Introduction
The uncontrolled production of free radicals is
involved in various diseases, including cancer and atherosclerosis,
and degenerative aging processes. It is important to develop
effective antioxidants with low toxicity that protect the human
body from free radicals and, thus, a number of chronic diseases.
Polysaccharides consist of polymeric structures composed of at
least ten monosaccharides sequentially connected by glycosidic
bonds. Polysaccharides can be classified as homopolymers, a term
used to indicate a polymer composed of identical monosaccharides,
or heteropolymers, a term used to classify polysaccharides composed
of two or more types of monosaccharides. Fungal polysaccharides are
a type of active organic compound that are found in the fruiting
bodies, mycelium and fermentation broth of large edible and
medicinal fungi (1,2). A large number of studies have
demonstrated that fungal polysaccharides exhibit a variety of
biological activities, including anti-aging, antitumor,
anti-oxidation and immunoregulatory activities, and are safe with
low toxicity (3–10).
Amanita caesarea is a type of fungi of the
Amanita genus, which grows in the Garzê county of Sichuan
province (China) at an elevation of 3,800 m. In this study, a
water-soluble polysaccharide (termed AC-1) was obtained from the
fruiting bodies of Amanita caesarea using a
diethylaminoethyl (DEAE)-cellulose column, Sephacryl S-300 gel
column and Sephadex G-200 column. To the best of our knowledge, its
chemical structure was characterized for the first time in the
present study. Structural analysis of the fraction was conducted
using chemical methods, infrared spectra spectroscopy and nuclear
magnetic resonance spectroscopy. The antioxidant activity of AC-1
was also evaluated by two antioxidant assays. The result of this
study introduced Amanita caesarea as a possible valuable
natural product, which exhibited unique antioxidant properties.
Materials and methods
Materials
Fresh Amanita caesarea was collected in the
Garzê county of Sichuan province, for which specific permission was
not required as it is an open village in China. The field studies
did not involve endangered or protected species as the endangered
or protected species protection zone was not entered for sampling.
Following vacuum freeze-drying, the Amanita caesarea were
crushed and stored at 4°C prior to use in the Key Laboratory of
Southwest China wild Resources Conservation (Ministry of
Education), College of Life Sciences, China West Normal University
(Nanchong, China). Trifluoroacetic acid (TFA), standard
monosaccharides and dextrans of different molecular weights were
purchased from Beijing Biodee Biotechnology Co., Ltd. (Beijing,
China). DEAE-cellulose column, Sephacryl S-300 gel column and
Sephadex G-200 column were purchased from Sigma-Aldrich; Merck
Millipore (Darmstadt, Germany). All other reagents used were of
analytical grade.
Extraction, isolation and purification of
polysaccharides from the fruiting bodies of Amanita caesarea
The fresh Amanita caesarea was thoroughly
washed with water, dried at 60°C, and then powdered with a
pulverizer. For conventional extraction, dried and powdered
Amanita caesarea (200 g) was accurately weighed and
extracted with 2,000 ml distilled water at 85°C for 6 h. The
extract was filtrated and centrifuged at 17,925 × g for 20
min in a high-speed centrifuge and concentrated in a vacuum. Then
the supernatant was added to 6X 95% EtOH to precipitate the crude
polysaccharides. After the deproteination as described previously
(11), the crude polysaccharides
were redissolved in 100 ml distilled water, purified with the
Sephacryl S-300 gel column and DEAE-cellulose column according to
the manufacturer's instructions. The polysaccharides were eluted
stepwise, fractionated with 0.1, 0.2, 0.3, 0.4 and 0.5 mol/l NaCl
and monitored using the phenol-sulfuric acid method (12). The 0.1 M NaCl elution was purified,
concentrated using the Sephadex G-200 column, passed through a
6-kDa membrane for 36 h to eliminate small molecular compounds and
lyophilized overnight in a Christ Alpha 1–2 LD freeze dryer (Martin
Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany).
AC-1 were obtained by vacuum freeze drying for further analysis of
their structure.
Determination of the molecular weight of
AC-1
The molecular weight of the polysaccharide fraction
was identified by high-performance gel permeation chromatography
(HPGPC) as described previously (13). Briefly, an aliquot (5 mg) of the
dry polysaccharide was dissolved in 5 ml double-distilled water and
filtered through a membrane filter (0.22 µm). The
calibration curve was prepared from the standard T-series Dextran.
The data were analyzed using GPC software (Millennium 32 software;
Agilent Technologies, Inc., Santa Clara, CA, USA).
Monosaccharide composition analysis of
AC-1
The polysaccharide AC-1 (5 mg) was hydrolyzed with 2
M TFA at 110°C for 6 h using acid-catalyzed hydrolysis (14). When hydrolysis was completed, the
products were dissolved with distilled water for monosaccharide
composition analysis. Monosaccharide composition was measured by a
high-performance liquid chromatography (HPLC) refractive index
detector (Agilent 1100 series; Agilent Technologies, Inc.). The
HPLC was performed under the following conditions: A concentration
of refined polysaccharide of 20 mg/ml, 75% acetonitrile as the
mobile phase at 1.4 ml/min and a column oven temperature of 35°C
(15). D-glucose, D-mannose,
D-fructose, D-galactose, L-rhamnose, L-arabinose and D-xylose were
used as standard sugars.
Fourier transform-infrared spectra
(FT-IR) analysis
FT-IR spectra of the polysaccharide AC-1 were
measured by grinding a mixture of polysaccharide with dry KBr, then
pressing into pellets. FT-IR spectra of the AC-1 was collected
using a Thermo Nicolet 6700 spectrometer (Thermo Fisher Scientific
Inc., Waltham, MA, USA) operating in the range of 400–4,000
cm−1 at a resolution of 2 cm−1.
Methylation analysis
The polysaccharide was methylated using methyliodide
as described previously (16). The
completeness of methylation was confirmed by the disappearance of
the hydroxyl absorption in IR spectrum at 3,400 cm−1.
The permethylated product was depolymerized with 90% formic acid at
100°C for 4 h, and further hydrolyzed with 2 M TFA at 100°C for 6
h. The resulting products were derivatized using the derivatization
reagent and analyzed by gas chromatography-mass spectrometry
(GC-MS) as described previously (17).
Nuclear magnetic resonance (NMR)
analysis
The poly-saccharide was dissolved in deuteroxide
accompanied by ultrasonic wave precessing for 10 min. Ultrasonic
wave precessing was conducted using a Bransonic CPX-3800H (Branson
Ultrasonics, Danbury, CT, USA) and precessing increases the
dissolution rate of polysaccharides. Then the Varian Unity INOVA
400/45 (Agilent Technologies, Inc.) was used to perform the
13C NMR spectra and 1H NMR spectra analyses
with tetramethylsilane as internal standard.
DPPH− radical scavenging
activity
The DPPH− radical scavenging activity of
the polysaccharide sample was measured by a decrease in absorbance
at 517 nm of a solution of purple-colored DPPH− in
methanol brought about by the sample (18). A lower absorbance of the reaction
mixture indicates higher free radical scavenging activity.
Absorbance at 517 nm was measured after 30 min using a UV-visible
spectrometer. The capability to scavenge the DPPH−
radical was calculated using the following equation:
Where Acontrol is the absorbance of the control reaction
and Atest is the absorbance in the presence of the
polysaccharide sample (). The
antioxidant activity of the extract was compared with that of
vitamin C (Vc) butylated hydroxytoluene (BHT).
ABTS radical scavenging activity
ABTS+ radical scavenging activity of the
polysaccharide extracts and fractions was measured using the
ABTS+ cation decolorization assay as described
previously (20). Briefly, the
ABTS+ radical cation was produced by reaction of 7 mM
stock solution ABTS+ with 2.45 mM ammonium persulphate
and then the mixture was incubated at room temperature in the dark
for 16 h. Then 2 ml of various concentrations of the sample and 2
ml of 0.7 mM ABTS+ radical solution were added. A
control reaction was conducted without the polysaccharide extracts.
The absorbance was measured immediately at 734 nm. The percentage
of scavenging of hydrogen radicals was calculated as follows:
Where Acontrol was the absorbance of the control group
in the ABTS+ radicals generation system,
Asample was the absorbance of the test group and
Asample blank was the absorbance of the samples only. VC
was used as a positive control.
Statistical analysis
All data are presented as the mean ± standard
deviation of three replications. Statistical analyses were
performed using Student's t-test and one-way analysis of variance
with SPSS software (version 20; IBM SPSS, Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Extraction, isolation and purification of
polysaccharides
The crude polysaccharide, termed AC-1, was obtained
as a water-soluble light yellow powder from the fruiting bodies of
Amanita caesarea, with a yield of 10.8%.
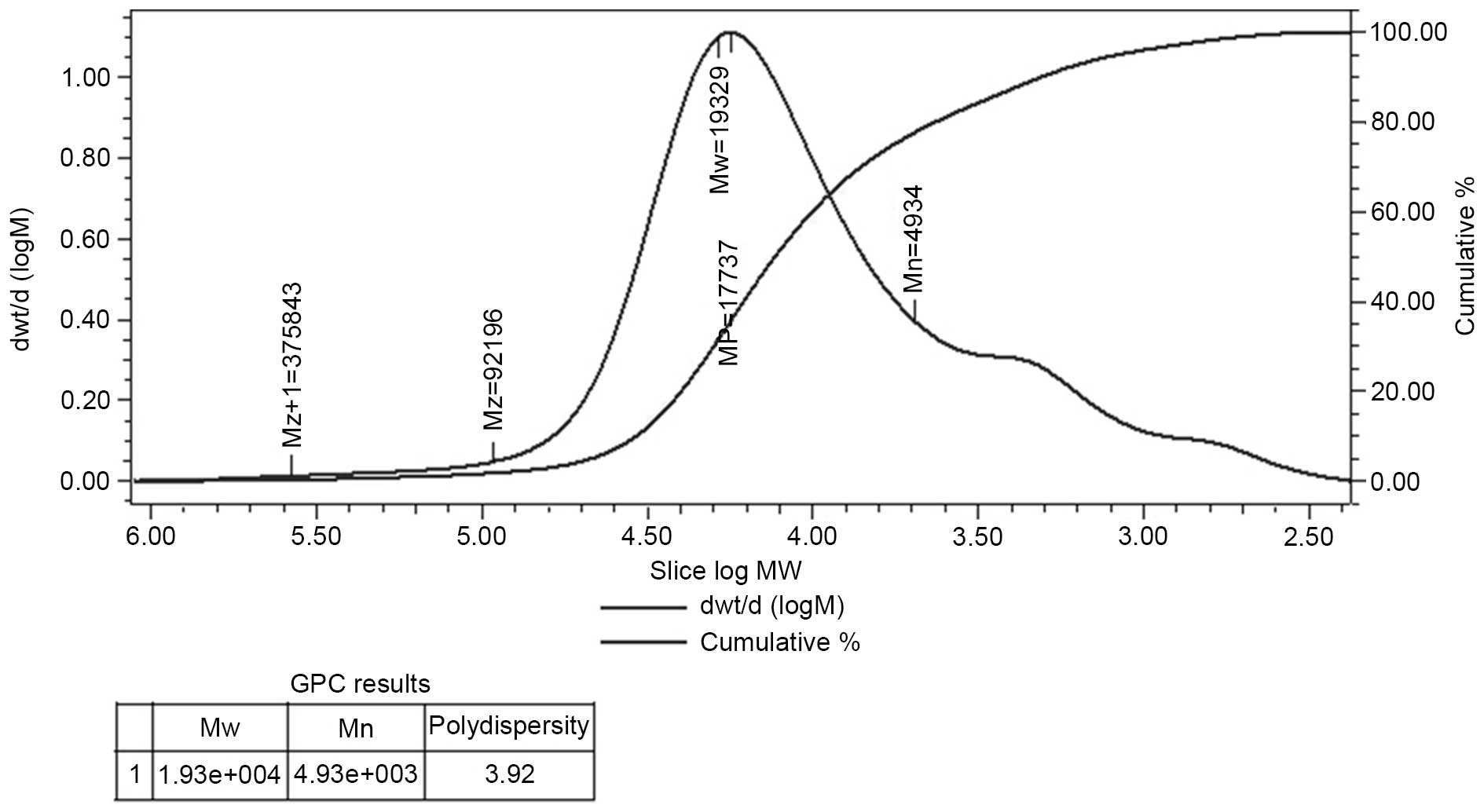
Determination of molecular weight
HPGPC of the polysaccharide fraction demonstrated
that each fraction was represented by a broad and symmetrical peak
on the chromatograms. The dextran standards were used to create a
calibration curve for elucidating the molecular weight of AC-1.
Weight-average molecular weight of AC-1 was ~19,329 Da and the
polydispersity was 3.92 (Fig.
1).
Monosaccharide composition analysis
The composition analysis of polysaccharides is an
important quality control step to determine basic information
regarding the polysaccharides. In this study, the AC-1
polysaccharide samples were hydrolyzed with TFA and then the
component monosaccharides were analyzed by HPLC with Agilent
refractive index detector. It is shown that the AC-1
polysaccharides were composed of D-Glucose and D-Xylose (Fig. 2).
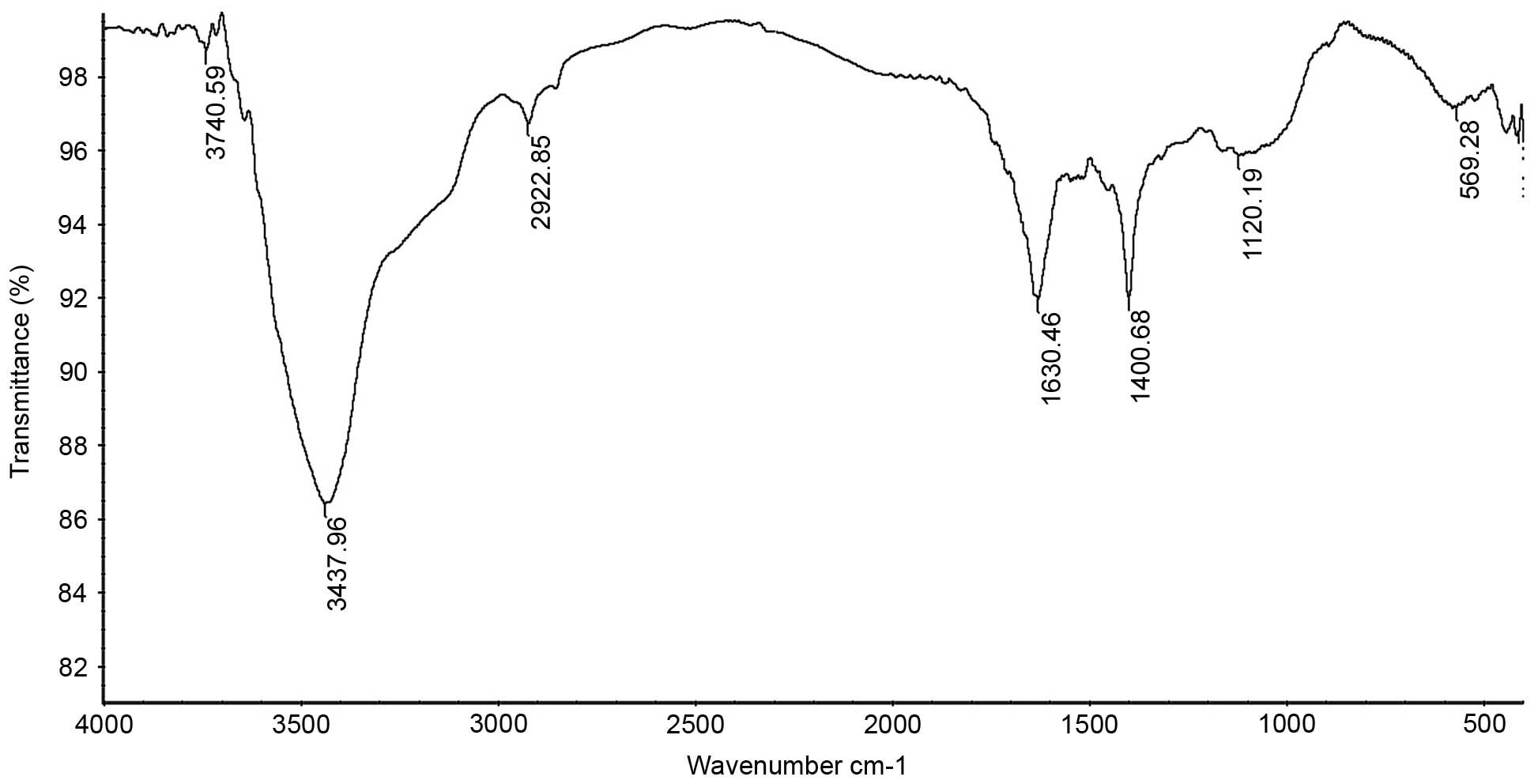
FT-IR analysis
The IR spectrum of the sample (Fig. 3) showed that the absorption was
greatest at >3,000 cm−1, which was caused by the
stretching and angular vibration of the O-H linkage. In addition,
the absorption peak observed at 2,922.85 cm−1 was due to
C-H stretching vibration, the absorption peak at 1,630.46
cm−1 was caused by OH deformation vibration and the
strong absorption peaks at 1,400.68 cm−1 were due to C-H
bending vibration. The absorption peaks at 1,120.19 cm−1
in the range of 1,200–1,000 cm−1 in the IR spectrum
suggested that the monosaccharides in the two samples had a
pyranosering. In addition, the peaks at 569.28 cm−1 were
due to C-H rocking vibration.
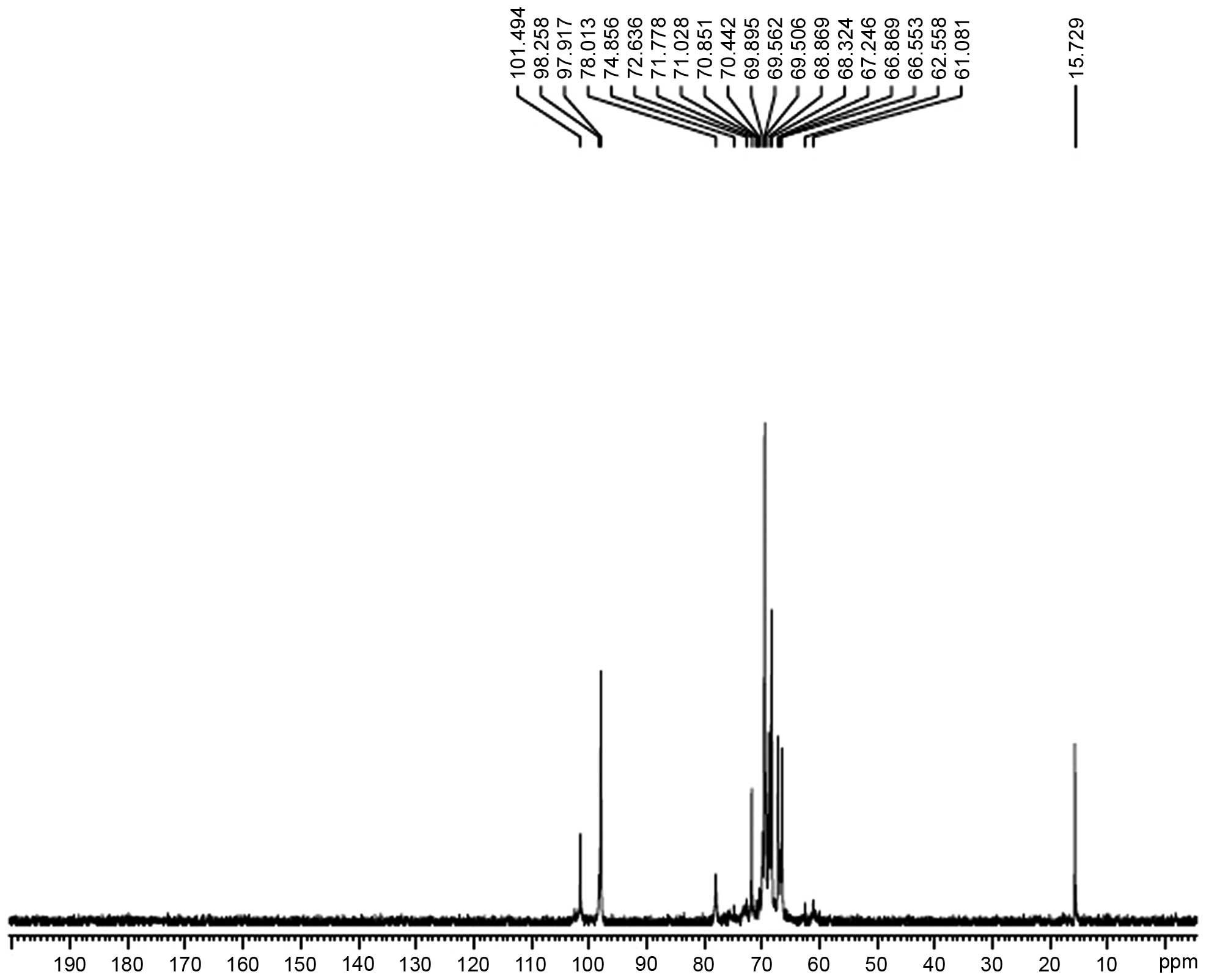
Analysis of the NMR results
The hydrogen spectrum of AC-1 is shown in Fig. 4. In the 1H NMR (400HZ)
spectrum, δ5.06 and δ4.98 indicate that there were two anomeric
hydrogens existing in AC-1. This suggests that AC-1 is composed of
two monosaccharides (α pyranose), as the H-1 chemical shift was
>4.95. The signals at δ3.647-δ4.196 are the signal peak overlaps
of remaining protons. δ4.793 and δ4.814 were the hydrogen signals
of water. The 13C NMR spectrum of AC-1 (Fig. 5) showed that the anomeric peaks
were centralized at δ97.917, δ98.258 and δ101.494 ppm, indicating
that there was only an α-anomeric configuration in AC-1. The
results are consistent with the analysis results of IR and
1H NMR. A chemical shift was not observed in the region
between δ160 and δ180 ppm, indicating that there was no hyaluronic
acid in AC-1. The presence of the AC-1 signal confirmed that all
monomers had a pyran ring, as furan ring signals should be around
δ107–109 ppm. According to the literature, the resonances in the
region of 97–101 ppm in the 13C NMR (400 MHz) spectrum
of AC-1 were attributed to the anomeric carbon atoms of
-D-lyxopyranose (-D-lyx) and -D-glucosepyranose (-D-Glup). In the
anomeric carbon region, the signal at δ97.92 could be attributed to
C-1 of →4)-α-D-Glu-(1→; the signal at δ98.26 to C-1 of
→3,6)-α-D-Glu-(1→; and the signal at δ101.50 to C-1 of α-D-lyx-(1→
(Fig. 5). The assignment of the
carbon atom signals is shown in Table
I.
 | Table I13C nuclear magnetic
resonance chemical shift data (δ, ppm) for polysaccharide AC-1 |
Table I
13C nuclear magnetic
resonance chemical shift data (δ, ppm) for polysaccharide AC-1
| Chemical shift, δ
(ppm)
|
|---|
| Sugar residue | C1 | C2 | C3 | C4 | C5 | C6 |
|---|
| →4)-α-D-Glcp-(1→ |
97.92 | 68.32 | 70.85 | 72.63 | 69.56 | 62.56 |
|
→3,6)-α-D-Glcp-(1→ |
98.26 | 68.87 | 71.03 | 74.86 | 69.90 | 69.90 |
| α-D-lyx-(1→ | 101.50 | 69.51 | 71.78 | 78.01 | 70.44 | 61.08 |
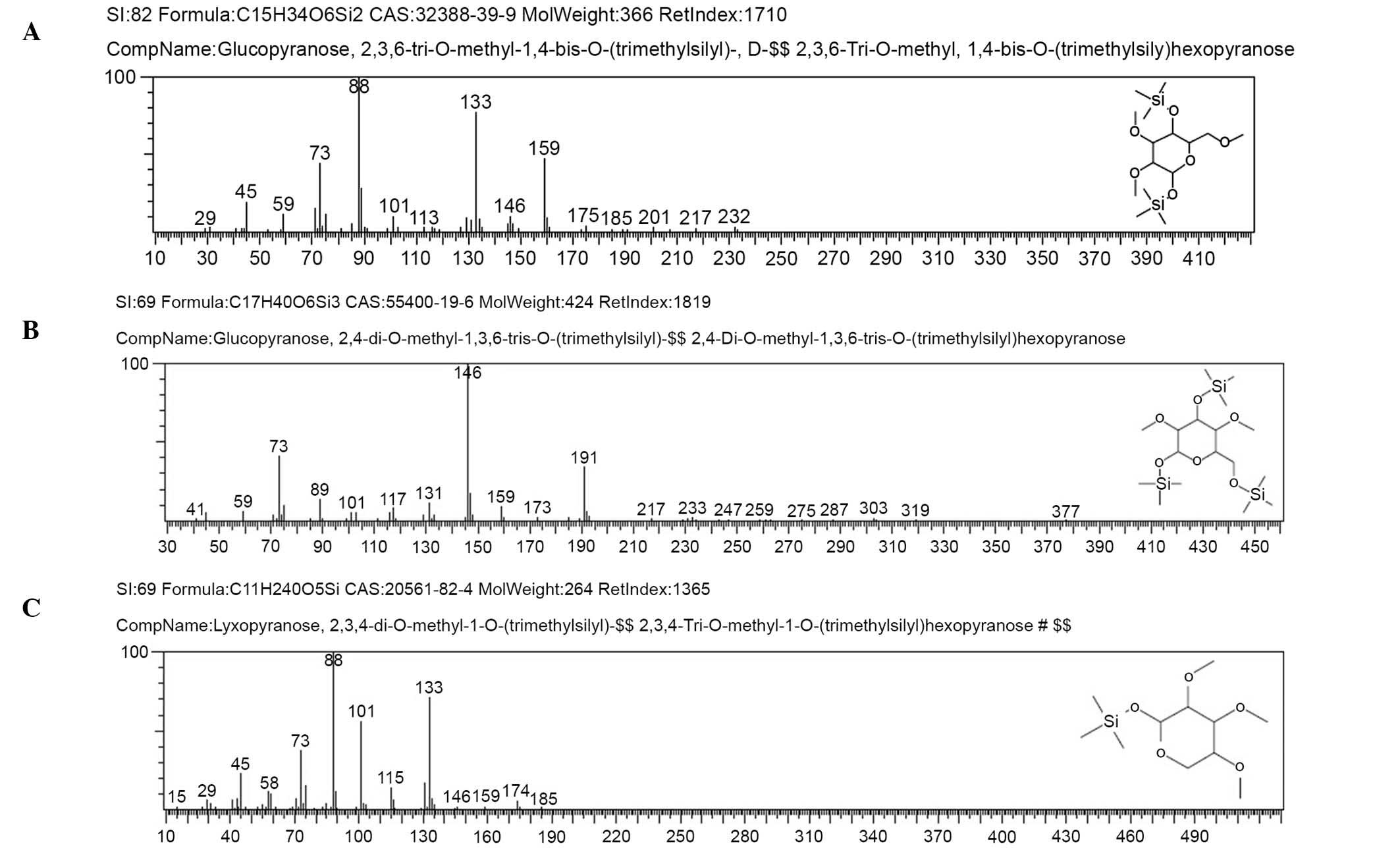
Methylation analysis
The methylated products of AC-1 were hydrolyzed with
acid, converted into alditol acetate, and analyzed by GC-MS.
Experimental data are shown in Table
II. The information in MS showed that fragment ion peaks were
consistent with data of D-configuration monosaccharide fragment ion
peaks, which suggested that the glucose and lyxose residues were
both of D-configuration. Methylation analysis for AC-1 demonstrated
that the α-D-glucosepyranose residues were 2,4-bis-substituted and
2,3,6-trisubsituted, and the α-D-lyxopyranose residues were
2,3,4-tri-substituted (Fig. 6 and
Table II). Results of methylated
linkage analysis of AC-1 indicated that
(1→4)-linked-α-D-glucosepyranose was one of the largest residues of
the polysaccharide structure, the branched residue was (1→3,
6)-linked-α-D-glucosepyranose revealing that
(1→3)-linked-α-D-glucosepyranose forms the backbone of the
structure. Residues of branch structure terminated with
α-D-lyxopyranose residues. It was concluded that a repeating unit
of AC-1 has a backbone of (1→4)-α-D-glucosepyranose and (1→3,
6)-α-D-glucosepyranose. The branches were composed of one →1)
α-D-lyxopyranose residue. The D-configuration monosaccharide of
AC-1 (Fig. 2) was in accordance
with the GC-MS analysis (Fig.
6).
 | Table IIGas chromatography-mass spectrometry
results of methylation analysis of AC-1. |
Table II
Gas chromatography-mass spectrometry
results of methylation analysis of AC-1.
| Methylated
sugar | Linkage | m/z |
|---|
|
2,3,6-Me3-Glcp |
1,4- | 45, 59, 73, 88,
101, 113, 133, 146, 159, 175, 185, 201, 217, 232 |
|
2,4-Me2-Glcp |
1,3,6- | 41, 59, 73, 89,
101, 117, 131, 146, 159, 173, 191, 217, 233 |
|
2,3,4-Me3-Lyx | 1- | 45, 58, 73, 88,
101, 115, 133, 146, 159, 174, 185 |
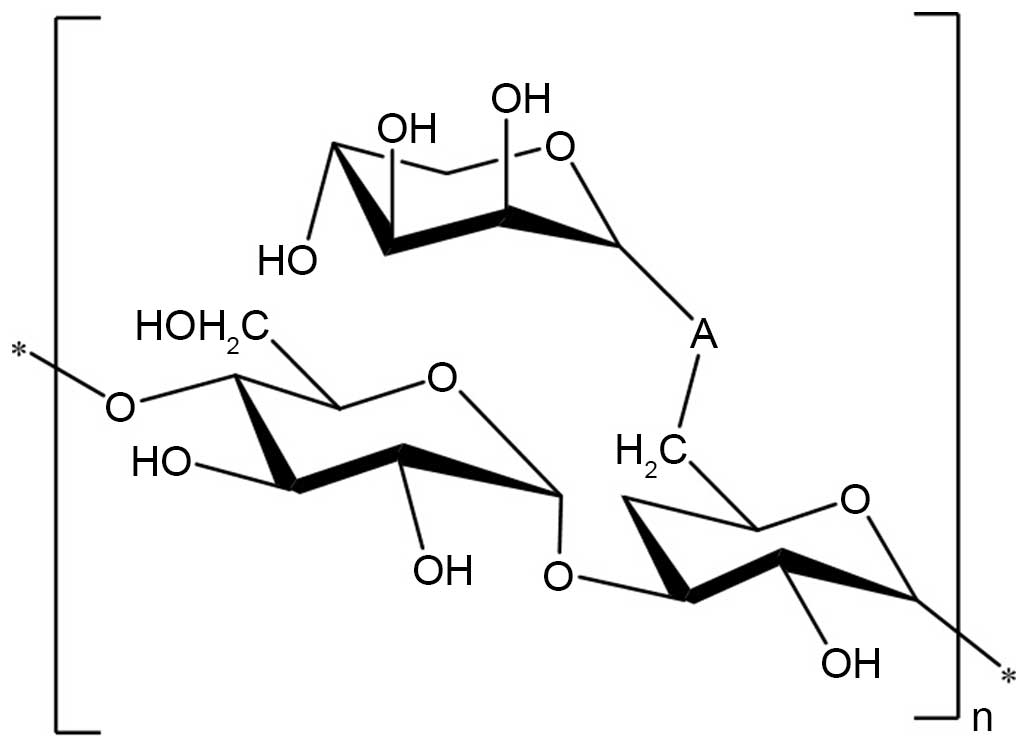
Structure elucidation of AC-1
On the basis of the above experimental data, it was
elucidated that the possible structure of AC-1 had a backbone of
1,4-linked D-glucose and 1,3, 6-linked D-glucose with branches
predominantly composed of one 1-linked D-lyxose residue (Fig. 7).
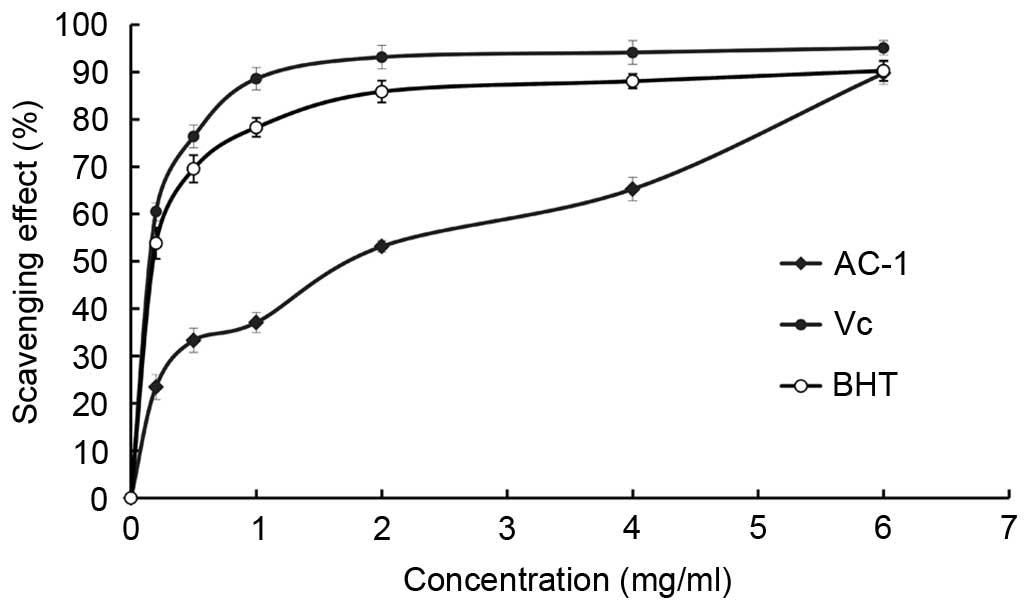
DPPH free radical scavenging activity of
AC-1
DPPH radicals have an odd (unpaired) number of
electrons and a strong absorption at 517 nm. When a radical
scavenger pairs to an unpaired electron, the strong absorption is
gradually decreased. It is visually noticeable as a change in color
from purple to yellow. Amanita caesarea exhibited a
comparable anti-oxidant activity with that of standard Vc at
varying concentrations tested. There was a dose-dependent increase
in the percentage antioxidant activity (Fig. 8). The extract of AC-1 at a
concentration of 1.0 mg/ml showed 37.09% free radical scavenging
activity and 89.74% at 6.0 mg/ml. The results showed that the
IC50 value of eliminating DPPH− radicals was
~1.69 mg/ml for AC-1, which indicated that AC-1 has a significant
effect on the scavenging DPPH− radical, particularly at
a high concentration. However, the scavenging ability was lower
than that of Vc. There was no significant difference
between the DPPH free radical scavenging activity when the
concentration of AC-1 was 6 mg/ml compared with the Vc and BHT.
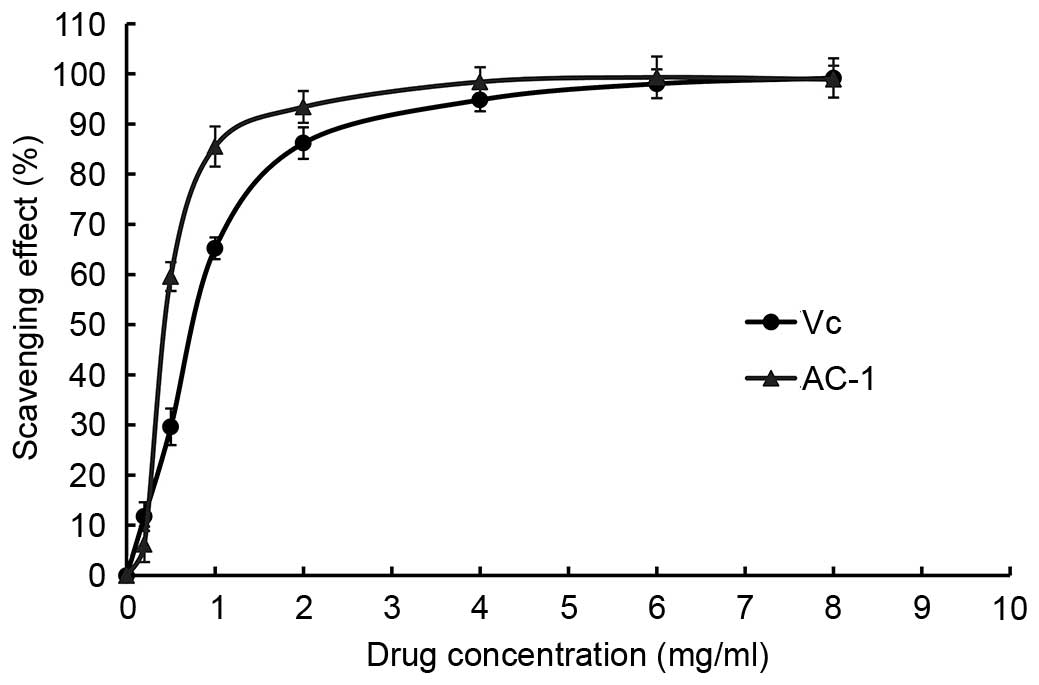
ABTS radical scavenging activity of
AC-1
The ABTS+ radical scavenging activity of
AC-1 was measured spectrophotometrically at 734 nm. The results of
antioxidant activity of AC-1 are shown in Fig. 9. It demonstrated that the
absorbance of the ABTS+ radical cation was decreased
dose dependently, upon interaction with various concentrations of
the extract, and the IC50 value of AC-1 was 0.92 mg/ml.
Notably, there was no significant difference between ABTS free
radical scavenging activity when the concentration of AC-1 was 6–8
mg/ml compared with Vc.
Discussion
The present study reveals that the polysaccharide
obtained from the fruiting bodies of Amanita caesarea,
termed AC-1, is a heteropolysaccharide. The purified polysaccharide
was confirmed to be of high purity. Structural analysis using GC-MS
indicated that AC-1 consists of α-D-glucose and α-D-lyxose at a
ratio of 2:1. The backbone of AC-1 is composed of 1,4-linked
α-D-glucose and 1,3, 6-linked α-D-glucose. The branches were
predominantly composed of one 1-linked α-D-lyxose residue.
Free radicals are atoms or groups of atoms with an
odd (unpaired) number of electrons and are formed when oxygen
interacts with certain molecules. Once formed these highly reactive
radicals can initiate a chain reaction. Damage is caused when free
radicals react with important cellular components, such as DNA or
the cell membrane. Cells may function poorly or die if this occurs
(21,22). Antioxidants are molecules which can
safely interact with free radicals and terminate the chain reaction
before vital molecules are damaged. Antioxidants are also thought
to serve a role in slowing the aging process, and preventing heart
disease and stroke (23,24). A large number of studies have
demonstrated that fungal polysaccharides exhibit antioxidative
activities and have low toxicity.
In the present study, DPPH free radical scavenging
activity was analyzed and compared with that of Vc. It was
demonstrated under the same experimental conditions, that the
IC50 value of Vc was 0.1 mg/ml while the IC50
value of AC-1 was 1.69 mg/ml. According to the respective
structural characteristics of the polysaccharide molecules, we can
infer that the weight-average molecular weight and the degree of
polymerization of polysaccharide extracted are greater, while the
amount of isolated hydroxyl are less. Therefore, the scavenging
activity of AC-1 through the direct reduction of the electron and
proton depend on the isolated hydroxyl, which make it possible to
decease the capacity of the N=N double bond in DPPH by oxidation-
reduction reaction. In DPPH scavenging assays, the fraction of AC-1
extract exhibit the great antioxidant activity, which may be due to
the amount of isolated hydroxyl.
Furthermore, in the ABTS radical scavenging activity
experiment, different polysaccharide doses resulted in different
scavenging ability of ABTS+. Under the experimental
conditions of the ABTS radical scavenging activity assays, the
IC50 value of AC-1 is 0.92 mg/ml. Overall the fruiting
bodies of Amanita caesarea may be used in nutritional or
pharmaceutical fields.
Acknowledgments
This project was supported by the National Natural
Science Foundation of China (grant nos. 31400016 and 31200012); the
Application Foundation Project of Sichuan Province (grant no.
2013JY0094); the Science and Technology Support Project of Sichuan
Province (grant nos. 2014SZ0020 and 2014FZ0024), the Cultivate
Major Projects of Sichuan Province (grant no. 14CZ0016); and the
Open Foundation of Microbial Resources and Drug Development of Key
Laboratory and of Guizhou Province (grant no. GZMRD-2014-002).
References
|
1
|
Hibbett DS, Binder M, Bischoff JF,
Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM,
Lücking R, et al: A higher-level phylogenetic classification of the
fungi. Mycol Res. 111:509–547. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bertozzi CR and Kiessling LL: Chemical
glycobioloy. Science. 291:2357–2364. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bittencourt VC, Figueiredo RT, da Silva
RB, Mourão-Sá DS, Fernandez PL, Sassaki GL, Mulloy B, Bozza MT and
Barreto-Bergter E: An alpha-glucan of Pseudallescheria boydii is
involved in fungal phagocytosis and toll-like receptor activation.
J Biol Chem. 281:22614–22623. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borchers AT, Stern JS, Hackman RM, Keen CL
and Gershwin ME: Mushrooms, tumors, and immunity. Proc Soc Exp Biol
Med. 221:281–293. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rudd PM, Elliott T, Cresswell P, Wilson IA
and Dwek RA: Glycosylation and the immune system. Science.
291:2370–2376. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brown GD, Herre J, Williams DL, Willment
JA, Marshall AS and Gordon S: Dectin-1 mediates the biological
effects of beta-glucans. J Exp Med. 197:1119–1124. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Angeli JP, Ribeiro LR, Gonzaga ML, Soares
Sde A, Ricardo MP, Tsuboy MS, Stidl R, Knasmueller S, Linhares RE
and Mantovani MS: Protective effects of beta-glucan extracted from
Agaricus brasiliensis against chemically induced DNA damage in
human lymphocytes. Cell Biol Toxicol. 22:285–291. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cordero RJ, Frases S, Guimaräes AJ, Rivera
J and Casadevall A: Evidence for branching in cryptococcal capsular
polysaccharides and consequences on its biological activity. Mol
Microbiol. 79:1101–1117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang F, Hou Y, Ding X, Hou W, Song B, Wang
T, Li J and Zeng Y: Structure elucidation and antioxidant effect of
a polysaccharide from Lactarius camphoratum (Bull.). Fr Int J Biol
Macromol. 62:131–136. 2013. View Article : Google Scholar
|
|
10
|
Wu XM and Tu PF: Isolation and
characterization of alpha-(1→6)-glucans from Cistanche deserticola.
J Asian Nat Prod Res. 7:823–828. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Staub AM: Removal of protein-Sevag method.
Carbohydr Chem. 5:5–6. 1965.
|
|
12
|
Kumar CG, Joo HS, Choi JW, Koo YM and
Chang CS: Purification and characterization of extracellular
polysaccharide from haloalkalophilic Bacillus sp I-450. Enzyme
Microb Technol. 34:673–681. 2004. View Article : Google Scholar
|
|
13
|
Pang XB, Yao WB, Yang XB, Xie C, Liu D,
Zhang J and Gao XD: Purification, characterization and biological
activity on hepatocytes of a polysaccharide from Flammulina
velutipes mycelium. Carbohydr Res. 70:291–297. 2007. View Article : Google Scholar
|
|
14
|
Yu RM, Yin Y, Yang W, Ma WL, Yang L, Chen
XJ, Zhang Z, Ye B and Song LY: Structural elucidation and
biological activity of a novel polysaccharide by alkaline
extraction from cultured Cordyceps militaris. Carbohydr Polym.
75:166–171. 2009. View Article : Google Scholar
|
|
15
|
Fan Y, He X, Zhou S, Luo A, He T and Chun
Z: Composition analysis and antioxidant activity of polysaccharide
from Dendrobium denneanum. Int J Biol Macromol. 45:169–173. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun Y and Liu J: Purification, structure
and immunobiological activity of a water-soluble polysaccharide
from the fruiting body of Pleurotus ostreatus. Bioresour Technol.
100:983–986. 2009. View Article : Google Scholar
|
|
17
|
Rongmin Y, Yin Y, Wei Yang, Weili M, Lin
Y, Xiujuan C, Zhang Z, Bin Y and Liyan S: Structural elucidation
and biological activity of a novel polysaccharide by alkaline
extraction from cultured Cordyceps militaris. Carbohydr Polymers.
75:166–171. 2009. View Article : Google Scholar
|
|
18
|
Ding X, Hou YL and Hou WR: Structure
elucidation and antioxidant activity of a novel polysaccharide
isolated from Boletus speciosus Forst. Int J Biol Macromol.
50:613–618. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen J, Hu T and Zheng R: Antioxidant
activities of Sophora subprosrate polysaccharide in
immunosuppressed mice. Int Immunopharmacol. 7:547–553. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo DH: Identification of structure and
antioxidant activity of a fraction of polysaccharide purified from
Dioscorea nipponica Makino. Carbohydr Polym. 71:544–549. 2008.
View Article : Google Scholar
|
|
21
|
Kumar V, Lemos M, Sharma M and Shriram V:
Antioxidant and DNA damage protecting activities of Eulophia nuda
Lindl. Free Radicals and Antioxidants. 3:55–60. 2013. View Article : Google Scholar
|
|
22
|
Blois MS: Antioxidant determination by use
of stable free radicals. Nature. 29:1199–1200. 1958. View Article : Google Scholar
|
|
23
|
Hertog MGL, Feskens EJM, Kromhout D,
Hertog MGL, Hollman PCH, Hertog MGL and Katan MB: Dietary
antioxidant flavonoids and risk of coronary heart disease: The
Zutphen Elderly Study. Lancet. 342:1007–1011. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kushi LH, Folsom AR, Prineas RJ, Mink PJ,
Wu Y and Bostick RM: Dietary antioxidant vitamins and death from
coronary heart disease in postmenopausal women. New Engl J Med.
334:1156–1162. 1996. View Article : Google Scholar : PubMed/NCBI
|