Introduction
Sepsis, which has been described as the response of
the host towards invading pathogens or their toxins, remains a
leading contributor to mortality rates in critically ill patients
(1–3). Previous reports have indicated that
this condition is occurring in increasing numbers of patients, with
the mortality rate within 48 h of admission to an intensive care
unit reaching 49.8% (4,5). There has been no marked reduction in
long-term mortality rates, despite advances in monitoring devices,
diagnostic tools and therapeutic strategies (6–8). In
this regard, novel effective therapeutic strategies for the
treatment of sepsis are in urgent demand.
The inflammatory response in sepsis is particularly
complex and is characterized by an imbalance in the cytokine
profile (9). It has been suggested
that sepsis is associated with an exacerbated release of
pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α
and interleukin (IL)-6 (10–12),
and these cytokines serve as markers of disease severity in sepsis
(13). Evidence also indicates
that the increase of pro-inflammatory cytokines is accompanied by
certain anti-inflammatory cytokines, including IL-10 (14). In this context, interventions
aiming at restoring the balance in the cytokine network may be
promising.
Mesenchymal stem cells (MSCs) are reported to have
immunomodulatory properties, and have emerged as a promising tool
for the treatment of sepsis. In previous years, the administration
of bone marrow-derived MSCs (BMSCs) has been shown to attenuate
sepsis-associated inflammation and multiple organ dysfunctions,
thereby improving survival rates in experimental sepsis (15–18).
Further evidence has suggested that MSC treatment reduces the
levels of pro-inflammatory cytokines, including TNF-α and IL-6,
accompanied by increased levels of the anti-inflammatory cytokine,
IL-10, which may counteract the marked pro-inflammatory response
and restore immunological equilibrium (13,17,18).
However, others have reported either no effect on the levels of
IL-10 or reported a reduction (15,19,20).
The beneficial effect of MSCs on sepsis may be associated with the
precise regulation targeted at different immunological statuses in
models of sepsis.
MSCs are non-hematopoietic precursor cells derived
from a variety of tissues, including bone marrow, adipose tissue,
the placenta and umbilical cord (21). BMSCs were initially considered the
primary source of MSCs for clinical application, and the protective
effect of BMSCs on sepsis has been demonstrated (17). However, bone marrow harvesting is
invasive and painful, and can be associated with donor-site
morbidity with potentially low cell yields (22). Compared with BMSCs, adipose
tissue-derived MSCs (ADMSCs) can be more readily obtained, isolated
and rapidly expanded in vitro to generate sufficient dosages
(23,24). Therefore, ADMSCs have been
investigated as an alternative to BMSCs in several animal models of
incurable disease (25,26).
Although it has been demonstrated that ADMSCs offer
protective effects against inflammation-associated injury (27), whether ADMSCs exhibit an identical
effect to BMSCs due to use of a different model remains to be
elucidated. In addition, MSCs derived from different sources may
exhibit different immunological characteristics and therapeutic
effects (28,29). Therefore, further investigations
are required to compare the effect of BMSC and ADMSC on dysfunction
and systemic inflammation in the same disease model. The present
study aimed to compare the therapeutic effect of BMSCs and ADMSCs
in a mouse model of lipopolysaccharide (LPS)-induced sepsis, and
examine their potential regulatory role on pro- and
anti-inflammatory cytokines underlying the sepsis-associated
inflammatory response.
Materials and methods
Animals
SPF BALB/c mice, provided by the Animal Experiment
Center of The Third Xiangya Hospital of Central South University
(Changsha, China) were used in the present study. The mice were fed
a standard diet and placed in isolation in the animal facility at
the Third Xiangya Hospital of Central South University, for
acclimation purposes, for 1 week prior to experimentation. The mice
were maintained at a 20–260°C, a relative humidity of 40–70% and 12
h light/dark cycles. All experimental protocols were approved by
the ethics committee of the Third Xiangya Hospital of Central South
University (2012S130).
BMSC isolation and culture
The BALB/c mice, weighing 12–22 g (4 weeks old;
n=5), were sacrificed by cervical dislocation. Following immersion
in 75% ethanol for 10 min, the femurs and tibiae of the mice were
removed and placed on a sterile glass platform. The ends of each
femur and tibia, just below the end of the marrow cavity, were cut
and the marrow was flushed out with phosphate-buffered saline (PBS;
GE Healthcare Life Sciences, Logan, UT, USA), followed by
centrifugation at 300 × g for 5 min at room temperature to remove
blood and unnecessary tissue (30). The cell pellet was then resuspended
with 5 ml of red blood cell lysis buffer (Sigma-Aldrich; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Following
centrifugation at as before, the cells were resuspended in 5 ml of
complete medium comprised of Dulbecco's modified Eagle's medium
(DMEM)/F12 (GE Healthcare Life Sciences) supplemented with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin. All cells were plated in a
25-cm2 cell culture flask (Greiner Bio-One GmbH,
Frickenhausen, Germany) and incubated at 37°C in 5% humidified
CO2. After 48 h, the non-adherent cells were discarded
and the adherent cells were washed twice with PBS. Fresh complete
medium was then added and replaced every 3 days. After 14 days of
initiating culture, the cells were washed with PBS and lifted by
incubation in 0.5 ml of 0.25% trypsin/1 mM EDTA (Gibco; Thermo
Fisher Scientific, Inc.) for ~1 min at 37°C [20]. Complete medium
(~1.5 ml) was then mixed with the cells to neutralize the trypsin,
following which the cells were centrifuged at as before and
resuspended in 15 ml of complete medium. The cells were passaged at
a split ratio of 1:3 and cultured in 25-cm2 cell culture
flasks, with culture medium replaced every 3 days (31). Cells in the third passage were used
for subsequent experimentation.
ADMSC isolation and culture
BALB/c mice weighing 12–22 g (4 weeks old; n=5) were
sacrificed by cervical dislocation and immersed in 75% ethanol for
10 min. Lumbar dorsal adipose tissue was then isolated and minced
into 1–2-mm3 fragments. The tissue was then digested in
a 10-ml centrifuge cube containing 5 ml 0.1% collagenase I
(Sigma-Aldrich; Thermo Fisher Scientific, Inc.) for 1 h in a
shaking incubator at 37°C and at a speed of 300 × g (32). Following centrifugation at 300 × g
for 5 min at room temperature, the cells were resuspended in 5 ml
of complete medium and seeded into a 25-cm2 cell culture
flask prior to culture in an incubator at 37°C with 5% humidified
CO2 for 48 h. The subsequent steps were identical to
those for the BMSCs described above.
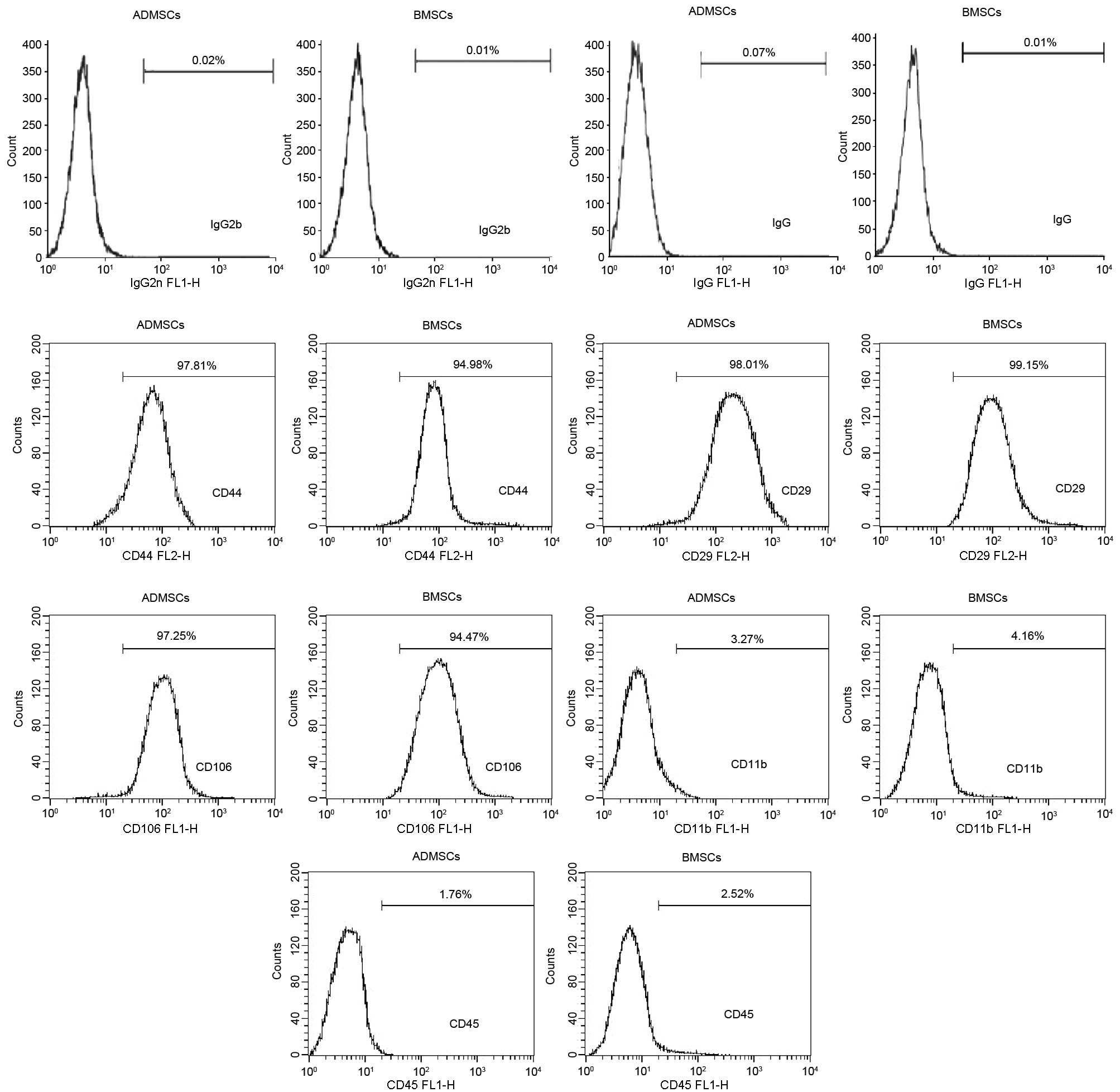
Phenotypic characterization of MSCs
The cells were harvested by trypsinization, as
described above, washed in cold PBS (4–8°C) and centrifuged for 5
min at 300 × g at room temperature. The cells (1×106)
were then suspended in 1 ml of cold PBS per tube, and stained with
fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD11b, CD45
and CD106; phycoerythrin (PE)-conjugated anti-mouse CD44 and CD29
(eBioscience, Inc., San Diego, CA, USA). The numbers of cells were
determined using a blood-cell counting chamber (Hausser Scientific,
Horsham, PA, USA). For the isotype controls, FITC- or PE-conjugated
non-specific rat and mouse IgG (eBioscience, Inc.) was substituted
for the primary antibody. Details of the antibodies used for flow
cytometry are shown in Table I.
The cells were incubated with the antibodies for 45 min at 4°C in
the dark, followed by washing in PBS and resuspension in 1 ml PBS
prior to examination using a FACSJazz flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA).
 | Table IList of antibodies. |
Table I
List of antibodies.
| Antigen | Labeling | Isotype | Dilution | Cat. no. |
|---|
| CD11b | FITC | Rat IgG2b | 0.5 mg/ml | 11-0112 |
| CD45 | FITC | Rat IgG2b | 0.5 mg/ml | 11-0451 |
| CD106 | FITC | Rat IgG2b | 0.5 mg/ml | 11-1061 |
| CD44 | PE | Rat IgG2b | 0.2 mg/ml | 12-0441 |
| CD29 | PE | Mouse IgG | 0.2 mg/ml | 12-0291 |
MSC multilineage differentiation
potential
The confluent BMSCs and ADMSCs were assessed for
their multi-lineage differentiation potential using standardized
protocols from Cyagen Biosciences, Inc. (Guangzhou, China).
Non-differentiated stained cells were used for comparison.
For chondrogenic differentiation, the MSCs
(1×106) were suspended in 1 ml of complete chondrogenic
medium (Cyagen Biosciences, Inc.). Following centrifugation at 300
× g for 5 min at room temperature, the cells were incubated at 37°C
in a humidified atmosphere of 5% CO2 with the tube caps
loosened by one half turn. The medium was replaced every 3 days.
The chondrogenic pellets were harvested following culture for 28
days. For examinations using microscopy, the pellets were
formalin-fixed and paraffin-embedded prior to Alcian blue staining
(Sigma-Aldrich; Thermo Fisher Scientific, Inc.).
For osteogenic differentiation, the MSCs were seeded
into 6-well tissue culture plates, which were pre-coated with
gelatin solution, at a density of 3×103
cells/cm2 in complete medium, and cultured at 37°C in 5%
CO2 for 7 days. The medium was then replaced with
osteogenic induction medium (Cyagen Biosciences, Inc.) for an
additional 21 days. The medium was replaced every 3–4 days.
Following 3 weeks of differentiation, the cells were formalin-fixed
and stained with Alizarin red (Sigma-Aldrich; Thermo FIsher
Scientific, Inc.).
For adipogenic differentiation, the MSCs were seeded
into 6-well tissue culture plates at a density of 2×104
cells/cm2 in complete medium, and were cultured at 37°C
in 5% CO2. The cells were maintained by replacing the
complete medium every 3 days until a confluent cell layer was
formed. The cells were then stimulated to differentiate into the
adipogenic lineage by exposing the cells to three cycles of
altering culture in adipogenic induction medium (Cyagen
Biosciences, Inc.) and maintenance medium (Cyagen Biosciences,
Inc.) according to the manufacturer's protocol. At the end of these
cycles, the cells were grown for another 7 days by replacing the
maintenance medium every 3 days. To verify adipogenic
differentiation, the cells were fixed with 4% formaldehyde solution
for 30 min and stained with oil red O (Sigma-Aldrich; Thermo Fisher
Scientific, Inc.). Following all staining procedures, images were
captured and analyzed using a an inverted system microscope
(Olympus, Tokyo, Japan) and were assessed using IX2-BSW Ver 01.07a
software.
Preparation of the murine model of
LPS-induced sepsis
SPF BALB/c mice weighing 22–28 g (8–12 weeks old;
n=120) were used for the model of LPS-induced sepsis. The mice were
induced with an injection of LPS (10 mg/kg, 1 mg/ml) extracted from
Escherichia coli (0127:B8; Sigma-Aldrich; Thermo Fisher
Scientific, Inc.) via the tail vein. A sham intervention was
performed using saline (10 mg/kg) in the control mice. To compare
the effect of MSCs on the septic mice, either saline (10 mg/kg),
BMSCs (1×107/ml; 100 µl) or ADMSCs
(1×107/ml; 100 µl) were injected via the tail
vein 5 min following administering LPS.
Survival analysis following LPS
administration
For survival analysis, 80 mice were randomly divided
into four groups (n=20/group): i) Saline only treatment (control
group); ii) LPS + saline treatment (LPS group); iii) LPS + BMSC
treatment (BMSC group), iv) LPS + ADMSC treatment (ADMSC group).
The mice were monitored for 48 h and the survival rates were
recorded. The rectal temperatures of the mice were also measured
every 4 h post-LPS administration.
Determination of serum cytokines and
biochemical markers
A total of 40 mice were randomly divided into the
four groups described above (n=20/group). Based on the data
obtained from the recording of survival rates and temperatures,
serum samples were collected 6 h post-LPS administration for
analysis, as no differences were observed between the four groups
at this time. The mice were sacrificed under anesthesia with 10%
chloral hydrate (30 mg/kg), and whole blood samples were collected
from the heart. Serum samples were obtained following blood
separation by centrifugation at 3,500 × g for 20 min at room
temperature and were stored at −70°C until analysis. Serum levels
of IL-2, IL-4, IL-6, IL-8, IL-10 and TNF-α were determined using an
enzyme-linked immunosorbent assay (ELISA) with mouse-specific kits
(Thermo Fisher Scientific, Inc.). Serum levels of the tissue
hypoperfusion indicator, lactate, the renal dysfunction indicator,
creatinine, and hepatic dysfunction indicators, alanine
aminotransferase (ALT) and aspartate aminotransferase (AST), were
determined using an automatic analyzer (7600-020; Hitachi, Tokyo,
Japan).
Statistical analysis
Data were analyzed using the SPSS 13.0 software
package (SPSS, Inc., Chicago, IL, USA) and are presented as the
mean ± standard deviation. Comparisons of mean body temperature,
levels of serum cytokines and biochemical markers between the four
groups were made using one-way analysis of variance followed by the
Bonferroni multiple-comparison post-hoc test. Survival data are
presented in Kaplan-Meier curves and statistical significance was
assessed using the Log rank test. P<0.05 was considered to
indicate a statistically significance difference.
Results
Characterization of BMSCs and ADMSCs
The BMSCs and ADMSCs reached 90% confluence between
days 5 and 7 (Fig. 1A)., and the
two cell types shared several features. The MSCs from the two cell
sources exhibited a spindle-shaped morphology and adhered well to
the plastic culture flasks. Additionally, the MSCs from the two
sources were demonstrated to differentiate into chondrocytes,
osteoblasts and adipocytes (Fig.
1B–D) following culture in appropriate induction medium.
Finally, the two cell types were negative for the expression of
CD11b and CD45 and positive for the expression of CD44, CD106 and
CD29 (Fig. 2).
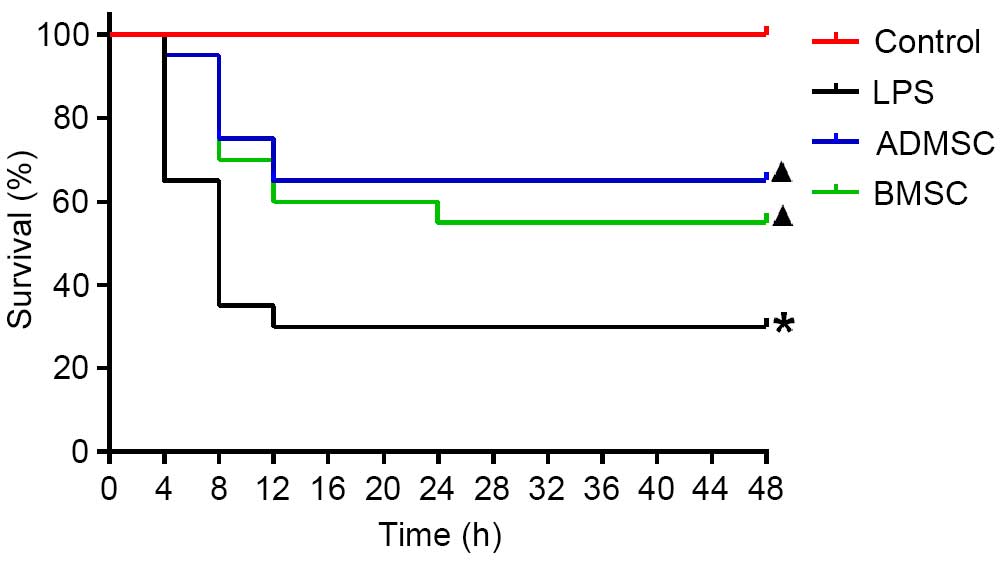
MSC treatment improves survival rates
following LPS induction
Survival analysis showed that all the mice in the
control group survived. The overall survival rates were
significantly improved in the BMSC and ADMSC groups, compared with
the LPS group (P<0.05). It was observed that, of the animals
that succumbed to mortality, all occurred within 24 h, with the
majority (13/14 for the LPS group, 5/7 for the ADMSC group and 6/9
for the BMSC group) occurring within 8 h. Therefore, the survival
rates were determined at 24 h. By 24 h, 6, 11 and 13 mice had
survived in the LPS, BMSC and ADMSC groups, respectively. The
survival rate was the highest in the ADMSC group (65%), followed by
the BMSC group (55%), and was lowest in the LPS group (30%). The
survival rates were significantly higher in the BMSC and ADMSC
groups, compared with the LPS group (P<0.05). Although the
survival rate was higher in the ADMSC group, compared with the BMSC
group, this difference was not significant (P>0.05; Fig. 3).
MSC treatment reduces body temperature
fluctuations following LPS induction
To evaluate changes in body temperature, the body
temperatures of the mice were recorded every 4 h within the 48 h
period following LPS exposure. With the exception of the control
group, the mice in the remaining three groups exhibited a decrease
in mean body temperature within 16 h. The temperature reached the
nadir at 8 h in the LPS group, and at 12 h in the two MSC treatment
groups. The body temperature was significantly lower in the LSP
group, compared with the MSC treatment groups, and significantly
higher in the BMSC group, compared with the ADMSC group at 4, 8 and
16 h (P<0.05). By 24 h, the temperature in the LPS group was
significantly higher, compared with those in the other three groups
(P<0.05). These results showed that MSC administration
attenuated the temperature decline, and ADMSCs appeared to be more
effective than BMSCs (Fig. 4).
 | Figure 4Temperature changes in mice with
LPS-induced sepsis following MSC treatment. Core mean body
temperatures were measured at different time points in the control
group (n=20), LPS group, BMSC and ADMSC group. By 4 h, the LPS,
BMSC and ADMSC groups contained 13, 19 and 19 mice, respectively.
By 8 h, the LPS, BMSC and ADMSC groups contained 7, 14 and 15 mice,
respectively. By 16 h, the LPS, BMSC and ADMSC groups contained 6,
12 and 13 mice, respectively. Treatment with BMSCs and ADMSCs
attenuated temperature decline at 4, 8 and 12 h post-LPS.
*P<0.05, vs. control group; ▲P<0.05,
vs. LPS group; □P<0.05, vs. BMSC group. BMSCs, bone
marrow-derived mesenchymal stem cells; ADMSCs, adipose
tissue-derived mesenchymal stem cells; LPS, lipopolysaccharide. |
MSC treatment attenuates organ damage
following LPS induction
As sepsis is life-threatening due to being
associated with organ failure, biochemical markers of the major
organs often injured in humans were examined to determine whether
MSCs had a beneficial effect on organ dysfunction. The serum levels
of lactate, creatinine, ALT and AST were measured 6 h following LPS
exposure. These markers were significantly increased in the mice
with LPS-induced sepsis, compared with the mice in the control
group. The concentrations of serum lactate, creatinine and liver
enzymes, including ALT and AST, which are released into the
circulation upon injury, were significantly decreased in the
MSC-treated mice, compared with mice in the LPS group (P<0.05).
However, no significant differences were found in the
concentrations of these biochemical markers between the BMSC and
ADMSC groups (P>0.05; Fig.
5).
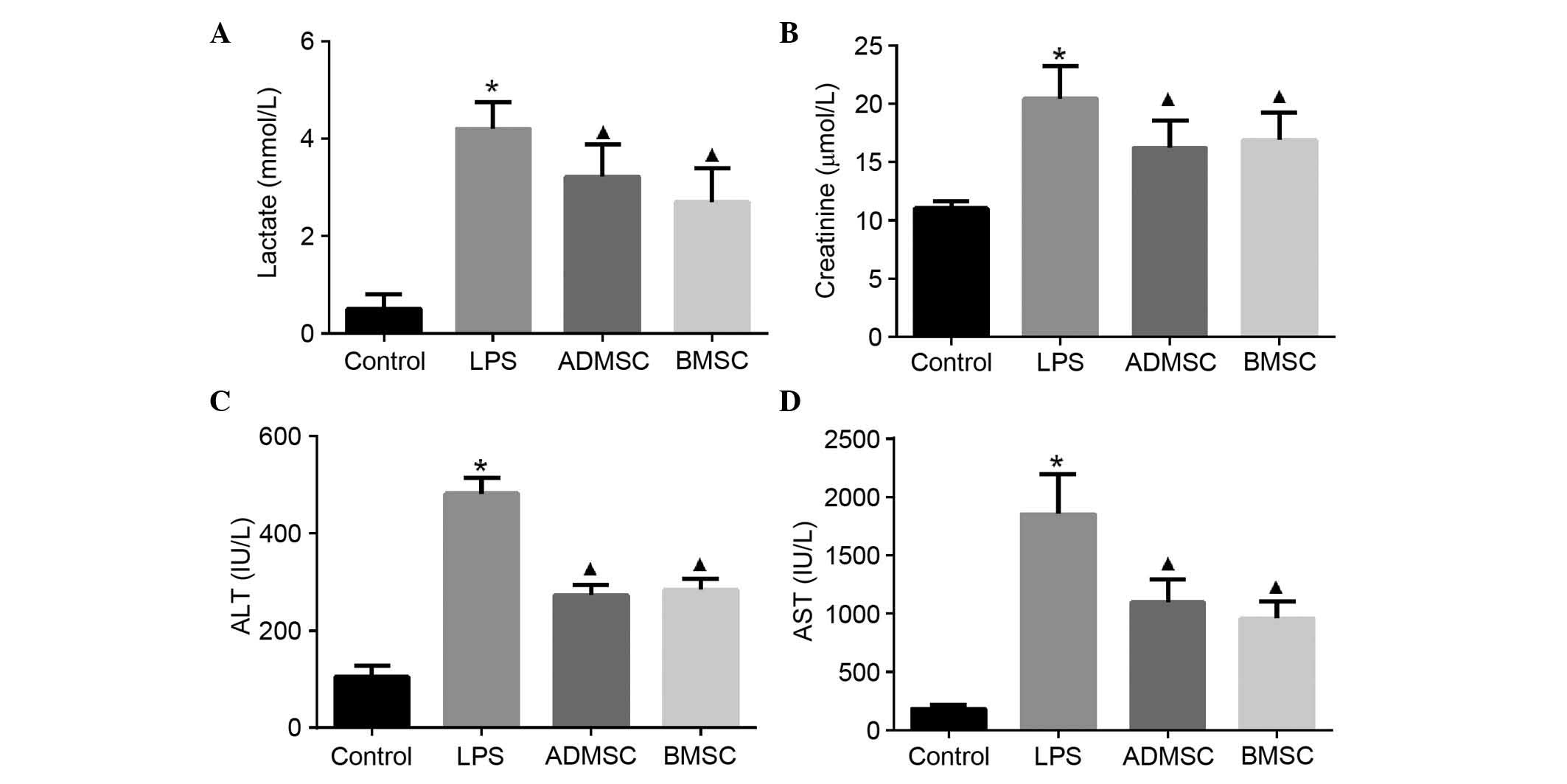 | Figure 5Serum levels of organ function
indicators in mice with LPS-induced sepsis following MSC treatment.
Intravenous administration of BMSCs or ADMSCs significantly reduced
the increases in (A) lactate, (B) creatinine, (C) ALT and (D) AST 6
h post-LPS exposure. The control, LPS, BMSC and ADMSC groups
contained 20, 13, 15 and 15 mice, respectively;
*P<0.05, vs. control group; ▲P<0.05,
vs. LPS group. BMSCs, bone marrow-derived mesenchymal stem cells;
ADMSCs, adipose tissue-derived mesenchymal stem cells; LPS,
lipopolysaccharide; ALT, alanine aminotransferase; AST, aspartate
aminotransferase. |
Effect of MSCs on serum cytokine
concentrations
To examine the serum levels of pro- and
anti-inflammatory cytokines, ELISA was performed 6 h post-LPS
exposure. The serum concentrations of all cytokines in the LPS
group were significantly increased, compared with those in the
control group (P<0.05). The serum levels of TNF-α, IL-2, IL-6
and IL-10 were significantly lower, whereas the level of IL-8 was
significantly higher in the BMSC group, compared with the LPS group
(P<0.05). The serum levels of TNF-α, IL-2, IL-4, IL-6, IL-8 and
IL-10 were significantly lower in the ADMSC group, compared with
the LPS group (P<0.05). Of note, the levels of TNF-α, IL-4 and
IL-8 were significantly higher in the BMSC group, compared with the
ADMSC group (P<0.05). In terms of the three remaining cytokines
(IL-2, IL-6 and IL-10), although their levels were higher in the
BMSC group compared with the ADMSC group, this result was not
significant (P>0.05; Fig.
6).
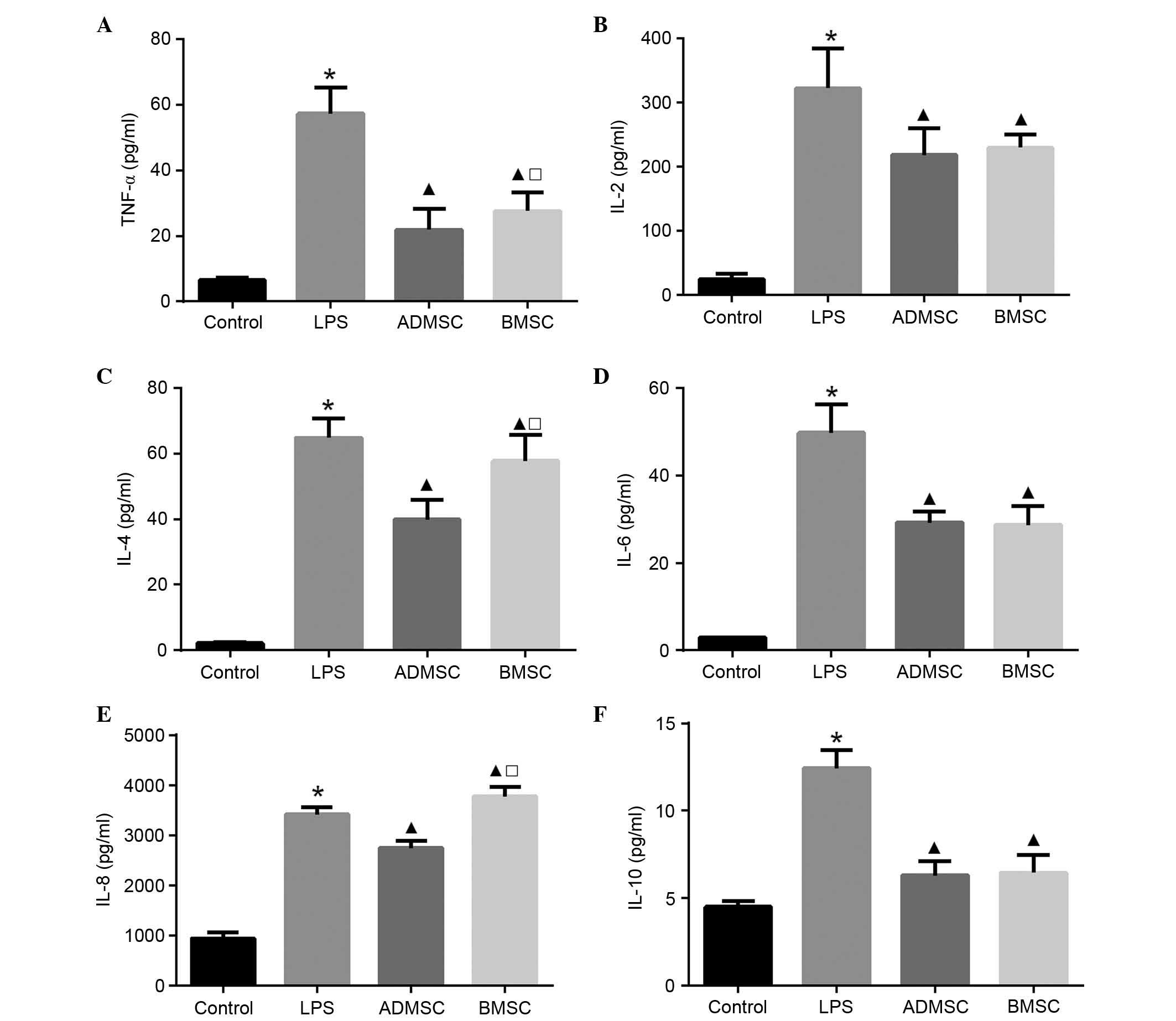 | Figure 6Serum cytokine concentrations in mice
with LPS-induced sepsis following MSC treatment. Serum levels of
(A) TNF-α, (B) IL-2, (C) IL-4, (D) IL-6, (E) IL-8 and (F) IL-10 6 h
following LPS exposure. The control, LPS, BMSC and ADMSC groups
contained 20, 12, 15 and 16 mice, respectively;
*P<0.05, vs. control group; ▲P<0.05,
vs. LPS group; □P<0.05, vs. ADMSC group. BMSCs, bone
marrow-derived mesenchymal stem cells; ADMSCs, adipose
tissue-derived mesenchymal stem cells; LPS, lipopolysaccharide;
TNF, tumor necrosis factor-α; IL, interleukin. |
Discussion
The present study focused on the therapeutic
potential and differences between two types of MSCs, BMSCs and
ADMSCs, in a murine model of LPS-induced sepsis. The data
demonstrated that the intravenous injection of BMSCs or ADMSCs
improved animal survival rates, and alleviated temperature
fluctuations and moderate multiple organ damage accompanied with a
reduction in the production of the majority of pro- and
anti-inflammatory cytokines. In addition, compared with the BMSCs,
the ADMSCs reduced, rather than increased the serum levels of IL-8,
and it appeared more potent at alleviating hypothermia in
LPS-induced sepsis.
The morality rate observed in the LPS group was
similar to that reported in a previous study (33). MSC treatment significantly improved
the survival rates of the mice from 30 to ≥55% at 24 h post-LPS
exposure. Additionally, the mice in the LPS group developed severe
hypothermia between 4 and 12 h post-LPS exposure, during which time
the majority of the mice succumbed to mortality. Hypothermia is
reported to be associated with increased mortality rates, septic
shock and organ failure in patients with severe sepsis (34,35),
and is therefore considered a marker of severe illness and poor
prognosis. In a cecal ligation and puncture model of sepsis,
Granger et al (36) also
found that body temperature was significantly reduced in the first
few hours prior to increasing. Consistent with this previous study,
the data obtained in the present study showed the same trend. By 8
h, the majority of the mice in the LPS group had developed severe
hypothermia. However, the mice treated with MSCs exhibited a less
marked decrease in their body temperature and the nadir was delayed
to 12 h, suggesting that MSCs may assist in alleviating sepsis.
A substantial increase in serum concentrations of
lactate, ALT, AST and creatine was observed in the mice with
LPS-induced sepsis, indicating that multiple organ injuries or
dysfunction had developed. This was also confirmed in previous
studies (16,37). The data obtained in the present
study suggested that there was a reduction in organ damage when
treated with MSCs, despite the MSC sources differing.
Previous evidence suggests that an independent
response pattern, a mixed antagonist response, exists at the onset
of sepsis, which encompasses signs of hyperinflammation and
immunosuppression (38,39). Consistent with a previous study
(40), the results of the present
study demonstrated a concomitant increase in the serum levels of
TNF-α, IL-6 and IL-10, typical cytokines in inflammatory and
anti-inflammatory responses, in the murine model of sepsis,
suggestive of the existence of a mixed antagonist response. A
tightly regulated balance in the cytokine network is crucial for
eliminating invading pathogens and limiting the excessive tissue
damage caused by inflammation (41). Of note, the results of the present
study showed that MSC treatment reduced not only the serum levels
of pro-inflammatory cytokines, including TNF-α and IL-6, but also
anti-inflammatory cytokines, including IL-2 and IL-10. These
results suggested that MSCs regulated pro- and anti-inflammatory
responses. Therefore, MSCs may regulate the disturbance of
homeostasis by affecting the cytokine network, which may further
alleviate the injury to organ function caused by an excessive
inflammatory response and the proliferation of infection resulting
from the overwhelming anti-inflammatory response. This may
contribute to the beneficial role of MSCs on sepsis in the model in
the present study.
Compared with the LPS group, the serum levels of
IL-8 were significantly increased in the BMSC group, however, they
were significantly decreased in the ADMSC group. IL-8 appears to be
important in the chemotaxis and activation of immune cells
(42), and is reported to be
closely associated with neutrophilic infiltration, which is
fundamental in the pathogenesis and progression of sepsis (43–45).
The serum level of IL-8 was increased in the mice treated with
BMSCs, suggesting that BMSCs may enhance the migration of
neutrophils to the site of inflammation, thus promoting the
clearance of microbial pathogens. However, this excessive increase
in neutrophil recruitment may cause severe oxidative stress injury
(46). ADMSC treatment appeared to
alleviate this effect by lowering the serum level of IL-8. Again,
further investigation is required to confirm the role of MSCs on
the levels of IL-8 and neutrophils.
Several limitations of the present study must be
considered when interpreting the findings. First, the LPS-induced
sepsis animal model, in which septic mice showed transient injury,
cannot not completely reflect septic conditions in humans. Second,
the timing of exogenous stem cell infusion following injury may be
important, however, the optimal time point has not yet been
established. Consequently, future investigations are required to
examine the effects of administering BMSCs and ADMSCs at different
time points following the induction of sepsis. Thirdly, injection
with different doses of LPS led to different levels of severity in
the sepsis mode used, therefore, the effect and mechanism of MSCs
may differ with respect to the severity of sepsis. Further
investigations are required to examine effects of administering
MSCs at different time points on the survival rates, organ function
and systemic inflammatory response in models of sepsis with varying
severities. Furthermore, the levels of the cytokines, ALT, AST and
creatinine were only measured 6 h post-LPS administration. Dynamic
observation of these factors at different time points may be
required to fully understand the mechanism. Further investigations
are required to determine the changes of these factors during the
development of sepsis.
In conclusion, the present study suggested that
BMSCs and ADMSCs have similar roles in the regulation of
inflammation, reducing organ injury and mortality rates in mice
with sepsis. One difference identified between the BMSCs and ADMSCs
was that the serum levels of IL-8 were increased and decreased,
respectively. The present comparitive study also indicated that
ADMSCs were more potent than BMSCs in alleviating LPS-induced
sepsis. Therefore, BMSCs and ADMSCs may serve as novel treatment
modalities for sepsis.
Acknowledgments
This study was supported by the Science and
Technology Project of Hunan Province, China (grant nos. 2012FJ4313
and 2013WK2009) and the Natural Science Foundation of Hunan
Province, China (grant no. 13JJ6019).
References
|
1
|
ARISE; ANZICS APD Management Committee:
The outcome of patients with sepsis and septic shock presenting to
emergency departments in Australia and New Zealand. Crit Care
Resusc. 9:8–18. 2007.PubMed/NCBI
|
|
2
|
Kumar G, Kumar N, Taneja A, Kaleekal T,
Tarima S, McGinley E, Jimenez E, Mohan A, Khan RA, Whittle J, et
al: Nationwide trends of severe sepsis in the 21st century
(2000–2007). Chest. 140:1223–1231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hartman ME, Linde-Zwirble WT, Angus DC and
Watson RS: Trends in the epidemiology of pediatric severe
sepsis*. Pediatr Crit Care Med. 14:686–693. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lagu T, Rothberg MB, Shieh MS, Pekow PS,
Steingrub JS and Lindenauer PK: Hospitalizations, costs, and
outcomes of severe sepsis in the United States 2003 to 2007. Crit
Care Med. 40:754–761. 2012. View Article : Google Scholar
|
|
5
|
Sakr Y, Elia C, Mascia L, Barberis B,
Cardellino S, Livigni S, Fiore G, Filippini C and Ranieri VM:
Epidemiology and outcome of sepsis syndromes in Italian ICUs: A
muticentre, observational cohort study in the region of Piedmont.
Minerva Anestesiol. 79:993–1002. 2013.PubMed/NCBI
|
|
6
|
Caironi P, Tognoni G, Masson S, Fumagalli
R, Pesenti A, Romero M, Fanizza C, Caspani L, Faenza S, Grasselli
G, et al: Albumin replacement in patients with severe sepsis or
septic shock. N Engl J Med. 370:1412–1421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
ProCESS Investigators; Yealy DM, Kellum
JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang
HE, Hou PC, et al: A randomized trial of protocol-based care for
early septic shock. N Engl J Med. 370:1683–1693. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mitka M: Drug for severe sepsis is
withdrawn from market, fails to reduce mortality. JAMA.
306:2439–2440. 2011.PubMed/NCBI
|
|
9
|
Schulte W, Bernhagen J and Bucala R:
Cytokines in sepsis: Potent immunoregulators and potential
therapeutic targets-an updated view. Mediators Inflamm.
2013:1659742013. View Article : Google Scholar
|
|
10
|
Shahkar L, Keshtkar A, Mirfazeli A, Ahani
A and Roshandel G: The role of IL-6 for predicting neonatal sepsis:
A systematic review and meta-analysis. Iran J Pediatr. 21:411–417.
2011.
|
|
11
|
Schrag B, Roux-Lombard P, Schneiter D,
Vaucher P, Mangin P and Palmiere C: Evaluation of C-reactive
protein, procalcitonin, tumor necrosis factor alpha, interleukin-6,
and interleukin-8 as diagnostic parameters in sepsis-related
fatalities. Int J Legal Med. 126:505–512. 2012. View Article : Google Scholar
|
|
12
|
Song R, Kim J, Yu D, Park C and Park J:
Kinetics of IL-6 and TNF-α changes in a canine model of sepsis
induced by endotoxin. Vet Immunol Immunopathol. 146:143–149. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bozza FA, Salluh JI, Japiassu AM, Soares
M, Assis EF, Gomes RN, Bozza MT, Castro-Faria-Neto HC and Bozza PT:
Cytokine profiles as markers of disease severity in sepsis: A
multiplex analysis. Crit Care. 11:R492007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chuang TY, Chang HT, Chung KP, Cheng HS,
Liu CY, Liu YC, Huang HH, Chou TC, Chang BL, Lee MR, et al: High
levels of serum macrophage migration inhibitory factor and
interleukin 10 are associated with a rapidly fatal outcome in
patients with severe sepsis. Int J Infect Dis. 20:13–17. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mei SH, Haitsma JJ, Dos Santos CC, Deng Y,
Lai PF, Slutsky AS, Liles WC and Stewart DJ: Mesenchymal stem cells
reduce inflammation while enhancing bacterial clearance and
improving survival in sepsis. Am J Respir Crit Care Med.
182:1047–1057. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nemeth K, Leelahavanichkul A, Yuen PS,
Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller
BH, Brown JM, et al: Bone marrow stromal cells attenuate sepsis via
prostaglandin E(2)-dependent reprogramming of host macrophages to
increase their interleukin-10 production. Nat Med. 15:42–49. 2009.
View Article : Google Scholar
|
|
17
|
Gupta N, Su X, Popov B, Lee JW, Serikov V
and Matthay MA: Intrapulmonary delivery of bone marrow-derived
mesenchymal stem cells improves survival and attenuates
endotoxin-induced acute lung injury in mice. J Immunol.
179:1855–1863. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu J, Qu J, Cao L, Sai Y, Chen C, He L and
Yu L: Mesenchymal stem cell-based angiopoietin-1 gene therapy for
acute lung injury induced by lipopolysaccharide in mice. J Pathol.
214:472–481. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Krasnodembskaya A, Samarani G, Song Y,
Zhuo H, Su X, Lee JW, Gupta N, Petrini M and Matthay MA: Human
mesenchymal stem cells reduce mortality and bacteremia in
gram-negative sepsis in mice in part by enhancing the phagocytic
activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol.
302:L1003–L1013. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sepúlveda JC, Tomé M, Fernández ME,
Delgado M, Campisi J, Bernad A and González MA: Cell senescence
abrogates the therapeutic potential of human mesenchymal stem cells
in the lethal endotoxemia model. Stem Cells. 32:1865–1877. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
in't Anker PS, Noort WA, Scherjon SA,
Kleijburg-van der Keur C, Kruisselbrink AB, van Bezooijen RL,
Beekhuizen W, Willemze R, Kanhai HH and Fibbe WE: Mesenchymal stem
cells in human second-trimester bone marrow, liver, lung, and
spleen exhibit a similar immunophenotype but a heterogeneous
multilineage differentiation potential. Haematologica. 88:845–852.
2003.
|
|
22
|
Dmitrieva RI, Minullina IR, Bilibina AA,
Tarasova OV, Anisimov SV and Zaritskey AY: Bone marrow- and
subcutaneous adipose tissue-derived mesenchymal stem cells:
differences and similarities. Cell Cycle. 11:377–383. 2012.
View Article : Google Scholar
|
|
23
|
Zappia E, Casazza S, Pedemonte E,
Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti
F, Frassoni F, et al: Mesenchymal stem cells ameliorate
experimental autoimmune encephalomyelitis inducing T-cell anergy.
Blood. 106:1755–1761. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: Implications for cell-based
therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gonzalez-Rey E, Anderson P, Gonzalez MA,
Rico L, Büscher D and Delgado M: Human adult stem cells derived
from adipose tissue protect against experimental colitis and
sepsis. Gut. 58:929–939. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Puissant B, Barreau C, Bourin P, Clavel C,
Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, et
al: Immunomodulatory effect of human adipose tissue-derived adult
stem cells: Comparison with bone marrow mesenchymal stem cells. Br
J Haematol. 129:118–129. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shin S, Kim Y, Jeong S, Hong S, Kim I, Lee
W and Choi S: The therapeutic effect of human adult stem cells
derived from adipose tissue in endotoxemic rat model. Int J Med
Sci. 10:8–18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fu X, Chen Y, Xie FN, Dong P, Liu WB, Cao
Y, Zhang WJ and Xiao R: Comparison of immunological characteristics
of mesenchymal stem cells derived from human embryonic stem cells
and bone marrow. Tissue Eng Part A. 21:616–626. 2015. View Article : Google Scholar :
|
|
29
|
Elman JS, Li M, Wang F, Gimble JM and
Parekkadan B: A comparison of adipose and bone marrow-derived
mesenchymal stromal cell secreted factors in the treatment of
systemic inflammation. J Inflamm (Lond). 11:12014. View Article : Google Scholar
|
|
30
|
Zhao J, Wang G, Zhang H and Fu X:
Isolation, cultivation, purification and identification of mice
bone marrow mesenchyaml stem cells in vitro. Chin J Lab Diagn.
16:11–13. 2012.In Chinese.
|
|
31
|
Soleimani M and Nadri S: A protocol for
isolation and culture of mesenchymal stem cells from mouse bone
marrow. Nat Protoc. 4:102–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu S, Lei J, Li Y, Xiang P and Zhou D:
Isolation, identification and biological characteristics of mouse
adipose-derived stromal cells. J Sun Yat-Sen University (Medical
Sciences). 33:299–304. 2012.In Chinese.
|
|
33
|
Kim SR, Ha YM, Kim YM, Park EJ, Kim JW,
Park SW, Kim HJ, Chung HT and Chang KC: Ascorbic acid reduces HMGB1
secretion in lipopolysaccharide-activated RAW 264.7 cells and
improves survival rate in septic mice by activation of Nrf2/HO-1
signals. Biochem Pharmacol. 95:279–289. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kushimoto S, Gando S, Saitoh D, Mayumi T,
Ogura H, Fujishima S, Araki T, Ikeda H, Kotani J, Miki Y, et al:
The impact of body temperature abnormalities on the disease
severity and outcome in patients with severe sepsis: An analysis
from a multicenter, prospective survey of severe sepsis. Crit Care.
17:R2712013. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tiruvoipati R, Ong K, Gangopadhyay H,
Arora S, Carney I and Botha J: Hypothermia predicts mortality in
critically ill elderly patients with sepsis. BMC Geriatr.
10:702010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Granger JI, Ratti PL, Datta SC, Raymond RM
and Opp MR: Sepsis-induced morbidity in mice: Effects on body
temperature, body weight, cage activity, social behavior and
cytokines in brain. Psychoneuroendocrinology. 38:1047–1057. 2013.
View Article : Google Scholar :
|
|
37
|
Shi DW, Zhang J, Jiang HN, Tong CY, Gu GR,
Ji Y, Summah H and Qu JM: LPS pretreatment ameliorates multiple
organ injuries and improves survival in a murine model of
polymicrobial sepsis. Inflamm Res. 60:841–849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Novotny AR, Reim D, Assfalg V, Altmayr F,
Friess HM, Emmanuel K and Holzmann B: Mixed antagonist response and
sepsis severity-dependent dysbalance of pro- and anti-inflammatory
responses at the onset of postoperative sepsis. Immunobiology.
217:616–621. 2012. View Article : Google Scholar
|
|
39
|
Osuchowski MF, Welch K, Siddiqui J and
Remick DG: Circulating cytokine/inhibitor profiles reshape the
understanding of the SIRS/CARS continuum in sepsis and predict
mortality. J Immunol. 177:1967–1974. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mei SH, McCarter SD, Deng Y, Parker CH,
Liles WC and Stewart DJ: Prevention of LPS-induced acute lung
injury in mice by mesenchymal stem cells overexpressing
angiopoietin 1. PLoS Med. 4:e2692007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hack CE, Aarden LA and Thijs LG: Role of
cytokines in sepsis. Adv Immunol. 66:101–195. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hechtman DH, Cybulsky MI, Fuchs HJ, Baker
JB and Gimbrone MA Jr: Intravascular IL-8. Inhibitor of
polymorpho-nuclear leukocyte accumulation at sites of acute
inflammation. J Immunol. 147:883–892. 1991.PubMed/NCBI
|
|
43
|
Collins PD, Jose PJ and Williams TJ: The
sequential generation of neutrophil chemoattractant proteins in
acute inflammation in the rabbit in vivo. Relationship between C5a
and proteins with the characteristics of IL-8/neutrophil-activating
protein 1. J Immunol. 146:677–684. 1991.PubMed/NCBI
|
|
44
|
Chen HC, Lin HC, Liu CY, Wang CH, Hwang T,
Huang TT, Lin CH and Kuo HP: Neutrophil elastase induces IL-8
synthesis by lung epithelial cells via the mitogen-activated
protein kinase pathway. J Biomed Sci. 11:49–58. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Harada A, Sekido N, Akahoshi T, Wada T,
Mukaida N and Matsushima K: Essential involvement of interleukin-8
(IL-8) in acute inflammation. J Leukoc Biol. 56:559–564.
1994.PubMed/NCBI
|
|
46
|
Ramaiah SK and Jaeschke H: Role of
neutrophils in the pathogenesis of acute inflammatory liver injury.
Toxicol Pathol. 35:757–766. 2007. View Article : Google Scholar : PubMed/NCBI
|