus fruits contain sugar, organic acids and a
number of physiologically functional components, including citric
acid, ascorbic acid, minerals, coumarins and flavonoids (naringin,
hesperidin, neohesperidin, rutin, naringenin, hesperetin, nairutin,
and tangeretin) (36,37). C. unshiu is commonly known
as the tangerine or mandarin orange. Traditionally, the C.
unshiu peel has been used as a folk remedy to treat the common
cold, dyspepsia, coughs and phlegm production (38). C. unshiu peel contains an
abundance of flavonoids, which are known to possess a number of
different beneficial effects (39–41).
Hesperidin, naringin, and nobiletin (42,43)
levels are high in citrus fruits (including C. unshiu peel),
and are used as chemical quality control markers for C.
unshiu peel products. Hesperidin is the most abundant flavonoid
in citrus peel (42,43). In Korea, extracts of dried C.
unshiu peel are sold as commercially available medicines for
the treatment of a variety of GI disorders, and single extract
doses of 0.5–15 g are generally recommended (21). However, despite the abundance of
these biomolecules in citrus fruits, to the best of our knowledge,
there is currently no data regarding the prokinetic activity of
CPE. In addition, the molecular and physiological mechanisms
underlying the therapeutic effects of C. unshiu peel on GI
disorders has not yet been elucidated.
ICCs are known to be the pacemaker cells that
modulate GI motility by generating pacemaker currents that produce
slow wave potentials. ICCs are connected to each other and to
neighboring smooth muscle cells via gap junctions (23,24).
Numerous neurotransmitters, including acetylcholine and
5-hydroxytryptamine, and diverse drugs or traditional herbal
medicines (e.g. Ge-Gen-Tang) have been demonstrated to elicit
excitatory or inhibitory effects on the pacemaker activity of ICCs
(44,45), which supports the notion that ICCs
are a critical in the control of smooth muscle motility in the GI
tract.
In the present study, CPE was observed to modulate
the pacemaker potential of ICCs. CPE produced pacemaker
depolarization in current clamp mode. In addition, exposure of ICCs
to the M3 muscarinic receptor antagonist, 4-DAMP,
inhibited CPE-induced pacemaker depolarization, whereas exposure to
the M2 receptor antagonist, methoctramine, did not. When
GDP-β-S was present in the pipette solution, CPE induced minor
pacemaker depolarization. In addition, membrane depolarization by
CPE was inhibited following treatment of ICCs with the active PLC
inhibitor U-73122. Furthermore, in the presence of MAPK inhibitors
PD98059, SB203580 and SP600125, CPE produced slight membrane
depolarization. These results suggest that CPE affects GI motility
by modulating ICC pacemaker activity through G protein-dependent
PLC and MAPK pathway-mediated activation of muscarinic
M3 receptors.
In the GI tract, M2 and M3
muscarinic receptors are involved in GI motility (46). However, no effect on CPE-induced
pacemaker membrane depolarization was observed following exposure
of ICCs to methoctramine in the present study. The GI tract is
composed of smooth muscle, the enteric nervous system and ICCs.
Therefore, we hypothesize that CPE may function to activate the
M3 receptor in ICCs, and the M2 receptor may
be involved in modulating smooth muscle or enteric nervous system
functions. In support of this notion, So et al (47) suggested that the modulation of
pacemaker currents by the muscarinic agonist carbachol is mediated
by only muscarinic M3 receptors and not M2
receptors in ICCs. In addition, during the recording of
intracellular Ca2+ concentrations using fluo-3-AM dye,
carbachol increased intracellular Ca2+ concentrations
and Ca2+ oscillations. Therefore, it is possible that
CPE may modulate ICC pacemaker potentials through muscarinic
M3 receptors only, through an intracellular
Ca2+ release-dependent mechanism. Future studies will
aim to investigate the effects of CPE in Ca2+
regulation. Acetylcholine muscarinic receptors are a family of G
protein-couples receptors, and are composed of five subtypes
(M1–M5). Of these, three (M1,
M3, and M5) are coupled with PLC through a
Gq protein, whereas the M2 and M4
subtypes inhibit adenylate cyclase through Gi or
Go proteins (35).
Stimulation of muscarinic receptors in a variety of cellular
systems has been demonstrated to activate MAPKs (35), which are a family of protein
kinases that with central roles in signal transduction (48). MAPKs regulate a variety of cellular
responses, including inflammation, cell cycle progression,
proliferation, differentiation and protein synthesis (49). However, the mechanisms underlying
MAPK activation in response to muscarinic receptor stimulation
remain to be elucidated. M2 and/or M3
receptors have been shown to mediate activation of the MAPK pathway
(50,51) and muscarinic receptors and the MAPK
signaling pathway are known to mediate proliferative responses in
various cell types (52–61). Matthiesen et al (52) suggested that these proliferative
effects are due to M2 receptor and Gi
protein-mediated MAPK activation, however, several G
protein-coupled-MAPK activation pathways have been identified
(53,54). Acetylcholine stimulates the
proliferation of colon carcinoma cell lines through M3
receptor-dependent phosphorylation of MAPK (55–57).
In addition, cholinergic neurotransmitters stimulate the growth of
astrocytoma and breast cancer cells through the AKT
serine/threonine kinase or MAPK signaling pathways (58,59).
Furthermore, acetylcholine stimulates ovarian or lung cancer growth
through muscarinic receptor-mediated phosphorylation of MAPK
(60,61). In a previous study, the effect of
C. unshiu peel on the production of proinflammatory
mediators in lipopolysaccharide (LPS)-stimulated RAW264.7
macrophage cells was investigated (16). The results demonstrated that C.
unshiu peel significantly reduced the phosphorylation of all
LPS-stimulated MAPKs in a dose-dependent manner (16). Therefore, we hypothesize that MAPKs
are important for the effect of C. unshiu peel on ICC
membrane depolarization.
In conclusion, the results of the present study
suggest that C. unshiu peel may be a suitable candidate for
the development of prokinetic agents that prevent or alleviate GI
disorders.
Acknowledgments
The present study was supported by the Korean
National Research Foundation (grant no. 2014R1A5A2009936), which is
funded by the Ministry of Science, ICT and Future Planning (Korean
Government).
References
|
1
|
Bai D: Traditional Chinese medicines and
new drug development. Pure Appl Chem. 65:1103–1112. 1993.
View Article : Google Scholar
|
|
2
|
Lim DW, Lee Y and Kim YT: Preventive
effects of Citrus unshiu peel extracts on bone and lipid metabolism
in OVX rats. Molecules. 19:783–794. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choi IY, Kim SJ, Jeong HJ, Park SH, Song
YS, Lee JH, Kang TH, Park JH, Hwang GS, Lee EJ, et al: Hesperidin
inhibits expression of hypoxia inducible factor-1 alpha and
inflammatory cytokine production from mast cells. Mol Cell Biochem.
305:153–161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang G, Lee J, Jung ED, Ham I and Choi HY:
Lipid lowering activity of Citri unshii pericarpium in hyperlipemic
rats. Immunopharmacol Immunotoxicol. 30:783–791. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Braddock RJ: Byproducts of citrus fruits.
Food Technol. 49:74–77. 1995.
|
|
6
|
Giannuzzo AN, Boggetti HJ, Nazareno MA and
Mishima HT: Supercritical fluid extraction of naringin from the
peel of Citrus paradisi. Phytochem Anal. 14:221–223. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jeong SM, Kim SY, Kim DR, Jo SC, Nam KC,
Ahn DU and Lee SC: Effect of heat treatment on the antioxidant
activity of extracts from citrus peels. J Agric Food Chem.
52:3389–3393. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim DK, Lee KT, Eun JS, Zee OP, Lim JP,
Eum SS, Kim SH and Shin TY: Anti-allergic components from the peels
of Citrus unshiu. Arch Pharm Res. 22:642–645. 1999. View Article : Google Scholar
|
|
9
|
Park SH, Park EK and Kim DH: Passive
cutaneous anaphylaxis-inhibitory activity of flavanones from Citrus
unshiu and Poncirus trifoliata. Planta Med. 71:24–27. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jo CR, Park BJ, Chung SH, Kim CB, Cha BS
and Byun MW: Antibacterial and anti-fungal activity of citrus
(Citrus unshiu) essential oil extracted from peel by-products. Food
Sci Biotechnol. 13:384–386. 2004.
|
|
11
|
Min KY, Kim HJ, Lee KA, Kim KT and Paik
HD: Antimicrobial activity of acid-hydrolyzed Citrus unshiu peel
extract in milk. J Dairy Sci. 97:1955–1960. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee S, Ra J, Song JY, Gwak C, Kwon HJ, Yim
SV, Hong SP, Kim J, Lee KH, Cho JJ, et al: Extracts from Citrus
unshiu promote immune mediated inhibition of tumor growth in a
murine renal cell carcinoma model. J Ethnopharmacol. 133:973–979.
2011. View Article : Google Scholar
|
|
13
|
Park HJ, Jung UJ, Cho SJ, Jung HK, Shim S
and Choi MS: Citrus unshiu peel extract ameliorates hyperglycemia
and hepatic steatosis by altering inflammation and hepatic glucose-
and lipid-regulating enzymes in db/db mice. J Nutr Biochem.
24:419–427. 2013. View Article : Google Scholar
|
|
14
|
Lee YH, Kim YS, Song M, Lee M, Park J and
Kim H: A herbal formula HT048, Citrus unshiu and Crataegus
pinnatifida, prevents obesity by inhibiting adipogenesis and
lipogenesis in 3T3-L1 preadipocytes and HFD-induced obese rats.
Molecules. 20:9656–9670. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim KS, Rhee HI, Park EK, Jung K, Jeon HJ,
Kim JH, Yoo H, Han CK, Cho YB, Ryu CJ, et al: Anti-inflammatory
effects of Radix Gentianae Macrophyllae (Qinjiao), Rhizoma Coptidis
(Huanglian) and Citri Unshiu Pericarpium (Wenzhou migan) in animal
models. Chin Med. 3:102008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oh YC, Cho WK, Jeong YH, Im GY, Yang MC,
Hwang YH and Ma JY: Anti-inflammatory effect of Citrus unshiu peel
in LPS-stimulated RAW 264.7 macrophage cells. Am J Chin Med.
40:611–629. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bocco A, Cuvelier ME, Richard H and Berset
C: Antioxidant activity and phenolic composition of citrus peel and
seed extracts. J Agri Food Chem. 46:2123–2129. 1998. View Article : Google Scholar
|
|
18
|
Jeong SM, Kim SY, Kim DR, Jo SC, Nam KC,
Ahn DU and Lee SC: Effect of heat treatment on the antioxidant
activity of extracts from Citrus peels. J Agri Food Chem.
52:3389–3393. 2004. View Article : Google Scholar
|
|
19
|
Yang X, Kang SM, Jeon BT, Kim YD, Ha JH,
Kim YT and Jeon YJ: Isolation and identification of an antioxidant
flavonoid compound from citrus-processing byproduct. J Sci Food
Agric. 91:1925–1927. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suzuki M, Sasaki K, Yoshizaki F, Oguchi K,
Fujisawa M and Cyong JC: Anti-hepatitis C virus effect of Citrus
unshiu peel and its active ingredient nobiletin. Am J Chin Med.
33:87–94. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim CM, Shin MK, Ahn DG and Lee KS:
Chungyak Daesajun. Jungdam; Seoul: 8. pp. 4026–4030. 1997
|
|
22
|
Lyu JH and Lee HT: Effects of dried Citrus
unshiu peels on gastrointestinal motility in rodents. Arch Pharm
Res. 36:641–648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ward SM, Burns AJ, Torihashi S and Sanders
KM: Mutation of the proto-oncogene c-kit blocks development of
interstitial cells and electrical rhythmicity in murine intestine.
J Physiol. 480:91–97. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huizinga JD, Thuneberg L, Klüppel M,
Malysz J, Mikkelsen HB and Bernstein A: W/kit gene required for
interstitial cells of Cajal and for intestinal pacemaker activity.
Nature. 373:347–349. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim BJ, Kwon YK, Kim E and So I: Effects
of histamine on cultured interstitial cells of cajal in murine
small intestine. Korean J Physiol Pharmacol. 17:149–156. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim BJ, Lim HH, Yang DK, Jun JY, Chang IY,
Park CS, So I, Stanfield PR and Kim KW: Melastatin-type transient
receptor potential channel 7 is required for intestinal pacemaking
activity. Gastroenterology. 129:1504–1517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ahn DK: Illustrated guide to clinical
medical herbs. Hyeonamsa Publ. Corp.; Seoul: pp. 147–148. 2012
|
|
28
|
Goto K, Matsuoka S and Noma A: Two types
of spontaneous depolarizations in the interstitial cells freshly
prepared from the murine small intestine. J Physiol. 559:411–422.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huizinga JD, Chang G, Diamant NE and
El-Sharkawy TY: Electrophysiological basis of excitation of canine
colonic circular muscle by cholinergic agents and substance P. J
Pharmacol Exp Ther. 231:692–699. 1984.PubMed/NCBI
|
|
30
|
Inoue R and Chen S: Physiology of
muscarinic receptor operated nonselective cation channels in
guinea-pig ileal smooth muscle. EXS. 66:261–268. 1993.
|
|
31
|
Epperson A, Hatton WJ, Callaghan B,
Doherty P, Walker RL, Sanders KM, Ward SM and Horowitz B: Molecular
markers expressed in cultured and freshly isolated interstitial
cells of Cajal. Am J Physiol Cell Physiol. 279:C529–C539.
2000.PubMed/NCBI
|
|
32
|
Komori S, Kawai M, Takewaki T and Ohashi
H: GTP-binding protein involvement in membrane currents evoked by
carbachol and histamine in guinea-pig ileal muscle. J Physiol.
450:105–126. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ogata R, Inoue Y, Nakano H, Ito Y and
Kitamura K: Oestradiol-induced relaxation of rabbit basilar artery
by inhibition of voltage-dependent Ca channels through GTP-binding
protein. Br J Pharmacol. 117:351–359. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sakamoto T, Unno T, Matsuyama H, Uchiyama
M, Hat tori M, Nishimura M and Komori S: Characterization of
muscarinic receptor-mediated cationic currents in longitudinal
smooth muscle cells of mouse small intestine. J Pharmacol Sci.
100:215–226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Slack BE: The M3 muscarinic acetylcholine
receptor is coupled to mitogen-activated protein kinase via protein
kinase C and epidermal growth factor receptor kinase. Biochem J.
348:381–387. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tanizawa H, Ohkawa Y, Takino Y, Miyase T,
Ueno A, Kageyama T and Hara S: Studies on natural antioxidants in
citrus species. I Determination of antioxidative activities of
citrus fruits. Chem Pharm Bull (Tokyo). 40:1940–1942. 1992.
View Article : Google Scholar
|
|
37
|
Kawaii S, Tomono Y, Katase E, Ogawa K and
Yano M: Quantitation of flavonoid constituents in citrus fruits. J
Agric Food Chem. 47:3565–3571. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kimura T, But PPH, Guo JX and Sung CK:
International collation of traditional and folk medicine: Northeast
Asia. River Edge: World Scientific Publishing Co; 1996, View Article : Google Scholar
|
|
39
|
Jung UJ, Lee MK, Park YB, Kang MA and Choi
MS: Effect of citrus flavonoids on lipid metabolism and
glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int
J Biochem Cell Biol. 38:1134–1145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Abeysinghe DC, Li X, Sun C, Zhang W, Zhou
C and Chen K: Bioactive compounds and antioxidant capacities in
different edible tissues of citrus fruit of four species. Food
Chem. 104:1338–1344. 2007. View Article : Google Scholar
|
|
41
|
Funaguchi N, Ohno Y, La BL, Asai T,
Yuhgetsu H, Sawada M, Takemura G, Minatoguchi S, Fujiwara T and
Fujiwara H: Narirutin inhibits airway inflammation in an allergic
mouse model. Clin Exp Pharmacol Physiol. 34:766–770. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lu Y, Zhang C, Bucheli P and Wei D: Citrus
flavonoids in fruit and traditional Chinese medicinal food
ingredients in China. Plant Food Hum Nutr. 61:57–65. 2006.
View Article : Google Scholar
|
|
43
|
Ma YQ, Ye XQ, Fang ZX, Chen JC, Xu GH and
Liu DH: Phenolic compounds and antioxidant activity of extracts
from ultrasonic treatment of Satsuma Mandarin (Citrus unshiu Marc)
peels. J Agri Food Chem. 56:5682–5690. 2008. View Article : Google Scholar
|
|
44
|
Wu MJ, Kee KH, Na J, Kim SW, Bae Y, Shin
DH, Choi S, Jun JY, Jeong HS and Park JS: Pituitary adenylate
cyclase-activating polypeptide inhibits pacemaker activity of
colonic interstitial cells of cajal. Korean J Physiol Pharmacol.
19:435–440. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee S, Gim H, Shim JH, Jung Kim H, Lee JR,
Kim SC, Kwon YK, Ha KT, So I and Kim BJ: The traditional herbal
medicine, Ge-Gen-Tang, inhibits pacemaker potentials by nitric
oxide/cGMP dependent ATP-sensitive K(+) channels in cultured
interstitial cells of Cajal from mouse small intestine. J
Ethnopharmacol. 170:201–209. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ehlert FJ, Pak KJ and Griffin MT:
Muscarinic agonists and antagonists: Effects on gastrointestinal
function. Handb Exp Pharmacol. 208:343–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
So KY, Kim SH, Sohn HM, Choi SJ, Parajuli
SP, Choi S, Yeum CH, Yoon PJ and Jun JY: Carbachol regulates
pacemaker activities in cultured interstitial cells of Cajal from
the mouse small intestine. Mol Cells. 27:525–531. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Garrington TP and Johnson GL: Organization
and regulation of mitogen-activated protein kinase signaling
pathways. Curr Opin Cell Biol. 11:211–218. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lukacs NW, Strieter RM, Chensue SW, Widmer
M and Kunkel SL: TNF-alpha mediates recruitment of neutrophils and
eosinophils during airway inflammation. J Immunol. 154:5411–5417.
1995.PubMed/NCBI
|
|
50
|
Cook AK, Carty M, Singer CA, Yamboliev IA
and Gerthoffer WT: Coupling of M(2) muscarinic receptors to ERK MAP
kinases and caldesmon phosphorylation in colonic smooth muscle. Am
J Physiol Gastrointest Liver Physiol. 278:G429–G437.
2000.PubMed/NCBI
|
|
51
|
Budd DC, Willars GB, McDonald JE and Tobin
AB: Phosphorylation of the Gq/11-coupled M3-muscarinic receptor is
involved in receptor activation of the ERK-1/2 mitogen-activated
protein kinase pathway. J Biol Chem. 276:4581–4587. 2001.
View Article : Google Scholar
|
|
52
|
Matthiesen S, Bahulayan A, Kempkens S,
Haag S, Fuhrmann R, Stichnote C, Juergens UR and Racké K:
Muscarinic receptors mediate stimulation of human lung fibroblast.
Am J Respir Cell Mol Biol. 35:621–627. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Luttrell LM: Activation and targeting of
mitogen-activated protein kinases by G-protein-coupled receptors.
Can J Physiol Pharmacol. 80:375–382. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gu J and Iyer VR: PI3K signaling and mRNA
expression during the response of quiescent human fibroblasts to
distinct proliferative stimuli. Genome Biol. 7:R422006. View Article : Google Scholar
|
|
55
|
Frucht H, Jensen RT, Dexter D, Yang WL and
Xiao Y: Human colon cancer cell proliferation mediated by the M3
muscarinic cholinergic receptor. Clin Cancer Res. 5:2532–2539.
1999.PubMed/NCBI
|
|
56
|
Cheng K, Zimniak P and Raufman JP:
Transactivation of the epidermal growth factor receptor mediates
cholinergic agonist-induced proliferation of H508 human colon
cancer cells. Cancer Res. 63:6744–6750. 2003.PubMed/NCBI
|
|
57
|
Ukegawa JI, Takeuchi Y, Kusayanagi S and
Mitamura K: Growth-promoting effect of muscarinic acetylcholine
receptors in colon cancer cells. J Cancer Res Clin Oncol.
129:272–278. 2003.PubMed/NCBI
|
|
58
|
Guizzetti M and Costa LG: Activation of
phosphatidylinositol 3 kinase by muscarinic receptors in
astrocytoma cells. Neuroreport. 12:1639–1642. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jimenez E and Montiel M: Activation of MAP
kinase by muscarinic cholinergic receptors induces cell
proliferation and protein synthesis in human breast cancer cells. J
Cell Physiol. 204:678–686. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Oppitz M, Möbus V, Brock S and Drews U:
Muscarinic receptors in cell lines from ovarian carcinoma: Negative
correlation with survival of patients. Gynecol Oncol. 85:159–164.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Song P, Sekhon HS, Lu A, Arredondo J,
Sauer D, Gravett C, Mark GP, Grando SA and Spindel ER: M3
muscarinic receptor antagonists inhibit small cell lung carcinoma
growth and mitogen-activated protein kinase phosphorylation induced
by acetylcholine secretion. Cancer Res. 67:3936–3944. 2007.
View Article : Google Scholar : PubMed/NCBI
|