Introduction
Postoperative cognitive dysfunction (POCD) is a
common complication involving transient or permanent impairment of
cognition in elderly patients following surgery (1). POCD affects a wide variety of
cognitive domains, including attention, memory, executive function
and speed of information processing, resulting in a reduction in
the patient's quality of life and contributing substantially to
healthcare costs. The International Multicenter Study on POCD
reported that, following noncardiac surgery, cognitive impairments
occur in 25.8% of patients 1 week postoperatively and in 9.9% of
patients 3 months postoperatively in patients >60 years of age
(1). Following cardiac surgery,
the short-term (2 weeks postoperatively) rate ranges between 26 and
80%, whereas the long-term rate (5 years following cardiac surgery)
is up to 37% (2). With a steady
increase in the geriatric surgical population, postoperative
cognitive decline is rapidly becoming a major global health burden
(3). Certain risk factors have
been identified to contribute to postoperative cognitive
dysfunction, including increasing age, anesthetics, surgical
intervention and postoperative pain (4). Increasing age and the extent of
surgical trauma are the only univocal risk factors (1,5),
however, the molecular mechanisms underlying POCD remain to be
fully elucidated. There is evidence to suggest that POCD and
neurodegenerative diseases, including Alzheimer's disease (AD), may
share certain neuropathological and biochemical mechanisms.
Mammalian target of rapamycin (mTOR) is a conserved
serine/threonine protein kinase and is a member of the
phosphoinositide-3-kinase-related family. It is involved in cell
growth, proliferation, metabolism and protein synthesis, which
integrates a variety of signals under physiological conditions
(6). In the nervous system, mTOR
is critical in maintaining brain function. A series of studies have
found that mTOR promotes learning and memory formation via the
protein synthesis-dependent strengthening of synapses (7). For example, mice deficient in mTOR
have impaired learning, memory and social behavior (8), and the dysregulation of mTOR has been
shown to cause learning deficits (9). Several studies have demonstrated that
mTOR signaling may be linked to several neurodegenerative diseases
(10,11). Neurodegenerative diseases,
including AD, are characterized by the aberrant accumulation of
misfolded proteins, leading to memory and cognitive impairment.
mTOR signaling has been shown to be involved in the synthesis of
β-amyloid and tau protein. The upregulation of mTOR and p70S6 K has
been found to be associated with the accumulation of
hyperphosphorylated tau in AD (12), and the inhibition of mTOR by
rapamycin has been shown to improve learning and memory abilities,
and reduce levels of β-amyloid by inducing the autophagic removal
of proteins (13,14). POCD is a prolonged change in
cognition, with similar clinical manifestations and
physiopathologic mechanisms to other neurodegenerative disorders.
Therefore, the present study hypothesized that mTOR signaling may
also be involved in the development of POCD.
In the present study, an animal model was used to
examine whether surgical trauma leads to the activation of mTOR
signaling within the hippocampal area, and whether inhibition of
the mTOR signaling pathway within the hippocampal area ameliorates
the cognitive impairment. The present study also aimed to determine
whether the activation of mTOR signaling causes the accumulation of
Aβ1-42 and hyperphosphorylated tau protein, which can
lead to memory and cognitive impairment.
Materials and methods
Animals
Male C57BL/6J mice (n=104), aged 12–14 weeks,
weighing 20–25 g (Vital River Laboratory Animal Technology Co.,
Ltd., Beijing, China), were used for all experiments. The animals
were housed under controlled conditions (21±2°C; 50±10% humidity,
12:12 h light:dark cycle) with access to food and water ad
libitum. All mice were allowed to adapt to the environment for
7 days prior to beginning the experiments. All experimental
protocols were approved by the animal ethics committee of Capital
Medical University (Beijing, China), and were in accordance with
the guidelines for animal experiments of the local Animal Care and
Use Committee.
Experiment 1 protocol
Based on preliminary time course experiments
(15–17), to investigate the effects of
orthopedic surgery on the activation of mTOR, β-amyloid
accumulation and tau phosphorylation, the present study examined
the changes of relevant proteins. The animals were divided into
three groups (n=8/group). Normal, untreated animals were used as a
control group. In the surgery group, the mice underwent orthopedic
surgery of left hindpaw under isoflurane anesthesia and analgesia.
In the sham surgery group, mice received the same anesthesia and
analgesia as the surgery group. The mice in each group received
contextual fear conditioning (CFC) to estimate the learning and
memory abilities of the mice, following which the animals were
sacrificed by cervical dislocation, and hippocampal tissue samples
were obtained for further experiments. Animals were anesthetized
prior to cervical dislocation through a single intraperitoneal
injection of chloral hydrate (10%; 0.3 ml/100 g).
Experiment 2 protocol
The experiments described above showed the
activation of mTOR signaling with β-amyloid accumulation and tau
phosphorylation in response to surgical stimulation. Therefore,
subsequent experiments were performed to examine the effects of
pretreatment with rapamycin, an inhibitor of mTOR, on postoperative
cognitive function, and to determine the levels of
Aβ1-42 and tau phosphorylation. This was performed to
confirm whether the hyperactivation of mTOR signaling was involved
in cognitive defects following surgery via the upstream regulation
of Aβ1-42 and the phosphorylation of tau. To investigate
the effects, the mice were divided into four groups (n=8/group):
Ctrl group (mice received injections of vehicle without surgery),
Ctrl+rapa group (mice received rapamycin pretreatment without
surgery), Sur group (mice received orthopedic surgery with
injections of vehicle) and Sur+rapa group (mice received orthopedic
surgery with rapamycin pretreatment). The animals received CFC 1
day following surgery, and the mice were then sacrificed by
cervical dislocation for western blot and enzyme-linked
immunosorbent assays.
Anesthesia, orthopedic surgery and
pharmacological treatment
Anesthesia was prepared using a procedure described
by Degos et al (18). In
brief, the animals were placed in a sealed plastic box and
anesthesia was induced with 5% isoflurane mixed with air. The
anesthesia was maintained with 1.2–1.5% isoflurane, which was
delivered through a nose cone to the mouse for 15 min. The gas
concentrations and respiratory rate were continuously monitored
using a multi-function monitor (Datex-Ohmeda, Helsinki, Finland).
In the surgery group, an open tibial fracture of the left hind paw
with intramedullary fixation was performed in aseptic conditions
under general anesthesia with isoflurane (19,20).
Buprenorphine was used to provide supplemental analgesia (0.1 mg/kg
administered subcutaneously). The surgical aspect of the left
hindpaw was sterilized with povidone-iodine, and a median incision
on the surgical region was made. Following incision, a 0.38 mm pin
was inserted in the intramedullary canal, the periosteum was
stripped and the wound was irrigated. Finally, the skin of the
wound was closed with 5–0 nylon sutures and covered with antibiotic
ointment. Following surgery, the mice were moved back to their
original cage for recovery with a sufficient supply of food and
water. Throughout the entire anesthesia and surgical procedures,
all vital signs were monitored, and blood gas analysis was
performed following anesthesia and surgery. For inhibition of the
mTOR signal, rapamycin was used to reduce the activity of mTORC1.
The dose of rapamycin used was selected based on previous studies
(21,22). The mice received intraperitoneal
injections of 0.5 mg/kg/day rapamycin for 3 days prior to
undergoing orthopedic surgery, with the final dose administered 2 h
prior to surgery. In the negative control group, animals received
an intraperitoneal injection of 0.5 mg/kg/day rapamycin for 3 days
without surgery.
CFC
The animals were transported to the laboratory at
least 2 h prior to CFC training. For CFC, a clear plexiglas chamber
was placed in a soundproof box and a camera (Meidi Electronic Co.,
Ltd., Shenzhen, China) was fixed to the top of the box to capture
videos of each animal during CFC training using ANY-maze software
(Stoelting Co., Wood Dale, IL, USA). Foot shocks were delivered
through a grid floor, which comprised 28 stainless steel bars.
Prior to and following each session, the chambers were cleaned
using pine solution.
Training was performed 24 h prior to surgery. The
animals were allowed free movement in the chambers for 5 min prior
to training, following which they received three tone (2,000 Hz; 90
dB)-electric shock (0.85 mA for 2 sec) pairings, which were
separated by 60 sec. Following fear establishment, the fear
response of the animals was measured based on freezing times. The
percentage of freezing time was used to reflect the
hippocampal-dependent memory. Following the different treatments,
the mice were returned to the training environment to assess the
contextual fear memory. During CFC assessment test, each mouse was
placed once again into the chamber for three 3 min without tone or
shock. Freezing time was measured by two observers blinded to the
group assignments.
Tissue preparation
For western blot analysis and the enzyme-linked
immunosorbent assay, the mice were sacrificed under deep
anesthesia. The hippocampus was removed and stored at −80°C.
Western blot analysis
The hippocampal tissues were homogenized in RIPA
buffer containing protease and phosphatase inhibitors, and then
centrifuged at 4°C at 12,000 g for 25 min. The concentration of
protein in the supernatants was determined using a Bradford protein
assay kit (Beyotime Institute of Biotechnology Co., Ltd., Shanghai,
China). Equal quantities of the protein samples (30 μg per
sample) were denatured at 100°C for 5 min, and were then separated
by 8–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
and transferred electrophoretically onto a polyvinylidene fluoride
membrane (EMD Millipore, Billerica, MA, USA). The membranes were
blocked using 5% skim milk-Tris-buffered saline (TBS) buffer for 60
min and then incubated with the following primary antibodies:
Rabbit polyclonal anti-mTOR (1:1,000; cat. no. 2972; Cell Signaling
Technology, Inc., Boston, MA, USA), rabbit polyclonal
anti-phosphorylated (phospho)-mTOR (Ser2448; 1:1,000; cat. no.
2971; Cell Signaling Technology, Inc.), rabbit polyclonal
anti-P70S6K (1:1,000; cat. no. 9202; Cell Signaling Technology,
Inc.), rabbit polyclonal anti-phospho-P70S6K (Thr389; 1:1,000; cat.
no. 9205; Cell Signaling Technology, Inc.), rabbit poly-clonal
anti-Tau (1:500; cat. no. YT4554; Immunoway, Newark, DE, USA) and
rabbit polyclonal anti-phospho-Tau (Ser396; 1:500; cat. no. YP0263;
Immunoway) overnight at 4°C. Following three washes (10 min each)
in TBS with Tween solution, the membranes were incubated with
horseradish peroxidase conjugated goat anti-rabbit IgG (1:2,000;
Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing,
China) secondary antibodies at room temperature for 1 h. The bands
were treated with an enhanced chemiluminescence detection kit (EMD
Millipore), and the intensity of each band was quantified by
densitometric analysis. The relative expression levels of protein
were normalized by GAPDH (1:1,000; cat. no. 5174; Cell Signaling
Technology, Inc.).
Enzyme-linked immunosorbent assay
The hippocampal tissue samples were weighed and
sonicated in phosphate-buffered saline with 50 mM protease
inhibitor cocktail, followed by centrifugation at 20,000 g 4°C for
10 min. The supernatant was collected to quantify the protein
concentration in the samples using a BCA protein assay kit (cat.
no. 23225; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Equal
quantities of protein sample (50 μg) were used for the
measurement of Aβ1-42 (Wuxi Donglin Sci & Tech
Development Co., Ltd., Jiangsu, China) using an enzyme-linked
immunosorbent assay, according to the manufacturer's protocol. The
intensity of the color was measured at a wavelength of 450 nm using
an iMark microplate reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Statistical analysis
All data were analyzed using SPSS statistical
software, version 18.0 (IBM SPSS, Armonk, NY, USA). The data were
expressed as the mean ± standard deviation, and statistical
analysis was performed using one-way analysis of variance followed
by Newman-Keuls post-hoc test where appropriate. P<0.05 was
considered to indicate a statistically significant difference.
Results
Orthopedic surgery impairs learning and
memory function
To elucidate the effect of surgery on cognitive
function, CFC assessment was performed to assess learning and
memory function. Compared with the control group and sham group,
animals in the surgery group presented with significantly reduced
freezing time percentages on day 1 (42.4±9.8, vs. 65.9±13.8%,
respectively; P<0.05) and day 3 (48.95±9.97, vs. 73.88±13.9%,
respectively; P<0.05). No significant difference in freezing
time was found between the sham group and control group (Fig. 1).
 | Figure 1Effects of orthopedic surgery on
hippocampal-dependent memory, measured as the percentage of
freezing time. Surgery decreased hippocampal-dependent memory on
day 1 and day 3 (*P<0.05, compared with Ctrl;
#P<0.05, compared with Sham). In the Sham group, no significant
difference was observed in the freezing time following isoflurane
anesthesia, compared with the Ctrl (P>0.05). Values are
presented as the mean ± standard deviation (n=8 for each group).
Ctrl, control group; Sham, sham surgery group; Sur, surgery group.
D1, day 1; D3, day 3; D7, day 7. |
Orthopedic surgery induces the
upregulation of mTOR/p70S6K signaling in the hippocampus
To determine the effects of surgical trauma on
mTOR/p70S6K signaling, the present study detected the levels of
mTOR and p70S6K in the homogenates of the hippocampal tissue using
western blot analysis (Fig. 2).
Although there were no significant differences in the levels of
total mTOR or p70S6K (Fig. 2D and
E), the levels of phospho-mTOR at Ser2448 and phospho-p70S6K at
Thr396 were significantly increased on day 1 and day 3 in the
hippocampal tissues of the surgery group (P<0.05; n=8), as shown
in Fig. 2B and C. No significant
differences were found in the levels of phospho-mTOR at Ser2448 or
phospho-p70S6K at Thr396 between the sham surgery group and the
control group (Fig. 2B and C).
These data indicated that surgery elevated the activation of mTOR
signaling on day 1 and day 3. However, the effect of surgery on
mTOR signaling was reversed on day 7. Surgical trauma induced the
upregulation of mTOR/p70S6K signaling, which suggested that the
activation of mTOR signaling may be involved in the development of
postoperative dysfunction.
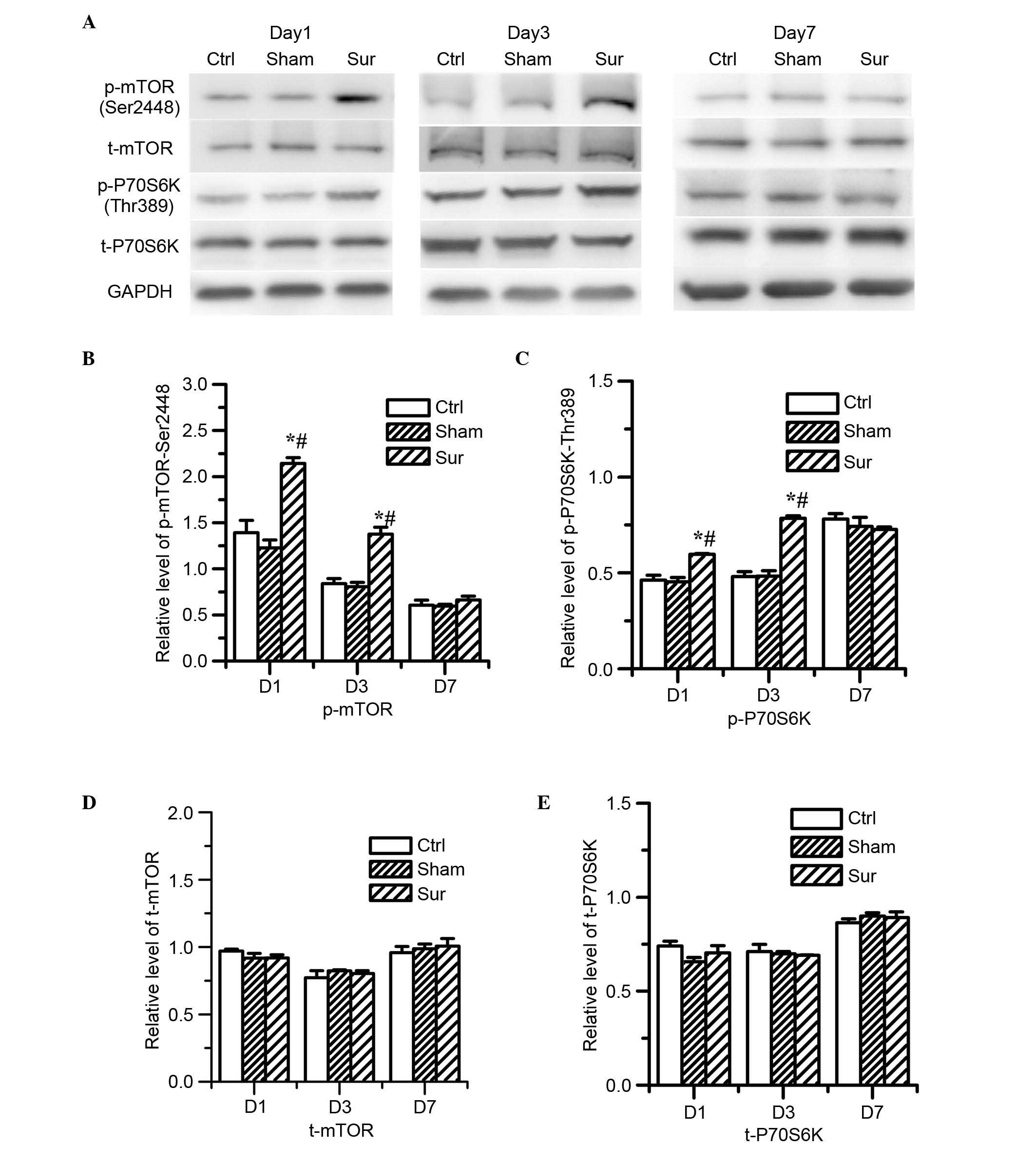 | Figure 2Effects of orthopedic surgery on the
activation of mTOR signaling in the hippocampus. The expression
levels of mTOR and p70S6K were determined by western blot analysis
on days 1, 3 and 7 post-surgery. (A) Typical immunoblot results
showed the changes in the levels of p-mTOR (Ser2448), total mTOR,
p-p70S6 K (Thr396) and total p70S6K at different points.
Quantitative analysis indicated that surgery significantly
increased the levels of (B) p-mTOR (Ser2448) and (C) p-p70S6K
(Thr396) on days 1 and 3 post-surgery (*P<0.05, vs.
Ctrl; #P<0.05 vs. Sham), however, the protein
expression levels of (D) t-mTOR and (E) t-p70S6K, were not
affected, and this difference was reversed on day 7 post-surgery.
In the Sham group, no significant difference in the levels of
p-mTOR (Ser2448) or p-p70S6K (Thr396) were observed following
isoflurane anesthesia, compared with the Ctrl (P>0.05, vs.
Ctrl). Values are presented as the mean ± standard deviation (n=8
for each group). mTOR, mammalian target of rapamycin; p-,
phosphoarylated; Ctrl, control group; Sham, sham surgery group;
Sur, surgery group; D1, day 1; D3, day 3; D7, day 7. |
Orthopedic surgery induces β-amyloid
accumulation and tau phosphorylation in the hippocampus
To investigate the effect of orthopedic surgery on
the production of β-amyloid, the present study measured the level
of Aβ1-42. Surgery increased the production of
Aβ1-42 at 24 h (2.88±0.34, vs. 1.93±0.19 pg/ml) and 3
days post-surgery (3.0±0.32, vs. 1.86±0.20 pg/ml; P<0.05; n=8;
Fig. 3). No significant
differences were observed between the sham surgery group and
control group (P>0.05; n=8).
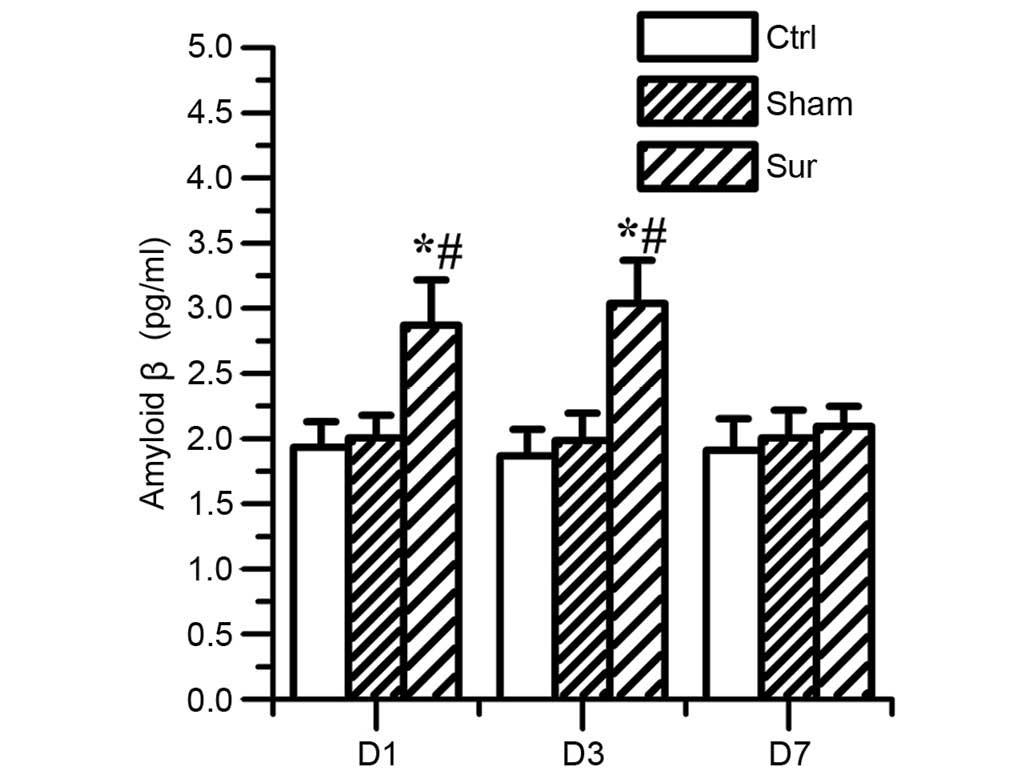 | Figure 3Effects of orthopedic surgery on the
levels of Aβ1-42 in the hippocampus. The expression of
Aβ1-42 was determined using an enzyme-immunosorbent
assay on days 1, 3 and 7 post-surgery. The level of
Aβ1-42 increased significantly on days 1 and 3
post-surgery (*P<0.05, vs. #P<0.05, vs.
Sham). No significant difference was found in the level of
Aβ1-42 in the Sham group, compared with the Ctrl group
(P>0.05, vs. Ctrl). Values are presented as the mean ± standard
deviation (n=8 for each group). Aβ1-42 amyloid β; Ctrl,
control group; Sham, sham surgery group; Sur, surgery group; D1,
day 1; D3, day 3; D7, day 7. |
Tau protein hyperphosphorylation is associated with
the upregulation of mTOR/p70S6K signaling in the development of AD.
The present study also investigated whether tau phosphorylation
occurred following surgery accompanying the increased mTOR
activity. The results showed that orthopedic surgery upregulated
the levels of phosphorylated protein at Ser396 on day 1 and day 3
post-surgery, compared with the sham surgery and control group
(P<0.05; n=8; Fig. 4B).
However, surgery did not alter the expression of total tau protein
(Fig. 4C).
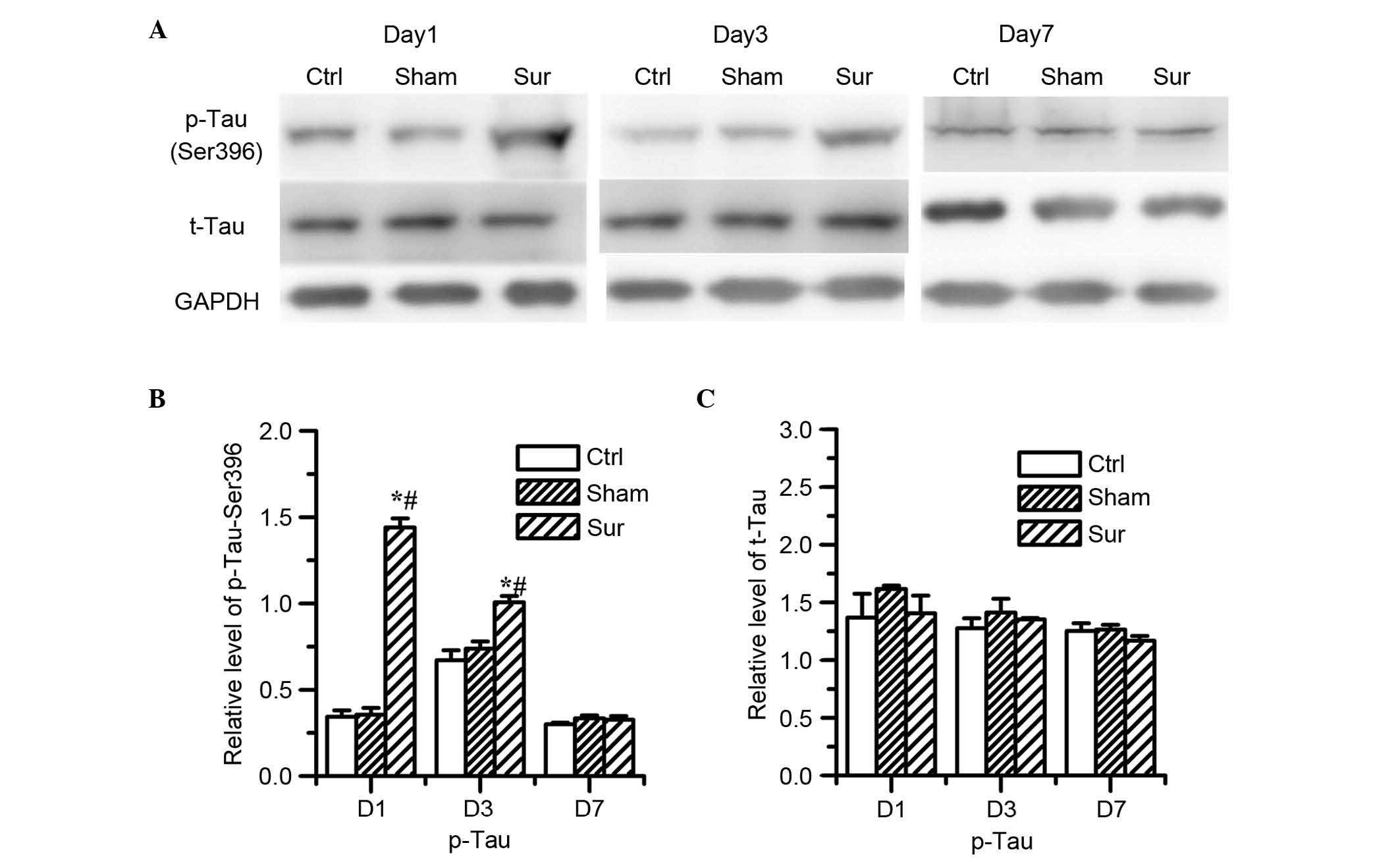 | Figure 4Effects of orthopedic surgery on the
protein levels of tau in the hippocampus. The expression levels of
p-tau and tau was determined using western blot analysis on days 1,
3 and 7 post-surgery. (A) Representative western blot results show
the changes in the protein levels of p-tau (Ser396) and t-tau. (B)
Quantitative analysis indicated that the level of p-tau (Ser396)
significantly increased on days 1 and 3 post-surgery
(*P<0.05, vs. Ctrl; #P<0.05, vs. Sham).
(C) Levels of t-tau remained unchanged in all groups In the Sham
group, no significant difference was found in the level of p-tau
(Ser396) following isoflurane anesthesia, compared with the Ctrl
group. (P>0.05 vs. Ctrl). Values are presented as the mean ±
standard deviation (n=8 for each group). Ctrl, control group; Sham,
sham surgery group; Sur, surgery group; p-, phosphorylated; D1, day
1; D3, day 3; D7, day 7. |
Rapamycin treatment inhibits the abnormal
mTOR/p70S6K signaling in the hippocampus induced by orthopedic
surgery
As mTOR/p70S6K signaling is hyperactive under the
condition of traumatic stimulation and rapamycin is a well known
mTOR inhibitor, the present study investigated the effect of
rapamycin administration on mTOR/p70S6K signaling. The data showed
that rapamycin treatment significantly reduced the levels of
phospho-mTOR at Ser2448 and phospho-p70S6K at Thr396 induced by
surgical trauma (P<0.05; n=8; Fig.
5B and C). However, rapamycin had no effect on the total levels
of mTOR or p70S6K (Fig. 5D and E).
Rapamycin treatment also reduced the levels of phospho-mTOR at
Ser2448 and phospho-p70S6K at Thr396 in the normal control mice
(P<0.05; n=8; Fig. 5B and
C).
 | Figure 5Effects of rapamycin pretreatment on
activation of mTOR signaling in the hippocampus following surgery.
The expression levels of mTOR and p70S6K were determined using
western blot analysis on day 1 post-surgery. (A) Typical western
blot results show changes in the expression levels of p-mTOR
(Ser2448), t-mTOR, p-p70S6K (Thr396) and t-p70S6K. (B and C) Image
analysis of band densities indicated that surgery significantly
increased the expression levels of p-mTOR (Ser2448) and p-p70S6K
(Thr396), (*P<0.05, vs. Ctrl). (B and C) Pretreatment
with rapamycin significantly reduced the surgery-induced
phosphorylation of p-mTOR (Ser2448) and p-p70S6K (Thr396) on day 1
post-surgery, (#P<0.05, vs. Surg). (D and E)
Pretreatment with rapamycin did not alter the protein expression
levels of t-mTOR or t-p70S6K. Values are presented as the mean ±
standard deviation (n=8 for each group). Ctrl, control group;
Ctrl+rapa, normal group with rapamycin treatment. Surg, surgery
group; Sur+rapa, surgery group with rapamycin pretreatment; t-,
total; p-, phosphorylated. |
Rapamycin treatment attenuates β-amyloid
accumulation and the hyperphosphorylation of tau protein triggered
by orthopedic surgery in the hippocampus
In order to investigate whether the hyperactivation
of mTOR/p70S6K signaling was involved in the accumulation of
β-amyloid and hyperphosphorylation of tau protein, the present
study examined the effect of rapamycin on the levels of
Aβ1-42, total tau and phospho-tau. It was found that
rapamycin significantly attenuated the production of
Aβ1-42 (2.32±0.18 pg/ml), compared with the surgery
group (2.99±0.27 pg/ml), as shown in Fig. 6 (P<0.01; n=8) and attenuated the
level of hyperphosphorylated tau at Ser396, (P<0.05; n=8;
Fig. 7A and B) which were
triggered by surgical injury. However, rapamycin had no effect on
the total level of tau protein (Fig.
7A and C). Rapamycin did not affect the levels of
Aβ1-42, total tau or phospho-tau in the normal control
mice (Figs. 6 and 7).
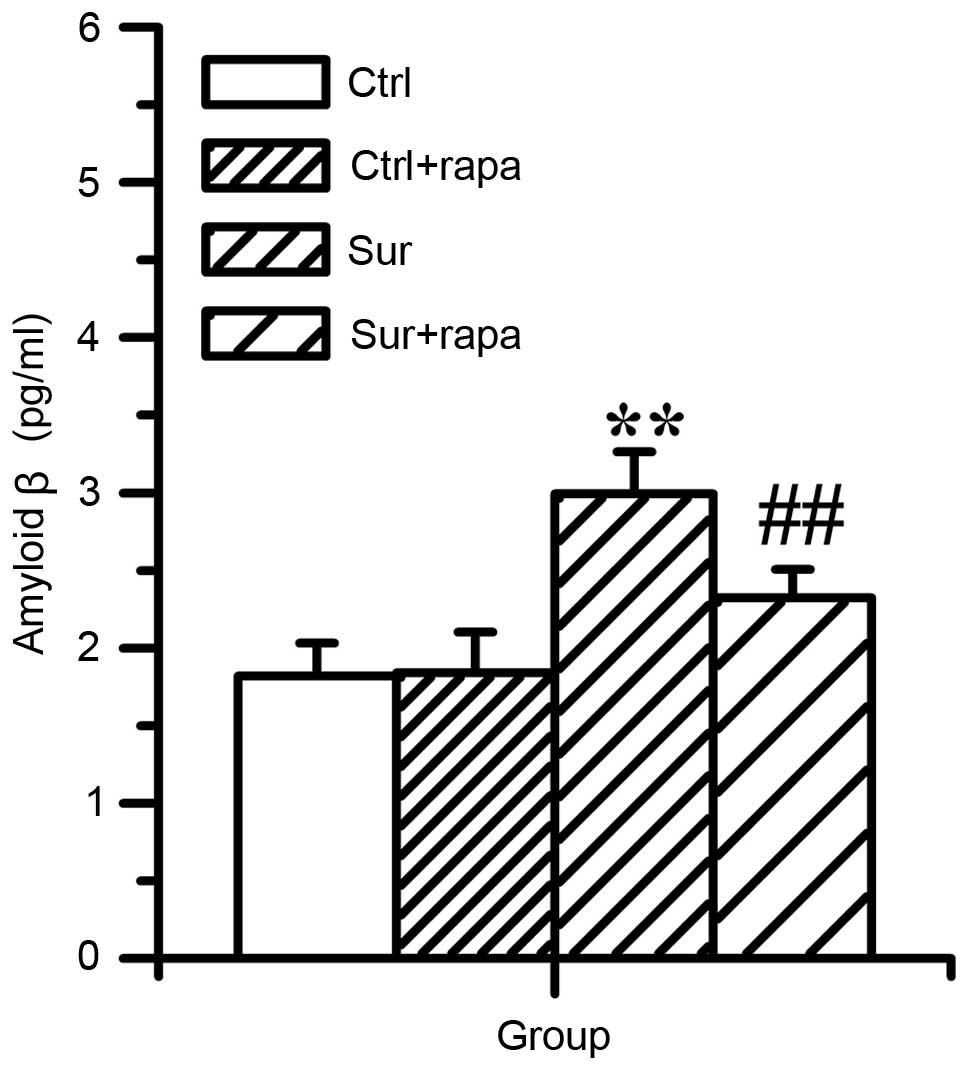 | Figure 6Effects of pretreatment of rapamycin
on the levels of Aβ1-42 in the hippocampus following
surgery. The expression of Aβ1-42 was determined using
an enzyme-linked immunosorbent assay 1 day following surgery.
Compared with the Ctrl group, the level of Aβ1-42 was
significantly elevated (**P<0.01, vs. Ctrl). In the
Sur+rapa group, the level of Aβ1-42 significantly
reduced on day 1 post-surgery, compared with the Sur group
(##P<0.01 vs. Sur). However, rapamycin treatment did
not affect level of Aβ1-42 in the Ctrl mice (P>0.05,
vs. Ctrl). Values are presented as the mean ± standard deviation
(n=8 for each group). Aβ1-42, amyloid β; Ctrl, control
group; Ctrl+rapa, normal group with rapamycin treatment. Sur,
surgery group; Sur+rapa, surgery group with rapamycin
pretreatment. |
 | Figure 7Effects of pretreatment with
rapamycin on the levels of tau protein in the hippocampus following
surgery. The expression of p-tau and tau protein was determined by
western blot analysis 1 day following surgery. (A) Representative
immunoblots show changes in the protein levels of p-tau (Ser396)
and t-tau. (B) Quantitative analysis indicated that surgery
significantly increased the level of p-tau (Ser396;
*P<0.05, vs. Ctrl). In the Sur+rapa group, the levels
of p-tau (Ser396) were significantly reduced 1 day following
surgery, compared with the surgery group (#P<0.05,
vs. Sur), but did not alter the expression of t-tau (P>0.05, vs.
Sur). (C) Rapamycin treatment did not affect the levels of t-tau or
p-tau in the control mice (P>0.05, vs. Ctrl). Values are
presented as the mean ± standard deviation (n=8 for each group).
Aβ1-42, amyloid β; Ctrl, control group; Ctrl+rapa,
normal group with rapamycin treatment. Sur, surgery group;
Sur+rapa, surgery group with rapamycin pretreatment; t-, total; p-,
phosphorylated. |
Rapamycin treatment ameliorates learning
and memory impairment induced by orthopedic surgery
The present study subsequently investigated whether
the increase in mTOR/p70S6K signaling contributed to the cognitive
dysfunction caused by surgery. The effect of rapamycin
administration on learning and memory function was determined using
a CFC assessment, which was also used to measure
hippocampal-dependent learning and memory. It was found that
surgical injury significantly reduced the percentage freezing time
(P<0.05; n=8), however, rapamycin pretreatment significantly
compromised the decreased freezing time caused by surgery
(60.0±8.1% in Sur+rapa group, vs. 43.4±8.0% in the Sur group), as
shown in Fig. 8 (P<0.05; n=8).
Rapamycin treatment had no significant effect in the normal mice
(P>0.05; n=8). These data suggested that rapamycin treatment
rescued the impairment of hippocampal-dependent memory induced by
surgery.
Discussion
In the present study, a mouse model was used to
investigate the cellular roles of mTOR signaling in the central
nervous system in response to surgical intervention. Surgical
trauma was found to induce the activation of mTOR signaling with
accumulation of Aβ1-42 and excessive tau protein
phosphorylation in the hippocampus. Inhibition of the mTOR
signaling pathway using the mTOR inhibitor, rapamycin, effectively
reduced the levels of Aβ1-42 and tau protein
phosphorylation triggered by surgery, and attenuated
hippocampal-dependent memory impairment. Therefore, the present
study demonstrated that surgical stimuli may lead to excessive
activity of mTOR, and hyperactivity of mTOR promoted the
accumulation of Aβ1-42 and phosphorylation of tau
protein, which are involved in memory and cognitive impairment.
In the present study, CFC assessments were used to
evaluate cognitive function in the animals. Several studies in
animals have shown that the hippocampus is critical in CFC
(23,24), and CFC assessment has become a
common method for investigating hippocampal-dependent associative
memory in models of POCD (18,25).
In previous studies, surgical trauma has been found to be associate
with impaired cognitive function (20,26).
In the present study, it was found that orthopedic surgery induced
similar effects. Compared with the control group and sham surgery
group, mice in the surgery group had a lower percentage of freezing
time on day 1 and day 3 following surgery, which indicated that
surgery led to hippocampal-dependent memory impairment. This was
consistent with previous studies in which surgical trauma was
associated with reduced freezing time (25,27).
Although orthopedic surgery has been found to be
associated with cognitive dysfunction, the effect of anesthetics in
POCD remain controversial. In certain studies, volatile anesthetics
have been shown to impair learning and memory ability. In addition,
no differences have been found in the occurrence of POCD between
surgery with and without general anesthesia (3). The present study found that
isoflurane led to no marked reduction in the percentage of freezing
time, compared with the control group, and this may be associated
with the duration of isoflurane exposure.
Rapamycin, an mTOR allosteric inhibitor, has been
shown to prevent the cognitive deficits induced by pathological
damage. Majumder et al (28) found that rapamycin can ameliorate
age-dependent cognitive deficits by reducing interleukin-1β (IL-1β)
and enhancing N methyl D aspartate signaling. In addition,
rapamycin administration can attenuate cognitive deficits by
ameliorating β-amyloid and tau pathology (14,29,30).
In the present study, it was found that rapamycin significantly
alleviated the hippocampal-dependent memory impairment induced by
surgical trauma. This suggested that surgery may induce the
hyperactivity of mTOR signaling, which may be involved in the
postoperative cognitive deficits.
mTOR is a highly conserved serine/threonine kinase.
It is critical in controlling metabolism, survival, protein
synthesis and phosphorylation via its downstream targets, including
p70S6K and 4E-BP1. Previous studies have suggested that the role of
mTOR signaling in protein homeostasis (synthesis and autophagic
degradation) appears to be particularly important in the brain in
learning and memory function. Synaptic plasticity is considered
important in learning and memory, and the activation of mTOR can
act directly at synapses to promote the synthesis of proteins,
which is necessary to facilitate plasticity. Hoeffer et al
(31) found that the enhanced
activity of mTORC1 in mice with FK-506 binding protein 12 removed
from hippocampal neurons, improved memory on assessment of
hippocampus-dependent memory. The inhibition of mTORC1 has also
been shown to disrupt the consolidation of memories, including
hippocampus-dependent spatial memory and auditory cortex-dependent
memory (32,33). By contrast, increasing mTORC1
activity can also disrupt memory processing. Increasing hippocampal
mTORC1 activity by increasing excitatory neurotransmission in the
hippocampus disrupts the formation of hippocampus-dependent memory
(34). Previous observations have
suggested that doses of rapamycin shown to attenuate pathology in
disease models may be below the threshold to disrupt memory
formation and may benefit health without detrimental cognitive
effects (13,14,35).
Caccamo et al (13)
suggested that there may be an optimal window of mTOR signaling for
learning and memory. The present study is the first, to the best of
our knowledge, to show that the levels of phospho-mTOR at Ser 2448
and phospho-p70S6K at Thr396 were increased in the hippocampi of
mice following orthopedic surgery, suggesting that the mTOR/p70S6K
signaling pathway was activated by surgical intervention.
As a well known mTOR inhibitor, the present study
also determined the effect of rapamycin pretreatment on activation
of the mTOR/p70S6K pathway. The levels of phospho-mTOR at Ser 2448
and phosphor-p70S6K at Thr396 were significantly reduced in the
hippocampus of the rapamycin-treated mice, compared with the
untreated mice following surgery. However, no significant
difference in the levels of total mTOR or p70S6K were observed. The
results of the present study demonstrated that the increase in
mTOR/p70S6K signaling in the hippocampus occurred following a
traumatic stimulus, and the enhancement of mTOR/p70S6K signaling
may be a key contributor to the postoperative cognitive
deficits.
For decades, the hallmarks of AD pathophysiology
have primarily been intracellular neurofibrillary tangles and
extracellular senile amyloid plaques (36). The intraneuronal accumulation of
soluble Aβ1-42 has been shown to be a good predictor of
AD pathogenesis (37).
Microtubule-associated tau, as the primary constituent of
neurofibrillary tangles, binds to and stabilizes microtubules in
neurons. A previous study found correlations between cognitive
decline and pathological markers of AD, including the production of
Aβ1-42 and the hyperphosphorylation of tau, in the
hippocampus of mice following major surgery (38). Another previous study demonstrated
that POCD correlated with increased levels of the AD biomarker,
Aβ1-42, in patients following cardiac surgery with
cardiopulmonary bypass (39).
Previous reports have shown that mTOR/p70S6K signaling regulates
the synthesis of β-amyloid and the translation of tau protein
(13,14,29,40).
In addition to regulating tau translation, previous studies have
shown that the mTOR/p70S6K pathway also modulates tau
phosphorylation, directly and indirectly (29,41).
Therefore, the present study hypothesized that the activation of
mTOR may involve β-amyloid accumulation and tau phosphorylation
following surgery. Based on this, the present study analyzed the
expression of Aβ1-42 and the level of tau
phosphorylation in the hippocampus. Increases in the two were found
in this region on day 1 and day 3 following surgery, accompanied by
the activation of mTOR. In addition, in order to determine whether
the hyperactivation of mTOR induced Aβ1-42 accumulation
and tau phosphorylation, the effect of rapamycin on levels of
Aβ1-42 and tau phosphorylation were examined in the
hippocampus of mice following surgery. It was found that rapamycin
treatment attenuated Aβ1-42 accumulation and decreased
the levels of phospho-tau at Thr396, however, no changes in total
tau were observed. These results suggested that the mTOR-mediated
synthesis of Aβ1-42 and phosphorylation of tau was
involved in POCD.
Although the present study demonstrated that
surgical injury upregulated the activity of mTOR signaling, and
then induced β-amyloid accumulation and tau phosphorylation, there
remain unanswered questions. It is difficult to determine how
trauma induces the hyperactivity of mTOR signaling. There is
evidence that mTORC1 can be regulated by amino acids, growth
factors, inflammatory mediators, hypoxia and DNA damage. The
neuroinflammation induced by trauma may be involved in the
hyperactivity of the mTOR signal. Previous investigations in
different tissues have shown that surgery induces inflammatory
responses, which are key in leading to cognitive change and decline
(42). For example, surgery
increases the levels of cytokines in neural tissue, including tumor
necrosis factor-α (TNF-α) and IL-1β, the increases of which are
associated with cognitive decline (25,43).
These proinflammatory cytokines, as with TNF-α, have been indicated
as upregulators in mTORC. Proinflammatory cytokines induce the
inhibitor of κB kinase β (IKKβ), which phosphorylates tuberous
sclerosis 1 (TSC1) leading to TSC1/2 inhibition. When TSC1/2 is
inhibited, it increases the activity of mTORC1 (44). The present study did not
investigate the autophagy mediated by mTOR, and future
investigations are required to determine whether trauma activates
mTOR through the IKKβ/TSC1/2 signal, the effect of surgical trauma
on autophagy and the effect of mTOR in this process.
In conclusion, the present study found that
orthopedic surgery may activate mTOR signaling within the
hippocampus, and the hyperactivity of mTOR promoted the
accumulation of Aβ1-42 and excessive phosphorylation of
the tau protein. Inhibiting the hyperactivity of mTOR may reduce
the production of these misfolded protein, and alleviate memory and
cognitive impairment. These findings provide novel insight into the
signaling transduction mechanisms in the development of
postoperative cognitive dysfunction and may represent a potential
therapeutic target to treat or prevent the development of POCD.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant. no. 81171025/H0902).
References
|
1
|
Moller JT, Cluitmans P, Rasmussen LS, Houx
P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD,
et al: Long-term postoperative cognitive dysfunction in the elderly
ISPOCD1 study. ISPOCD investigators International Study of
Post-Operative Cognitive Dysfunction. Lancet. 351:857–861. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rubens FD, Boodhwani M and Nathan H:
Interpreting studies of cognitive function following cardiac
surgery: A guide for surgical teams. Perfusion. 22:185–192. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Newman S, Stygall J, Hirani S, Shaefi S
and Maze M: Postoperative cognitive dysfunction after noncardiac
surgery: A systematic review. Anesthesiology. 106:572–590. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vacas S, Degos V, Feng X and Maze M: The
neuroinflammatory response of postoperative cognitive decline. Br
Med Bull. 106:161–78. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krenk L, Rasmussen LS and Kehlet H: New
insights into the pathophysiology of postoperative cognitive
dysfunction. Acta Anaesthesiol Scand. 54:951–956. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang C, Yu JT, Miao D, Wu ZC, Tan MS and
Tan L: Targeting the mTOR signaling network for Alzheimer's disease
therapy. Mol Neurobiol. 49:120–135. 2014. View Article : Google Scholar
|
|
7
|
Hoeffer CA and Klann E: mTOR signaling: At
the crossroads of plasticity, memory and disease. Trends Neurosci.
33:67–75. 2010. View Article : Google Scholar
|
|
8
|
Banko JL, Merhav M, Stern E, Sonenberg N,
Rosenblum K and Klann E: Behavioral alterations in mice lacking the
translation repressor 4E-BP2. Neurobiol Learn Mem. 87:248–256.
2007. View Article : Google Scholar
|
|
9
|
Ehninger D, Han S, Shilyansky C, Zhou Y,
Li W, Kwiatkowski DJ, Ramesh V and Silva AJ: Reversal of learning
deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med.
14:843–848. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garelick MG and Kennedy BK: TOR on the
brain. Exp Gerontol. 46:155–163. 2011. View Article : Google Scholar
|
|
11
|
Heras-Sandoval D, Pérez-Rojas JM,
Hernández-Damián J and Pedraza-Chaverri J: The role of
PI3K/AKT/mTOR pathway in the modulation of autophagy and the
clearance of protein aggregates in neurodegeneration. Cell Signal.
26:2694–2701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li X, Alafuzoff I, Soininen H, Winblad B
and Pei JJ: Levels of mTOR and its downstream targets 4E-BP1, eEF2,
and eEF2 kinase in relationships with tau in Alzheimer's disease
brain. FEBS J. 272:4211–4220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Caccamo A, Majumder S, Richardson A,
Strong R and Oddo S: Molecular interplay between mammalian target
of rapamycin (mTOR), amyloid-beta, and Tau: Effects on cognitive
impairments. J Biol Chem. 285:13107–13120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Spilman P, Podlutskaya N, Hart MJ, Debnath
J, Gorostiza O, Bredesen D, Richardson A, Strong R and Galvan V:
Inhibition of mTOR by rapamycin abolishes cognitive deficits and
reduces amyloid-beta levels in a mouse model of Alzheimer's
disease. PLoS One. 5:e99792010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao XZ, Ma H, Wang JK, Liu F, Wu BY, Tian
AY, Wang LL and Tan WF: Postoperative cognitive deficits and
neuroinflammation in the hippocampus triggered by surgical trauma
are exacerbated in aged rats. Prog Neuropsychopharmacol Biol
Psychiatry. 34:1426–1432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Laplante M and Sabatini DM: mTOR
signaling. Cold Spring Harb Perspect Biol. 4:22012. View Article : Google Scholar
|
|
17
|
Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK,
Wei Y, Sun HL, Li LY, Ping B, Huang WC, et al: IKK beta suppression
of TSC1 links inflammation and tumor angiogenesis via the mTOR
pathway. Cell. 130:440–455. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Degos V, Vacas S, Han Z, van Rooijen N,
Gressens P, Su H, Young WL and Maze M: Depletion of bone
marrow-derived macrophages perturbs the innate immune response to
surgery and reduces postoperative memory dysfunction.
Anesthesiology. 118:527–536. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vizcaychipi MP, Xu L, Barreto GE, Ma D,
Maze M and Giffard RG: Heat shock protein 72 overexpression
prevents early postoperative memory decline after orthopedic
surgery under general anesthesia in mice. Anesthesiology.
114:891–900. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vacas S, Degos V, Tracey KJ and Maze M:
High-mobility group box 1 protein initiates postoperative cognitive
decline by engaging bone marrow-derived macrophages.
Anesthesiology. 120:1160–1167. 2014. View Article : Google Scholar :
|
|
21
|
Erlich S, Alexandrovich A, Shohami E and
Pinkas-Kramarski R: Rapamycin is a neuroprotective treatment for
traumatic brain injury. Neurobiol Dis. 26:86–93. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang P, Hou H and Zhang L, Lan X, Mao Z,
Liu D, He C, Du H and Zhang L: Autophagy reduces neuronal damage
and promotes locomotor recovery via inhibition of apoptosis after
spinal cord injury in rats. Mol Neurobiol. 49:276–287. 2014.
View Article : Google Scholar
|
|
23
|
Broadbent NJ, Squire LR and Clark RE:
Spatial memory, recognition memory, and the hippocampus. Proc Natl
Acad Sci USA. 101:14515–14520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maren S, Phan KL and Liberzon I: The
contextual brain: Implications for fear conditioning, extinction
and psychopathology. Nat Rev Neurosci. 14:417–428. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cibelli M, Fidalgo AR, Terrando N, Ma D,
Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS
and Maze M: Role of interleukin-1beta in postoperative cognitive
dysfunction. Ann Neurol. 68:360–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Terrando N, Eriksson LI, Ryu JK, Yang T,
Monaco C, Feldmann M, Jonsson Fagerlund M, Charo IF, Akassoglou K
and Maze M: Resolving postoperative neuroinflammation and cognitive
decline. Ann Neurol. 70:986–995. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu N, Guo D, Wang H, Xie K, Wang C, Li Y,
Wang C, Wang C, Yu Y and Wang G: Involvement of the blood-brain
barrier opening in cognitive decline in aged rats following
orthopedic surgery and high concentration of sevoflurane
inhalation. Brain Res. 1551:13–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Majumder S, Caccamo A, Medina DX,
Benavides AD, Javors MA, Kraig E, Strong R, Richardson A and Oddo
S: Lifelong rapamycin administration ameliorates age-dependent
cognitive deficits by reducing IL-1β and enhancing NMDA signaling.
Aging Cell. 11:326–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Caccamo A, Magri A, Medina DX, Wisely EV,
López-Aranda MF, Silva AJ and Oddo S: mTOR regulates tau
phosphorylation and degradation: Implications for Alzheimer's
disease and other tauopathies. Aging Cell. 12:370–380. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang S, Zhou SL, Min FY, Ma JJ, Shi XJ,
Bereczki E and Wu J: mTOR-mediated hyperphosphorylation of tau in
the hippocampus is involved in cognitive deficits in
streptozotocin-induced diabetic mice. Metab Brain Dis. 29:729–736.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hoeffer CA, Tang W, Wong H, Santillan A,
Patterson RJ, Martinez LA, Tejada-Simon MV, Paylor R, Hamilton SL
and Klann E: Removal of FKBP12 enhances mTOR-Raptor interactions,
LTP, memory, and perseverative/repetitive behavior. Neuron.
60:832–845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dash PK, Orsi SA and Moore AN: Spatial
memory formation and memory-enhancing effect of glucose involves
activation of the tuberous sclerosis complex-Mammalian target of
rapamycin pathway. J Neurosci. 26:8048–8056. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schicknick H, Schott BH, Budinger E,
Smalla KH, Riedel A, Seidenbecher CI, Scheich H, Gundelfinger ED
and Tischmeyer W: Dopaminergic modulation of auditory
cortex-dependent memory consolidation through mTOR. Cereb Cortex.
18:2646–2658. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Puighermanal E, Marsicano G,
Busquets-Garcia A, Lutz B, Maldonado R and Ozaita A: Cannabinoid
modulation of hippocampal long-term memory is mediated by mTOR
signaling. Nat Neurosci. 12:1152–1158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Halloran J, Hussong SA, Burbank R,
Podlutskaya N, Fischer KE, Sloane LB, Austad SN, Strong R,
Richardson A, Hart MJ and Galvan V: Chronic inhibition of mammalian
target of rapamycin by rapamycin modulates cognitive and
non-cognitive components of behavior throughout lifespan in mice.
Neuroscience. 223:102–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Braak H and Braak E: Neuropathological
stageing of Alzheimer-related changes. Acta Neuropathol.
82:239–259. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Blasko I, Kemmler G, Jungwirth S, Wichart
I, Krampla W, Weissgram S, Jellinger K, Tragl KH and Fischer P:
Plasma amyloid beta-42 independently predicts both late-onset
depression and Alzheimer disease. Am J Geriatr Psychiatry.
18:973–982. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wan Y, Xu J, Meng F, Bao Y, Ge Y, Lobo N,
Vizcaychipi MP, Zhang D, Gentleman SM, Maze M and Ma D: Cognitive
decline following major surgery is associated with gliosis,
β-amyloid accumulation and tau phosphorylation in old mice. Crit
Care Med. 38:2190–2198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Reinsfelt B, Westerlind A, Blennow K,
Zetterberg H and Ricksten SE: Open-heart surgery increases
cerebrospinal fluid levels of Alzheimer-associated amyloid β. Acta
Anaesthesiol Scand. 57:82–88. 2013. View Article : Google Scholar
|
|
40
|
Ma YQ, Wu DK and Liu JK: mTOR and tau
phosphorylated proteins in the hippocampal tissue of rats with type
2 diabetes and Alzheimer's disease. Mol Med Rep. 7:623–627.
2013.
|
|
41
|
Tang Z, Bereczki E, Zhang H, Wang S, Li C,
Ji X, Branca RM, Lehtiö J, Guan Z, Filipcik P, et al: Mammalian
target of rapamycin (mTor) mediates tau protein dyshomeostasis:
Implication for Alzheimer disease. J Biol Chem. 288:15556–15570.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lyman M, Lloyd DG, Ji X, Vizcaychipi MP
and Ma D: Neuro-inflammation: The role and consequences. Neurosci
Res. 79:1–12. 2014. View Article : Google Scholar
|
|
43
|
Terrando N, Monaco C, Ma D, Foxwell BM,
Feldmann M and Maze M: Tumor necrosis factor-alpha triggers a
cytokine cascade yielding postoperative cognitive decline. Proc
Natl Acad Sci USA. 107:20518–20522. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu XC, Yu JT, Jiang T and Tan L:
Autophagy modulation for Alzheimer's disease therapy. Mol
Neurobiol. 48:702–714. 2013. View Article : Google Scholar : PubMed/NCBI
|






















