Introduction
Atrial fibrillation (AF) is one of the most common
arrhythmias encountered in clinical practice (1). The prevalence of AF in the general
population is ~1% and the risk increases with age. AF substantially
increases cardiovascular morbidity and mortality. There is a 5-fold
increase in the risk of stroke in patients with AF and 15–20% of
strokes are caused by AF. AF is an independent risk factor for
congestive heart failure and increases the mortality 2-fold.
Genetic defects may be responsible for the pathogenesis of AF in a
subset of patients. During AF, electrical and structural remodeling
occurs continually (2–4). The electrophysiological and
structural remodeling is crucial for the development, maintenance
and recurrence of AF. Remodeling shortens the atrial wavelength of
intraatrial reentry and leads to an increase in the potential
number of electrical reentrant cycles, which is responsible for AF
maintenance. Changes in the expression levels of ion channels,
including L-type calcium (Ca) channel (LTCC) and potassium (K)
channel, are important for early remodeling during AF (5). However, the pathogenic mechanisms
underlying atrial structural remodeling remain to be
elucidated.
Changes in atrial electrophysiology and structure,
referred to as remodeling, constitute the primary features of AF
occurrence, maintenance and recurrence (6,7).
Changes in ion currents, including Ca2+ and
K+, form the basis for early electrical remodeling in AF
(8,9). Calcium entry from the extracellular
space through LTCCs and the resultant intracellular Ca2+
elevation (calcium overload) was demonstrated to be crucial in the
regulation of atrial frequency-dependent action potential duration
(APD) and effective refractory period (ERP) (10). The transient outward K+
current mediated by the potassium channel Kv4.3 contributes to
early repolarization (11).
Emerging evidence indicates that abnormalities in
cardiovascular embryological development contribute to AF (12). Nkx2.5 is a critical transcription
factor and its mutation is associated with AF development (13–16).
As a member of the NK2 family, the expression and functions of
Nkx2.5 overlap with those of the GATA family during cardiovascular
development (17,18). However, whether Nkx2.5 affects ion
channel proteins in the context of AF remains to be elucidated. The
aim of the present study was to investigate the effect of rapid
pacing (rapid electrical stimulation used to simulate AF) on APD,
ion channel proteins and the Nkx2.5/CARP (cardiac ankyrin repeat
protein) signaling pathway.
Materials and methods
Isolation and culture of rat atrial
myocardial cells (AMCs)
The present study and all experimental protocols
involved were approved by the Institutional Animal Care and Use
Committee of the Third Military Medical University (Chongqing,
China). A total of 20 female Wistar rats (2 weeks old) were
purchased from the Experimental Animal Center of the Third Military
Medical University. Rats were maintained under constant temperature
and humidity conditions with a 12-h light/dark cycle and ad
libitum access to standard chow and water. Prior to the
experiment, rats were sacrificed by CO2 inhalation and
then fixed in a supine position. Following sterilization with 70%
ethanol, an incision was made along the right edge of the sternum
and the chest wall was removed. The heart was dissected out and
washed in cold phosphate-buffered saline (PBS). The left and right
atria were isolated and washed with serum-free Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with penicillin and streptomycin.
Under aseptic conditions, the right atrial appendage was cut into
small pieces with scissors, which were then digested with 0.08%
trypsin at 37°C for 5 min. The reaction was terminated by addition
of DMEM containing 10% fetal bovine serum (FBS; HyClone; GE
Healthcare Life Sciences, Logan, UT, USA). This solution was
maintained at room temperature and the supernatant was filtered
through a 100-μm mesh filter. Digestion was performed twice.
Finally, suspensions of single cells were prepared by treatment of
the digested product with 0.1% collagenase at 37°C for 15 min. The
cells were seeded into flasks at a density of ~1×108/l,
followed by incubation with DMEM containing 10% FBS at 37°C with 5%
CO2. In the control group, following 72 h of routine
culture, the medium was replaced with serum-free DMEM for 24 h, and
then rapid pacing was performed.
Rapid pacing of AMCs
When the cell confluence reached ~80%, the culture
dishes were placed in an electrical field and stimulated with 10
Hz, 1.5 V/cm using the BL-420E+ biological and functional
experimental system (Chengdu Techman Software, Co., Ltd., Chengdu,
China). The beating frequency of the cells was visually recorded.
The survival rate of cells prior to and following the rapid pacing
was assessed by 3-[4,5-dimethylthiazol-2-y1]-2,5-diphenytetrazolium
bromide assay (Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions, and the APD at 50 and 90%
repolarization was recorded with a patch clamp at the whole cell
mode.
Transmission electron microscopy
AMCs prior to and following rapid pacing were
collected, transferred into Eppendorf tubes, resuspended in cold
PBS and centrifuged at 200 × g for 5 min at 4°C. The supernatant
was removed and cells were fixed in 2% glutaraldehyde for 2 h and
post-fixed in 1% tetroxide osmium for 2 h. Following dehydration
with an alcohol gradient, cells were embedded in epoxy resin 618
(Shanghai Kang Lang Biological Technology Co., Ltd., Shanghai,
China). Ultrathin sections (100 nm) were prepared and contrast
stained with uranyl acetate and lead citrate. Images were captured
(magnification, ×6,000) using a transmission electron microscope
(H7500; Hitachi, Ltd., Tokyo, Japan).
RNA interference
The short interfering RNA (siRNA) for Nkx2.5 and
negative control siRNA duplexes were designed and synthesized by
Shanghai GenePharma Co., Ltd. (Shanghai, China). The sequences of
siRNA duplexes were as follows: Sense, 5′-UUCUCCGAACGUGUCACGUTT-3′
and anti-sense, 5′-ACGUGACACGUUCGGAGAATT-3′ for the negative
control; sense, 5′-CCCUCGGGCGGAUAAGAAATT-3′ and anti-sense,
5′-UUUCUUAUCCGCCCGAGGGTC-3′ for Nkx2.5-310. In each group,
1×105 cells were seeded into 60-mm culture dishes
without antibiotics. At 70% confluence, the siRNA duplexes for
Nkx2.5 and negative control were added with
Lipofectamine® RNAimax (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
mRNA expression levels were detected by RT-PCR.
Total cellular RNA was extracted from the rat AMCs of each group
using TRIzol® Reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and cDNA was prepared using a Superscript II
First-Strand cDNA synthesis kit (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. PCR
was performed using Taq DNA Polymerase (Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China). The primer sequences
and annealing temperatures were as follows: Forward,
5′-ATGGAGGCTGGAGCCCAGATTGA-3′ and reverse,
5′-GACATTGAGGTCCGCACCGAAGG-3′ for α1c (annealing temperature,
61.3°C); forward, 5′-GCAGCAACCTGAAATCTGAAACT-3′ and reverse,
5′-GATAAGCAATGAACCCATCTCCA-3′ for Kv4.3 (annealing temperature,
56.1°C); forward, 5′-TTGTTTCTGTCACCAGTAAC-3′ and reverse,
5′-GATGAGGAAGGAAGAGAAGC-3′ for connexin-43 (Cx43; annealing
temperature, 56.3°C); forward, 5′-GTAAGCGACAGCGGCAGGAC-3′ and
reverse, 5′-CGACGCCAAAGTTCACGAAG-3′ for Nkx2.5 (annealing
temperature, 58.7°C); forward, 5′-GGGGTACCAGCCAACATGATG-3′ and
reverse, 5′-CCCTCGAGGCCTCAGAATGTAGC-3′ for CARP (annealing
temperature, 60.1°C); and forward, 5′-TGAGAGGGAAATCGTGCGTGAC-3′ and
reverse, 5′-ATCTGCTGGAAGGTGGACAGTGAG-3′ for β-actin (annealing
temperature, 53.9°C). The amplification process was performed for
35 cycles following an initial 45 sec denaturation at 94°C,
annealed for 30 sec at the above-indicated temperatures and
extended for 5 min at 72°C. PCR products were separated by agarose
gel electrophoresis and stained with ethidium bromide. Band
intensities were measured by densitometry and normalized to β-actin
using ImageJ software version 1.5.0 (National Institutes of Health,
Bethesda, MD, USA).
Western blot analysis
Rat AMCs (1.5×106) from each group were
lysed with 0.5 ml radioimmunoprecipitation assay buffer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) containing 5 μl
phenylmethylsulfonyl fluoride. Cell lysates were centrifuged at
12,000 × g at 4°C for 30 min and the resulting supernatant (total
tissue homogenate) was stored at -80°C until further analysis.
Protein (15 μg) from each group was separated by 15%
SDS-PAGE (200 V, 45 min) and transferred to polyvinylidene
difluoride membranes. The membranes were blocked with 5% bovine
serum albumin (HyClone; GE Healthcare Life Sciences) in
Tris-buffered saline containing Tween 20 (TBST) for 1 h at room
temperature, and then were incubated with the following primary
antibodies: Rabbit anti-rat α1c (1:2,000; catalog no. AB5156; EMD
Millipore, Billerica, MA, USA), rabbit anti-rat Kv4.3 (1:1,000;
catalog no. AB5194; EMD Millipore), rabbit anti-rat Cx43 (1:1,000;
catalog no. sc-9059; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), rabbit anti-rat Nkx2.5 (1:500; catalog no. sc-14033; Santa
Cruz Biotechnology, Inc.), rabbit anti-CARP (1:500; catalog no.
sc-30181; Santa Cruz Biotechnology, Inc.) and rabbit anti-β-actin
(1:1,000; catalog no. ab8227; Abcam, Cambridge, MA, USA). Membranes
were washed three times in TBST and incubated with a horseradish
peroxidase-conjugated secondary goat anti-rabbit IgG antibody
(1:500; catalog no. DGSP-H-KIT-4; Beijing Dingguo Changsheng
Biotechnology Co., Ltd., Beijing, China). The protein bands were
visualized using SuperSensitive Enhanced Chemiluminescence solution
(Beijing Dingguo Changsheng Biotechnology Co., Ltd.) and quantified
using ImageJ software version 1.5.0. Band intensities were measured
by densitometry and normalized to β-actin.
Statistical analysis
Statistical analysis was performed in SPSS version
17.0 (SPSS, Inc., Chicago, IL, USA). Group comparisons were
performed by one-way analyses of variance, and the
Student-Newman-Keuls method was used as a post-hoc test.
Data are expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Observation of cultured atrial myocardial
cells (AMCs)
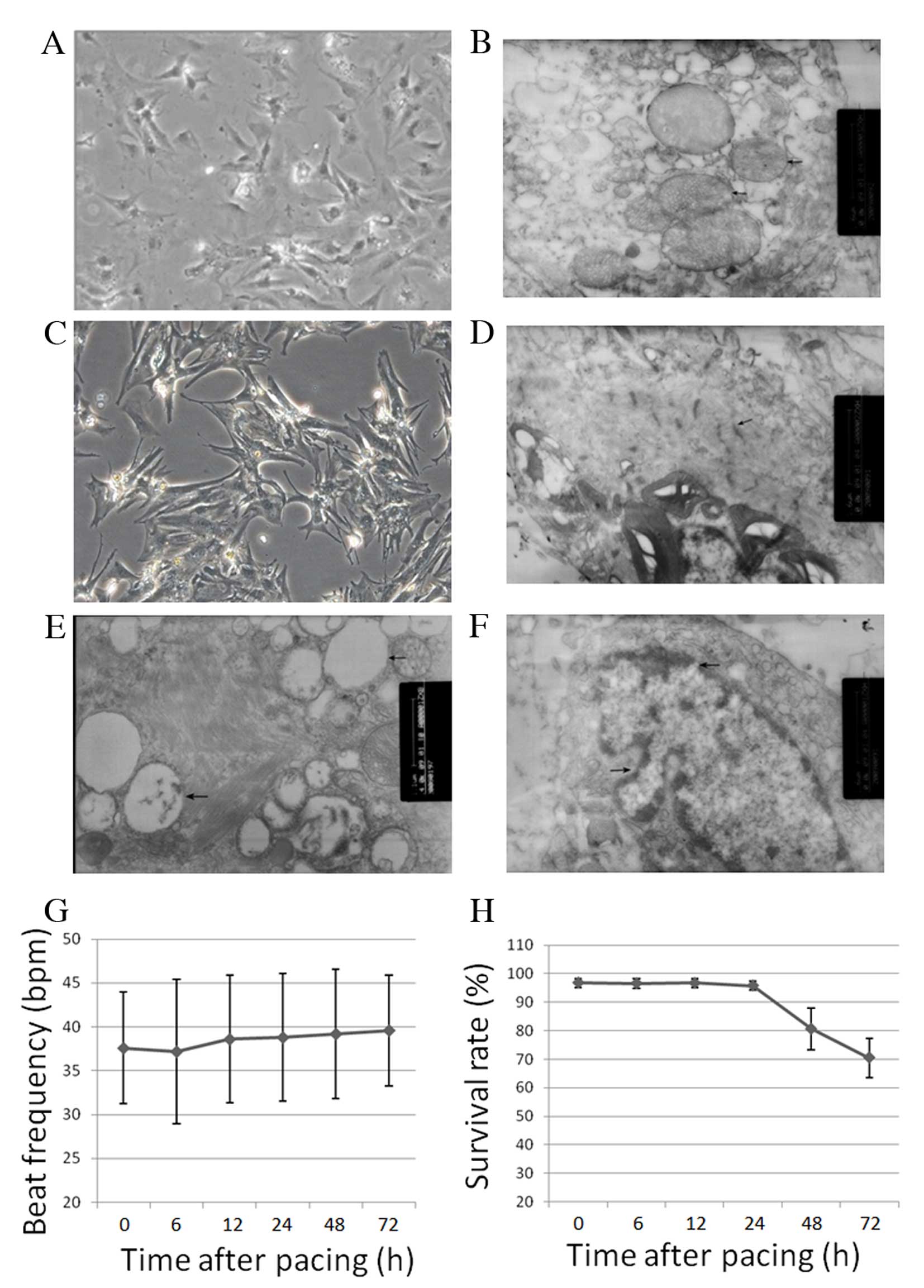
AMCs cultured for three days were heterogeneous in
shape, including rod, spindle, triangular and irregular (Fig. 1A). The number of spontaneously
beating cells increased following 48 h in culture. AMCs exhibited
clear ultrastructural features and regular arrangement of
mitochondrial cristae (Fig. 1B).
At 24 h following 3-h rapid pacing, AMCs presented with a more
polygonal shape, irregular myofibril arrangement and sparse
myofilaments (Fig. 1C and D). At
48 and 72 h following rapid pacing, vacuolar degeneration and
expanded bubbles were observed (Fig.
1E and F). The beat frequency did not alter significantly
following rapid pacing (Fig. 1G);
however the survival rate decreased from 48 h following rapid
pacing (Fig. 1H; P<0.001).
Under normal conditions, cells would proliferate for one month.
Therefore, the decrease in survival rate was as a result of rapid
pacing.
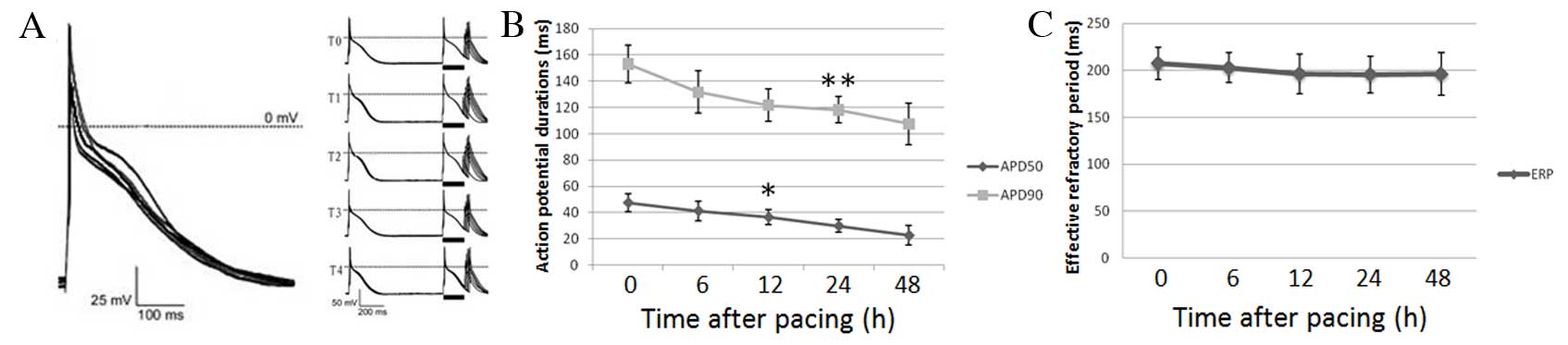
Electrophysiological changes in AMCs
The APD measured at 50% repolarization (APD 50) was
significantly decreased from 12 h following rapid pacing (12 h,
P=0.0235; 24 h, P=0.0014; 48 h, P=0.0005; Fig. 2A and B). The APD 90 was
significantly decreased at 24 (P=0.056) and 48 (P=0.0021) h
following rapid pacing (Fig. 2A and
B). No significant differences in ERP were observed following
rapid pacing (6 h, P=0.6647; 12 h, P=0.3858; 24 h, P=0.3438; 48 h,
P=0.3930; Fig. 2A and C).
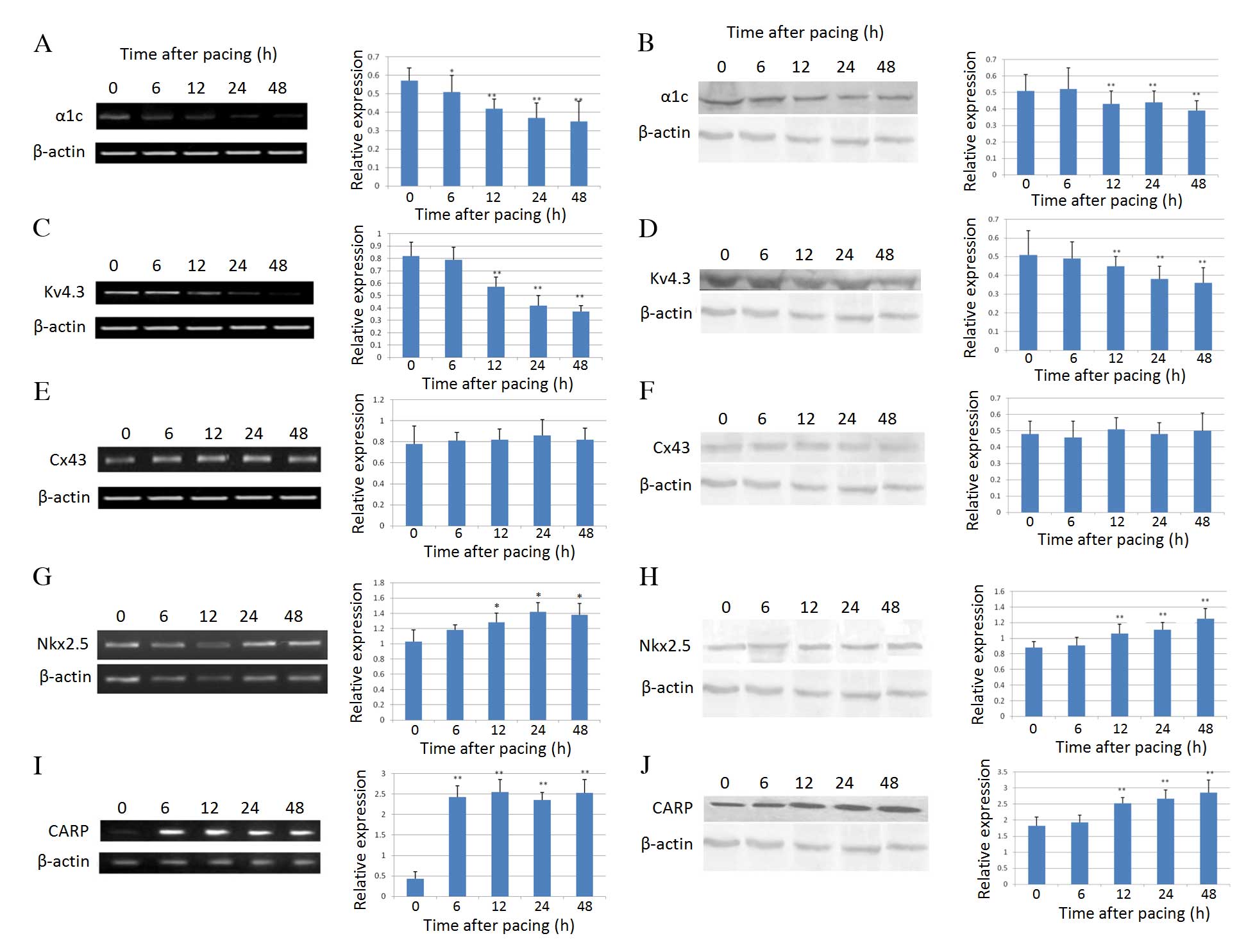
Effect of rapid pacing on the expression
levels of ion channels and nuclear proteins in AMCs
mRNA expression levels of the LTCC protein α1c were
significantly reduced at 6 h (P=0.023) following rapid pacing
compared with prior to rapid pacing, and this difference increased
at 12 (P=0.0053), 24 (P=0.0021) and 48 (P=0.0016) h (Fig. 3A). Western blotting correlated well
with RT-PCR data, with α1c protein expression levels significantly
reduced from 12 h following rapid pacing (P=0.0036; Fig. 3B). mRNA (P=0.0011) and protein
(P=0.0085) expression levels of the potassium channel Kv4.3 were
decreased from 12 h following rapid pacing (Fig. 3C and D). The mRNA and protein
expression levels of the important gap junction protein Cx43 were
not affected by rapid pacing (Fig. 3E
and F). The mRNA (P=0.022) and protein (P=0.0073) expression
levels of Nkx2.5, a critical cardiac transcription factor, were
upregulated from 12 h following rapid pacing (Fig. 3G and H). CARP, a downstream
molecule in the Nkx2.5 homeobox gene signaling pathway, exhibited a
similar pattern to Nkx2.5 (mRNA, P=0.0005 and protein, P=0.0032 at
12 h; Fig. 3I and J).
Effect of Nkx2.5 inhibition on the
expression levels of ion channel proteins in AMCs
As presented in Fig.
4A, transfection with Nkx2.5 siRNA inhibited the rapid
pacing-induced increase in Nkx2.5 expression at the mRNA level
(P=0.0089). In addition, the increase in mRNA expression levels of
CARP induced by rapid pacing was inhibited by Nkx2.5 siRNA
(P=0.0068; Fig. 4B and C). Protein
expression levels of Nkx2.5 (P=0.046) and CARP (P=0.031) followed
the same pattern (Fig. 4D and
E).
 | Figure 4Effect of Nkx2.5 inhibition on the
mRNA and protein expression levels of Nkx2.5 and CARP in atrial
myocardial cells, determined by reverse transcription-polymerase
chain reaction and western blotting, respectively. mRNA expression
levels of (A) Nkx2.5 and (B) CARP were reduced following
transfection with Nkx2.5, but not negative control, siRNA. (C)
Relative mRNA expression of Nkx2.5 and CARP. (D) Protein expression
levels of Nkx2.5 and CARP were reduced following transfection with
Nkx2.5, but not negative control, siRNA. (E) Relative protein
expression of Nkx2.5 and CARP. Data were normalized to β-actin.
Data are presented as the mean ± standard deviation (n=3).
*P<0.05 and **P<0.01 vs. RP and
negative groups. CARP, cardiac ankyrin repeat protein; RP, rapid
pacing; Neg, negative group; siRNA, Nkx2.5 siRNA transfection
group; Con, control group without pacing. |
Furthermore, treatment with Nkx2.5 siRNA attenuated
the decrease in α1c and Kv4.3 mRNA (α1c, P= 0.028; Kv4.3, P=0.043)
and protein (α1c, P=0.017; Kv4.3, P=0.019) expression levels
induced by rapid pacing (Fig.
5).
 | Figure 5Effect of Nkx2.5 inhibition on the
mRNA and protein expression levels of the ion channel proteins α1c
and Kv4.3 in atrial myocardial cells, determined by reverse
transcription-polymerase chain reaction and western blotting,
respectively. mRNA expression levels of (A) α1c and (B) Kv4.3 were
increased following transfection with Nkx2.5, but not negative
control, siRNA. (C) Relative mRNA expression of Nkx2.5 and CARP.
(D) Protein expression levels of α1c and Kv4.3 were increased
following transfection with Nkx2.5, but not negative control,
siRNA. (E) Relative protein expression of Nkx2.5 and CARP. Data
were normalized to β-actin. Data are presented as the mean ±
standard deviation (n=3). *P<0.05 vs. RP and negative
groups. RP, rapid pacing; Neg, negative group; siRNA, Nkx2.5 siRNA
transfection group; Con, control group without pacing. |
Discussion
AF, the most common form of sustained cardiac
arrhythmia, is characterized by uncoordinated atrial activation and
chaotic electrical activity, with consequent deterioration of
atrial mechanical function (19).
Using the in vitro rat AMC culture and rapid pacing model,
the present study demonstrated that rapid pacing shortened the APD
and downregulated the expression levels of LTCC and potassium
channels. Expression of Nkx2.5 and CARP were significantly
upregulated by rapid pacing at the mRNA and protein levels.
siRNA-mediated Nkx2.5 silencing rescued the rapid pacing-induced
downreglation of ion channel expression levels, suggesting that the
Nkx2.5/CARP signaling pathway contributes to the early electrical
remodeling process of AF.
In the current study, the APD of rat AMCs was
significantly reduced 12 h subsequent to rapid pacing, while no
effect on the ERP was observed at any time point. In AF patients,
shortened APD results in decreased wavelength of reentry circuits
and atrial electrical remodeling, thus facilitating the maintenance
of AF and inhibiting the natural termination of AF (20,21).
The lack of an effect on ERP may be due to differences in pacing
rate, electric field strength and varying cell sources. The causes
of APD shortening include: i) Increased outward K+
currents; ii) decreased inward Ca2+ current; and iii) a
combination of the above two factors (22). L-type Ca2+ current is
activated by membrane depolarization and contributes to the
formation of the action potential plateau phase (23). As in the ventricular muscle cells,
the transient outward K+ current (Ito)
is the basis of early rapid repolarization of atrial action
potential, while Kv4.3 is the major pore-forming subunit of
Ito channels (24). Atrial rapid pacing, as occurs in
atrial fibrillation, may lead to a decrease in the density of
functional ion channels (Na+, Ca2+ and
K+) (25); however, no
effect was observed on the intrinsic properties of single ion
channels.
A calcium channel current (ICa) is
essential for action potential and excitation-contraction coupling
of myocardial cells (26). The
voltage-dependent calcium channels are typically divided into L-
and T-type channels, and Ca2+ influx mediated by LTCCs
is an important factor regulating human atrial frequency-dependent
APD. The present study revealed that the expression level of α1c at
6 h subsequent to rapid pacing was significantly reduced compared
with prior to pacing, becoming stable at 24 h. The reduction in
LTCC currents is critical for the shortening of the action
potential cycle, and decreased calcium influx is harmful to atrial
mechanical contraction. The expression level of Kv4.3 was
significantly reduced from 12 h subsequent to rapid pacing. These
results are largely in accordance with other experimental models of
atrial fibrillation, and may reflect an attempt to prevent the
shortening of APD and ERP; however, the underlying mechanism
requires further study.
Gap junctions between cardiac cells provide
connections and a low-resistance pathway interconnecting
cardiomyocytes (27). These
coordinate myocardial action potential and synchronous contraction.
The gap junction Cx proteins present in heart cells include Cx40
and Cx43 (28). A change in the
structure and density of Cx may result in changes in conductivity
anisotropy and conduction velocity of atrial myocytes, ideal
conditions for reentrant arrhythmia (29). In the present study, no significant
changes in Cx43 were observed, which may be due to various factors:
The pacing duration may not have been long enough; changes in Cx43
may be the result of long-term AF; or changes in the distribution
of Cx43 may be of greater importance than its expression levels.
Further investigations are required to elucidate the role of gap
junction proteins in atrial electrical remodeling.
Anomalies in embryological cardiovascular
development contribute to the initiation of AF (30,31).
Various transcription factors, including Nkx2.5, were identified as
essential in cardiovascular genesis (32,33).
Gutierrez-Roelens et al (34) first identified an Nkx2.5 mutation
suggested to be associated with the atrial fibrillation phenotype.
Homeobox gene Nkx2.5, also referred to as cardiac-specific homeobox
gene, belongs to the NK-2 homeobox family. Nkx2.5 is crucial for
myocardial cell differentiation and heart tube formation, and is
involved in the atrioventricular separation and conduction system
(35). As a downstream mediator of
Nkx2.5, CARP contributes to the maintenance of complete sarcomere
structure and function, and is involved in the regulation of
intracellular calcium (36). The
present study demonstrated that the expression levels of Nkx2.5 and
CARP were significantly elevated during the early phase following
fast pacing, indicating that Nkx2.5 is important in
undifferentiated cells and in differentiated cardiomyocytes. As the
Nkx2.5/CARP signaling pathway is a critical regulator of cell
development, cell communication and intracellular calcium, it was
hypothesized that the Nkx2.5/CARP signaling pathway may be critical
for ion channel remodeling in the early stages of atrial
fibrillation.
In the present study, although Nkx2.5-siRNA
transfected AMCs inhibited the downregulation of α1c and Kv4.3
expression levels induced by rapid pacing, this downregulation was
not completely reversed, suggesting that ion channel remodeling is
regulated by multiple factors.
In conclusion, the results of the present study
demonstrated that rapid pacing may shorten APD and induce the
downregulation of the LTCC protein, α1c and potassium channel,
Kv4.3, resembling the electrophysiological properties of atrial
fibrillation. The Nkx2.5/CARP signaling pathway was upregulated by
rapid pacing, while Nkx2.5 siRNA-mediated gene silencing inhibited
the rapid pacing-induced ion channel downregulation. These results
indicate that the Nkx2.5/CARP signaling pathway may be involved in
the early channel remodeling process during rapid pacing. These
findings may have implications for the early detection of AF, and
suggest potential targets for prophylaxis.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 30600252).
References
|
1
|
Wang XH, Huang CX, Wang Q, Li RG, Xu YJ,
Liu X, Fang WY and Yang YQ: A novel GATA5 loss-of-function mutation
underlies lone atrial fibrillation. Int J Mol Med. 31:43–50.
2013.
|
|
2
|
Zhou YM, Zheng PX, Yang YQ, Ge ZM and Kang
WQ: A novel PITX2c loss-of-function mutation underlies lone atrial
fibrillation. Int J Mol Med. 32:827–834. 2013.PubMed/NCBI
|
|
3
|
Yoon N, Cho JG, Kim KH, Park KH, Sim DS,
Yoon HJ, Hong YJ, Park HW, Kim JH, Ahn Y, et al: Beneficial effects
of an angiotensin-II receptor blocker on structural atrial
reverse-remodeling in a rat model of ischemic heart failure. Exp
Ther Med. 5:1009–1016. 2013.PubMed/NCBI
|
|
4
|
Qiu XB, Xu YJ, Li RG, Xu L, Liu X, Fang
WY, Yang YQ and Qu XK: PITX2C loss-of-function mutations
responsible for idiopathic atrial fibrillation. Clinics (Sao
Paulo). 69:15–22. 2014. View Article : Google Scholar
|
|
5
|
Gan TY, Qiao W, Xu GJ, Zhou XH, Tang BP,
Song JG, Li YD, Zhang J, Li FP, Mao T and Jiang T: Aging-associated
changes in L-type calcium channels in the left atria of dogs. Exp
Ther Med. 6:919–924. 2013.PubMed/NCBI
|
|
6
|
Xu GJ, Gan TY, Tang BP, Chen ZH, Mahemuti
A, Jiang T, Song JG, Guo X, Li YD, Zhou XH, et al: Alterations in
the expression of atrial calpains in electrical and structural
remodeling during aging and atrial fibrillation. Mol Med Rep.
8:1343–1352. 2013.PubMed/NCBI
|
|
7
|
Fu G, Cao Y, Lu J, Li J, Liu L, Wang H, Su
F and Zheng Q: Programmed cell death-1 deficiency results in atrial
remodeling in C57BL/6 mice. Int J Mol Med. 31:423–429. 2013.
|
|
8
|
Heijman J and Dobrev D: Systems approaches
to post-operative atrial fibrillation-do they help us to better
understand the ionic basis of the arrhythmogenic substrate? J Mol
Cell Cardio. 53:320–322. 2012. View Article : Google Scholar
|
|
9
|
Nattel S and Dobrev D: The
multidimensional role of calcium in atrial fibrillation
pathophysiology: Mechanistic insights and therapeutic
opportunities. Eur Heart J. 33:1870–1877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ren Y, Zhang M, Zhang T and Huang R:
Effect of ouabain on myocardial remodeling in rats. Exp Ther Med.
6:65–70. 2013.PubMed/NCBI
|
|
11
|
Huo R, Sheng Y, Guo WT and Dong DL: The
potential role of Kv4.3 K+ channel in heart hypertrophy. Channels
(Austin). 8:203–209. 2014. View Article : Google Scholar
|
|
12
|
Yue L, Xie J and Nattel S: Molecular
determinants of cardiac fibroblast electrical function and
therapeutic implications for atrial fibrillation. Cardiovasc Res.
89:744–753. 2011. View Article : Google Scholar :
|
|
13
|
Huang RT, Xue S, Xu YJ, Zhou M and Yang
YQ: A novel NKX2.5 loss-of-function mutation responsible for
familial atrial fibrillation. Int J Mol Med. 31:1119–1126.
2013.PubMed/NCBI
|
|
14
|
Yuan F, Qiu XB, Li RG, Qu XK, Wang J, Xu
YJ, Liu X, Fang WY, Yang YQ and Liao DN: A novel NKX2-5
loss-of-function mutation predisposes to familial dilated
cardiomyopathy and arrhythmias. Int J Mol Med. 35:478–486.
2015.
|
|
15
|
Wang J, Zhang DF, Sun YM, Li RG, Qiu XB,
Qu XK, Liu X, Fang WY and Yang YQ: NKX2-6 mutation predisposes to
familial atrial fibrillation. Int J Mol Med. 34:1581–1590.
2014.PubMed/NCBI
|
|
16
|
Yu H, Xu JH, Song HM, Zhao L, Xu WJ, Wang
J, Li RG, Xu L, Jiang WF, Qiu XB, et al: Mutational spectrum of the
NKX2-5 gene in patients with lone atrial fibrillation. Int J Med
Sci. 11:554–563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Rath N, Hannenhalli S, Wang Z,
Cappola T, Kimura S, Atochina-Vasserman E, Lu MM, Beers MF and
Morrisey EE: GATA and Nkx factors synergistically regulate
tissue-specific gene expression and development in vivo.
Development. 134:189–198. 2007. View Article : Google Scholar
|
|
18
|
Wang J, Zhang DF, Sun YM and Yang YQ: A
novel PITX2c loss-of-function mutation associated with familial
atrial fibrillation. Eur J Med Genet. 57:25–31. 2014. View Article : Google Scholar
|
|
19
|
Shi HF, Yang JF, Wang Q, Li RG, Xu YJ, Qu
XK, Fang WY, Liu X and Yang YQ: Prevalence and spectrum of GJA5
mutations associated with lone atrial fibrillation. Mol Med Rep.
7:767–774. 2013.PubMed/NCBI
|
|
20
|
Iwasaki YK, Nishida K, Kato T and Nattel
S: Atrial fibrillation pathophysiology: Implications for
management. Circulation. 124:2264–2274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee HL, Chang PC, Chou CC, Wo HT, Chu Y,
Wen MS, Yeh SJ and Wu D: Blunted proarrhythmic effect of nicorandil
in a Langendorff-perfused phase-2 myocardial infarction rabbit
model. Pacing Clin Electrophysiol. 36:142–151. 2013. View Article : Google Scholar
|
|
22
|
Shaw RM and Rudy Y: Electrophysiologic
effects of acute myocardial ischemia: A theoretical study of
altered cell excitability and action potential duration. Cardiovasc
Res. 35:256–272. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang X, Wang X, Gu Y, Wang T and Huang C:
Wenxin Keli attenuates ischemia-induced ventricular arrhythmias in
rats: Involvement of L-type calcium and transient outward potassium
currents. Mol Med Rep. 7:519–524. 2013.
|
|
24
|
Zhang H, Wu S, Huang C and Li X: Long-term
treatment of spontaneously hypertensive rats with losartan and
molecular basis of modulating Ito of ventricular myocytes. Mol Med
Rep. 9:1959–1967. 2014.PubMed/NCBI
|
|
25
|
Nattel S, Burstein B and Dobrev D: Atrial
remodeling and atrial fibrillation: Mechanisms and implications.
Circ Arrhythm Electrophysio. 1:62–73. 2008. View Article : Google Scholar
|
|
26
|
Shi S, Liu T, Li Y, Qin M, Tang Y, Shen
JY, Liang J, Yang B and Huang C: Chronic N-methyl-D-aspartate
receptor activation induces cardiac electrical remodeling and
increases susceptibility to ventricular arrhythmias. Pacing Clin
Electrophysiol. 37:1367–1377. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Q, Deng C, Rao F, Modi RM, Zhu J,
Liu X, Mai L, Tan H, Yu X, Lin Q, et al: Silencing of desmoplakin
decreases connexin43/Nav1.5 expression and sodium current in HL-1
cardiomyocytes. Mol Med Rep. 8:780–786. 2013.PubMed/NCBI
|
|
28
|
Yan Y, Huang J, Ding F, Mei J, Zhu J, Liu
H and Sun K: Aquaporin 1 plays an important role in myocardial
edema caused by cardiopulmonary bypass surgery in goat. Int J Mol
Med. 31:637–643. 2013.PubMed/NCBI
|
|
29
|
Valderrábano M: Influence of anisotropic
conduction properties in the propagation of the cardiac action
potential. Prog Biophys MolBiol. 94:144–168. 2007. View Article : Google Scholar
|
|
30
|
Mommersteeg MT, Christoffels VM, Anderson
RH and Moorman AF: Atrial fibrillation: A developmental point of
view. Heart Rhythm. 6:1818–1824. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hong K and Xiong Q: Genetic basis of
atrial fibrillation. Curr Opin Cardiol. 29:220–226. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Searcy RD, Vincent EB, Liberatore CM and
Yutzey KE: A GATA-dependent nkx-2.5 regulatory element activates
early cardiac gene expression in transgenic mice. Development.
125:4461–4470. 1998.PubMed/NCBI
|
|
33
|
Mahida S: Transcription factors and atrial
fibrillation. Cardiovasc Res. 101:194–202. 2014. View Article : Google Scholar
|
|
34
|
Gutierrez-Roelens I, De Roy L, Ovaert C,
Sluysmans T, Devriendt K, Brunner HG and Vikkula M: A novel
CSX/NKX2-5 mutation causes autosomal-dominant AV block: Are atrial
fibrillation and syncopes part of the phenotype? Eur J Hum Genet.
14:1313–1316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moskowitz IP, Kim JB, Moore ML, Wolf CM,
Peterson MA, Shendure J, Nobrega MA, Yokota Y, Berul C, Izumo S, et
al: A molecular pathway including Id2, Tbx5, and Nkx2-5 required
for cardiac conduction system development. Cell. 129:1365–1376.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen B, Zhong L, Roush SF, Pentassuglia L,
Peng X, Samaras S, Davidson JM, Sawyer DB and Lim CC: Disruption of
a GATA4/Ankrd1 signaling axis in cardiomyocytes leads to sarcomere
disarray: Implications for anthracycline cardiomyopathy. PLoS One.
7:e357432012. View Article : Google Scholar : PubMed/NCBI
|