Introduction
Ischemia-reperfusion (I/R) injury-associated
diseases, including stroke, cardiovascular disease and acute renal
failure are among the leading causes of mortality or disability
worldwide (1,2). I/R injury is a complex
pathophysiological process (3) in
which a variety of factors are important, including the production
of reactive oxygen species (ROS), calcium overload and
mitochondrial dysfunction (3,4).
Increasing evidence indicates that I/R injury induces the
production of numerous cytokines and chemokines, which are key for
I/R-induced injury or cell death (3,5).
Renal I/R injury represents a major cause of acute
renal injury (ARI) (6). In ARI,
macrophages, immune cells that are widely distributed in tissues
and organs (7), rapidly enter the
site of injury and produce effector molecules, which initiate the
inflammatory response and recruit other immune cells (8). Accumulating evidence suggests that
inflammatory mediators produced by macrophages are critical for the
pathogenesis of I/R injury in ARI (8–10).
High levels of pro-inflammatory cytokines, including inducible
nitric oxide synthase (iNOS), interleukin (IL)-1β, IL-6 and tumor
necrosis factor-α (TNF-α) are present in ARI patients (10,11).
Notably, gene polymorphisms and the levels of certain cytokines may
have predictive value in clinical ARI (11,12).
Furthermore, inhibition of macrophage migration to the site of
injury or cytokine production are protective against ARI (9).
Macrophages are polarized into various distinct
populations according to the specific microenvironment (7,8).
Following stimulation by lipopolysaccharide (LPS) and interferon-γ
(IFN-γ), macrophages differentiate into the classically activated
pro-inflammatory (M1) subtype, which upregulate chemokine receptor
7 and produce iNOS, TNF-α and other pro-inflammatory cytokines
involved in tissue injury (13–16).
Conversely, following stimulation with IL-4 and IL-13, macrophages
differentiate into the alternatively activated (M2) subtype, which
are responsible for anti-inflammatory cytokine production and
tissue repair. M2 macrophages are characterized by high expression
of cluster of differentiation 206 (CD206; the mannose receptor) and
production of chitinase 3-like 3, arginase-1 and transforming
growth factor-β (13–16). In ARI, M1 and M2 macrophage
phenotypes are significant in kidney injury and repair (17): M1 macrophages contribute to I/R
injury, while M2 macrophages alleviate I/R injury and promote
tissue repair (17–19). Collectively, these results indicate
that regulation of the balance of M1 and M2 macrophages may
represent a promising therapeutic strategy for the treatment of
ARI.
Currently, no effective treatments for ARI are
available. The 3-hydroxy-3-methylglutaryl-coenzyme reductase
inhibitors (statins), including atorvastatin, are commonly used to
treat high cholesterol, and additionally reduce the risk of stroke
and other cardiovascular complications of type 2 diabetes (20). Recent studies have suggested that
atorvastatin may exert a beneficial effect in kidney-associated
diseases, including ARI (21,22).
Although the underlying molecular mechanisms remain to be fully
elucidated, atorvastatin has been demonstrated to reduce
LPS-induced upregulation of its receptor, Toll-like receptor 4
(23). In addition, our previous
study in an experimental rat I/R model suggested that atorvastatin
attenuates renal injury partially via its anti-inflammatory effects
(24). However, the direct
anti-inflammatory effects of atorvastatin in I/R-induced
macrophages remain unclear. In the present study, oxygen-glucose
deprivation (OGD)/reperfusion-stimulated RAW264.7 murine
macrophages were used as a model of I/R injury to investigate the
anti-inflammatory effects of atorvastatin, in order to develop
novel therapies for treatment of I/R associated disorders.
Materials and methods
Antibodies and chemicals
The primary antibodies raised in rabbits recognizing
CD206 (catalog no., sc-48758), PPARγ (catalog no., sc-7196) and
GAPDH (catalog no., sc-25778), and the secondary antibodies,
CruzFluor™ 488-conjugated goat anti-rabbit IgG (catalog no.,
sc-362262) and CruzFluor™ 594-conjugated goat anti-rabbit IgG
(catalog no., sc-362282) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Rabbit antibodies against
IFN-γ (catalog no., ab198801), iNOS (catalog no., ab15323) and
TNF-α (catalog no., ab6671) were purchased from Abcam (Cambridge,
MA, USA). Chemicals were obtained from Sigma-Aldrich (St. Louis,
MO, USA) unless indicated otherwise.
Cell culture
RAW264.7 murine macrophages were obtained from the
American Type Cell Culture Collection (Manassas, VA, USA) and
maintained in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and 1% penicillin/streptomycin
at 37°C in a humidified 5% CO2 atmosphere.
Overexpression/knockdown of PPARγ
The pBabe-puro-PPARγ expression vector was
constructed as described previously (25). Control and PPARγ expression vectors
were packaged into retroviruses by transient transfection of
ecotropic packaging cells (Phoenix™; Allele Biotechnology, San
Diego, CA, USA). RAW264.7 macrophages were infected with equal
titers of recombinant retrovirus at 50% confluence and stable cell
lines were selected with 2 µg/ml puromycin. For knockdown of PPARγ
expression in RAW264.7 cells, the retroviral vector-mediated short
hairpin RNA against PPARγ
(5′-TAATGTGGAGTAGAAATGCTGTTGATATCCGCAGCATTTCTACTCCACATTA-3′) was
cloned into pRNAT-H1.1/adeno vectors (catalog no., SD1213;
GenScript, New Brunswick, NJ, USA) and recombinant lentiviruses
were produced by cotransfecting 293T cells with the lentivirus
expression plasmid and packaging plasmids (psPAX2, catalog no.,
12260; pMD2.G, catalog no., 12259, both Addgene, Cambridge, MA,
USA) using the calcium phosphate method (26). Cells with stable knockdown of
expression were acquired following transfection of the plasmid into
cells and selection of stable cells with 1.5 µg/ml G418.
OGD/reperfusion
OGD/reperfusion was conducted as described
previously (27). Briefly, to
achieve OGD, cells were incubated in a humidified anaerobic chamber
(Reming Bioinstruments Co., Redfield, NY, USA) with an atmosphere
of 5% CO2 and 95% N2 for 60 min. The culture medium was replaced
three times (every 20 min each time) with a glucose-free, balanced
salt solution containing 116 mmol/l NaCl, 1.8 mmol/l
CaCl2, 0.8 mmol/l MgSO4, 5.4 mmol/l KCl, 1 mmol/l
NaH2PO4, 14.7 mmol/l NaHCO3 and 0.1 mol/L4-
(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.4). For
reoxygenation, OGD cells were incubated with medium containing 5.5
mg/ml glucose in an atmosphere of 5% CO2 and 95% air at 37°C for 60
min. Control cells were incubated in a normoxic incubator in medium
supplemented with 5.5 mmol/l glucose at 37°C for 120 min.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The mRNA expression levels of iNOS, CD206, PPARγ,
TNF-α and IFN-γ were detected by RT-qPCR. Briefly, total RNA was
extracted using RNAiso Plus (Takara Bio, Inc., Otsu, Japan)
according to the manufacturer's instructions and cDNA was
synthesized using a Bio Reverse Transcription kit (Takara Bio,
Inc.). RT-qPCR was performed using the SYBR Green kit of the Bio
Reaction System according to the manufacturer's instructions. The
primer sequences were as follows: Sense, 5′-CCAAGAACGTGTTCACCATG-3′
and anti-sense, 5′-GATGTCCAGGAAGTAGGTGAGG-3′ for iNOS; sense,
5′-CTAAGCCAAGGGGCAACC-3′ and anti-sense, 5′-GAACAGCGACCGGAATCAC-3′
for CD206; sense, 5′-CCTCCCTGATGAATAAAGATGG-3′ and anti-sense,
5′-GCAAACTCAAACTTAGGCTCCA-3′ for PPARγ; sense,
5′-CGTGGAACTGGCAGAAGAGG-3′ and anti-sense,
5′-AGACAGAAGAGCGTGGTGGC-3′ for TNF-α; sense,
5′-AGCAACAACATAAGCGTCAT-3′ and anti-sense,
5′-CCTCAAACTTGGCAATACTCA-3′ for IFN-γ; and sense,
5′-GCCATGTACGTAGCCATCCA-3′ and anti-sense,
5′-GAACCGCTCATTGCCGATAG-3′ for β-actin. Thermal cycling parameters
for the amplification were as follows: A denaturation step at 94°C
for 2 min, followed by 40 cycles at 95°C for 20 sec, 58°C for 20
sec and 72°C for 20 sec. Relative expression levels of target genes
were calculated with Mx3000P (Agilent Technologies, Inc., Santa
Clara, CA, USA) according to the expression of 2-∆∆Cq
(28).
Administration of atorvastatin and
grouping of cells
To evaluate the effect of atorvastatin on the PPARγ
associated pathways, PPARγ knockdown RAW264.7 cells were
administrated with atorvastin as follows: i) CTRL group, control
group, normal RAW264.7 cells; ii) Atorvastatin group, RAW264.7
cells incubated with 10 µM atorvastatin (Haoran Biological
Technolgy Co., Ltd., Shanghai, China) for 24 hat room temperature;
iii) Model group, RAW264.7 cells underwent OGD/R treatment; iv)
Model + atorvastatin group, OGD/R treated RAW264.7 cells incubated
with 10 µM atorvastatin for 24 h at room temperature. Subsequently,
the expression of PPARγ associated indicators was detected using
RT-qPCR, western blotting and immunofluorescence staining.
Western blot analysis
Cellular proteins were extracted using the Total
Protein Extraction kit according to the manufacturer's instructions
(Wanleibio, Shenyang, China) and analyzed by western blotting.
GAPDH was used as internal reference protein. Briefly, 40 µg
denatured protein in 20 µl solution samples were loaded onto a 13%
SDS-PAGE gel, electrophoresed for 2.5 h at 80 V and transferred to
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). Following blocking with non-fat milk, membranes were
incubated overnight at 4°C with antibodies recognizing iNOS
(1:1,000), CD206 (1:200), PPARγ (1:1,000), TNF-α (1:100), IFN-γ
(1:1,000) and GAPDH (1:5,000), and incubated with the anti-rabbit
horseradish peroxidase-conjugated secondary antibody (1:20,000,
diluted in 55 (M/V) skim milk powder). Protein detection was
performed using Enhanced Chemilumiscence Advance Western Blotting
Detection reagents (GE Healthcare Life Sciences, Chalfont, UK).
Immunofluorescence staining
Cells were fixed with 4% paraformaldehyde in
phosphate-buffered saline, permeabilized with 0.5% Triton X-100 and
then blocked with 5% normal goat serum. The cells were then
incubated with antibodies recognizing iNOS, CD206, PPARγ, TNF-α and
IFN-γ in 1% bovine serum albumin (Gibco; Thermo Fisher Scientific,
Inc.) at 4°C overnight. The bound antibodies were detected by the
secondary antibodies conjugated to CruzFluor 488 or CruzFluor 594.
Cell nuclei were indicated by staining with DAPI for 15 min. Images
were captured on a Zeiss fluorescence microscope (ICM-405; Carl
Zeiss AG, Oberkochen, Germany) at magnification, ×400.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistically significant differences between the groups were
identified by one-way analysis of variance followed by paired
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using SPSS version 19.0 (IBM SPSS, Armonk, NY, USA).
Results
Involvement of PPARγ in
OGD/reperfusion-induced alteration of iNOS, CD206, TNF-α and IFN-γ
mRNA expression levels
To investigate whether PPARγ is involved in
OGD/reperfusion-induced expression of inflammatory mediators in
RAW264.7 murine macrophages, knockdown and overexpression of PPARγ
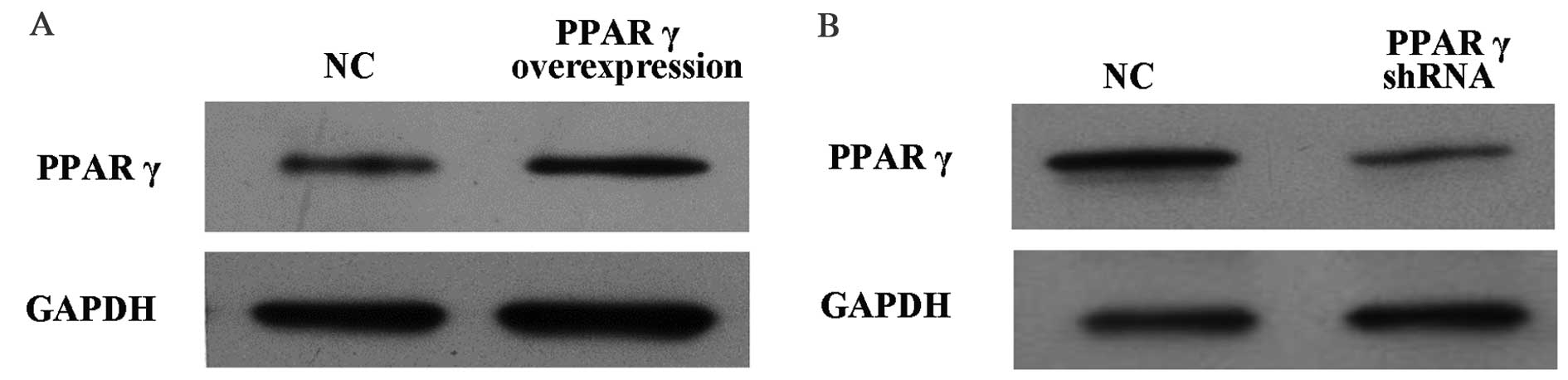
in RAW264.7 cells was performed. As presented in Fig. 1A, PPARγ protein expression levels
in RAW264.7 cells overexpressing PPARγ were increased compared with
cells that received the vehicle control. Conversely, as presented
in Fig. 1B, PPARγ protein
expression levels in RAW264.7 cells with PPARγ knocked down were
decreased compared with cells that received the vehicle
control.
OGD/reperfusion-stimulated RAW264.7 murine
macrophages were used as a model of I/R injury to examine the
effect of OGD on the expression of iNOS, TNF-α, IFN-γ and CD206. As
presented in Fig. 2, exposure of
RAW264.7 macrophages to 1 h of OGD followed by 1 h of reperfusion
induced the upregulation of iNOS, TNF-α and IFN-γ, as well as the
downregulation of CD206 at the mRNA level. Overexpression of PPARγ
significantly attenuated OGD/reperfusion-induced expression of
iNOS, TNF-α and IFN-γ at the mRNA levels (Fig. 2A-C; for iNOS, Model+PPARγ group vs.
Model group, P<0.001, Model+PPARγ-shRNA group, P<0.001; for
TNF-α, Model+PPARγ group vs. Model group, P<0.001,
Model+PPARγ-shRNA group, P<0.001; for IFN-γ, Model+PPARγ group
vs. Model group, P<0.001, Model+PPARγ-shRNA group, P<0.001).
Furthermore, overexpression of PPARγ significantly increased the
expression of CD206, a marker of M2 macrophages (Fig. 2D; Model+PPARγ group vs. Model
group, P=0.005, Model+PPARγ-shRNA group, P<0.001).
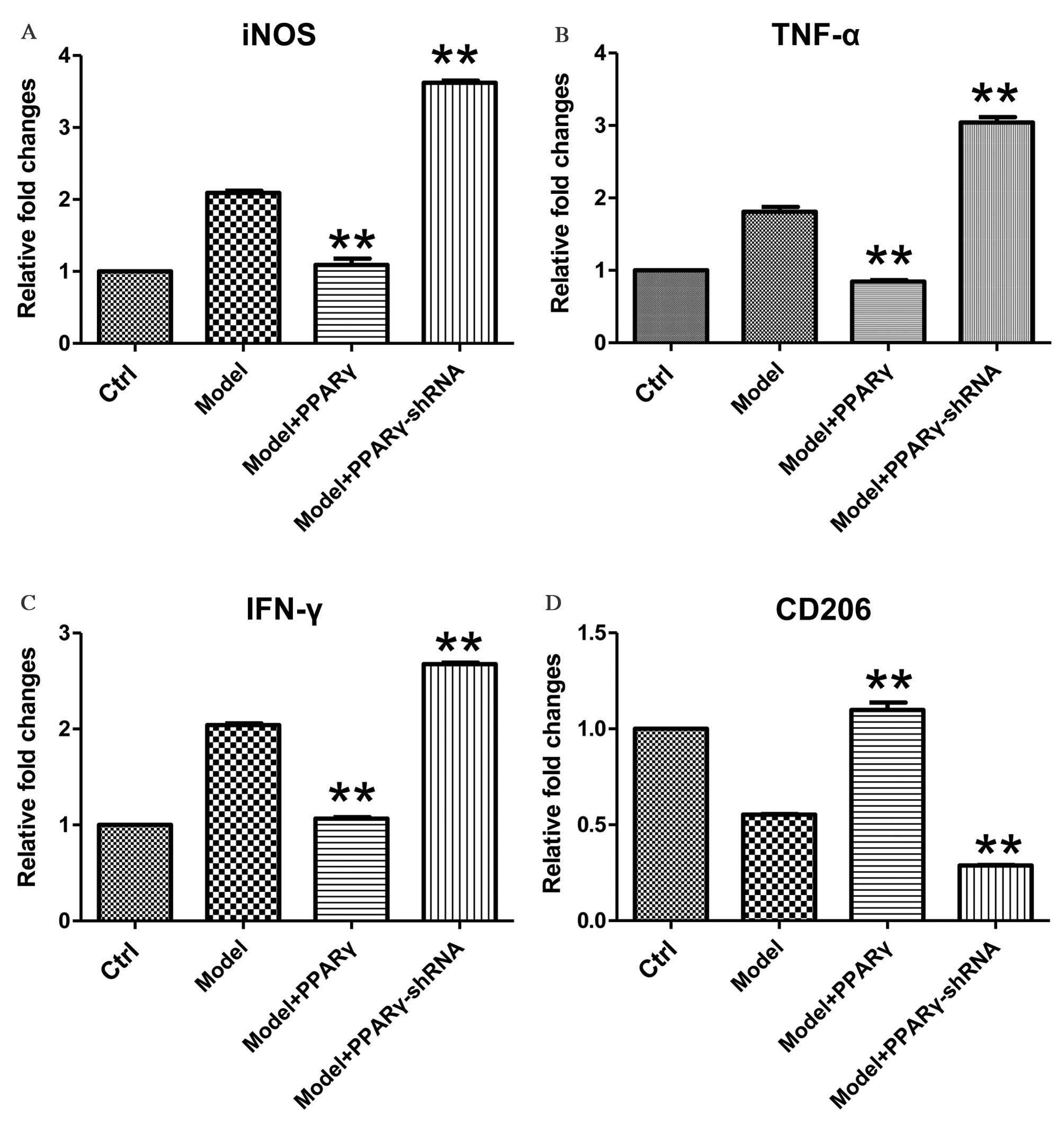 | Figure 2.Effects of overexpression and
knockdown of PPARγ on OGD/reperfusion-induced expression of (A)
iNOS, (B) TNF-α, (C) IFN-γ and (D) CD206 at the mRNA level in
RAW264.7 murine macrophages. mRNA expression levels were analyzed
by reverse transcription-quantitative polymerase chain reaction in
cells cultured under normal conditions (control), and in model
(OGD/R treated), PPARγ overexpressing and PPARγ knockdown cells
that had undergone OGD/reperfusion. The mRNA expression levels of
iNOS, TNF-α, IFN-γ and CD206 were determined. PPARγ overexpression
significantly decreased the expression of iNOS, TNF-α and IFN-γ,
and significantly increased the expression of CD206 at the mRNA
level compared with model cells. PPARγ knockdown had significant
and opposite effects. Data are presented as the mean ± standard
deviation (n=3). **P<0.01 vs. model. PPARγ; peroxisome
proliferator activated receptor-γ; OGD, oxygen-glucose deprivation;
iNOS, inducible nitric oxide synthase; TNF-α, tumor necrosis
factor-α; IFN-γ, interferon-γ; CD206, cluster of differentiation
206; Ctrl, control; shRNA, short hairpin RNA. |
By contrast, knockdown of PPARγ expression
significantly increased OGD-induced expression of iNOS, TNF-α and
IFN-γ mRNA levels (Fig. 2A-C;
P=0.008, P=0.009, P=0.006 for iNOS, TNF-α and IFN-γ, respectively),
and significantly decreased OGD-induced downregulation of CD206
mRNA expression levels (Fig. 2D;
P=0.009, P=0.007, P=0.007 for iNOS, TNF-α and IFN-γ, respectively).
Together, these data demonstrate that PPARγ is important for the
regulation of OGD/reperfusion-induced effects on the mRNA
expression levels of iNOS, TNF-α, IFN-γ and CD206.
Involvement of PPARγ in
OGD/reperfusion-induced alterations of iNOS, CD206, TNF-α and IFN-γ
protein expression levels
To evaluate whether PPARγ is essential for
OGD/reperfusion-induced alterations of iNOS, CD206, TNF-α and IFN-γ
protein expression levels, western blotting was performed.
OGD/reperfusion induced the upregulation of iNOS, TNF-α and IFN-γ,
and the downregulation of CD206, at the protein level (Fig. 3). Notably, overexpression of PPARγ
in RAW264.7 cells abrogated the OGD/reperfusion-induced expression
of iNOS, TNF-α and IFN-γ. In addition, western blotting indicated
that overexpression of PPARγ reversed the OGD/reperfusion-induced
downregulation of CD206 expression (Fig. 3). By contrast, knockdown of PPARγ
expression increased OGD-induced expression of iNOS, TNF-α and
IFN-γ protein, and decreased the OGD-induced downregulated
expression of CD206 protein (Fig.
3).
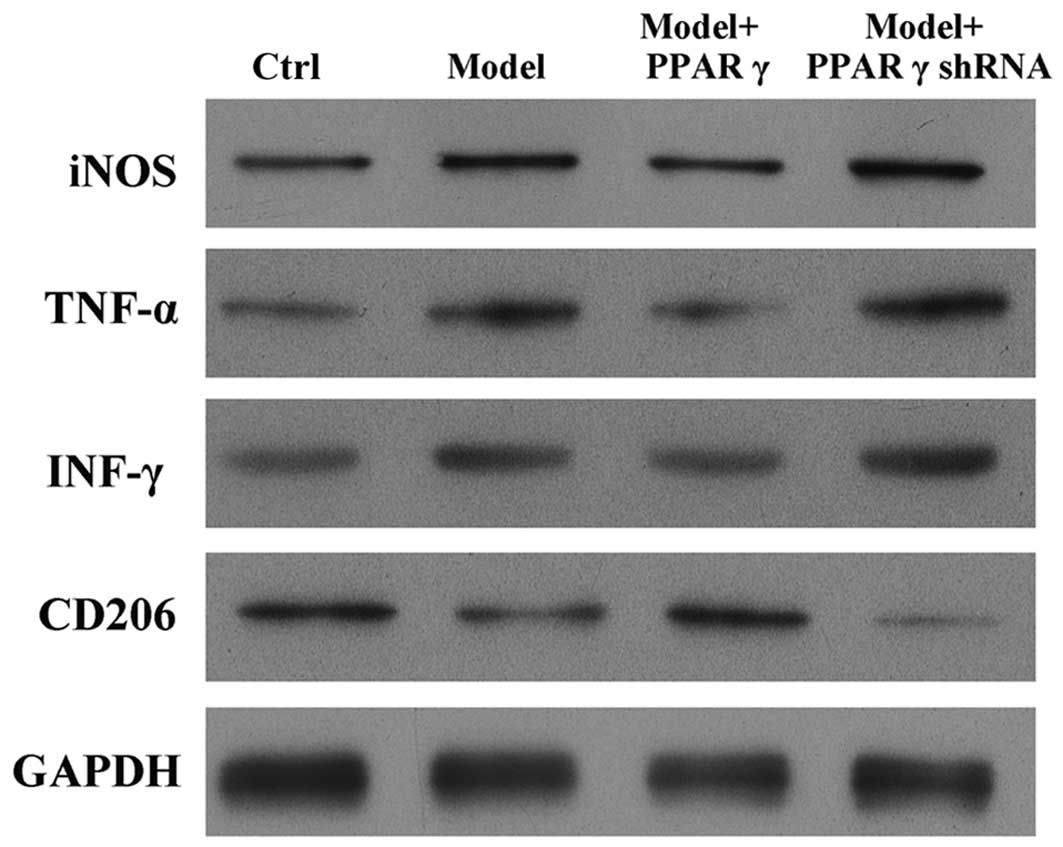 | Figure 3.Effects of overexpression and
knockdown of PPARγ on OGD/reperfusion-induced expression of iNOS,
TNF-α, IFN-γ and CD206 at the protein level in RAW264.7 murine
macrophages. Protein expression was detected by western blotting in
cells cultured under normal conditions (control), and in model
(OGD/R treated), PPARγ overexpressing and PPARγ knockdown cells
that had undergone OGD/reperfusion. PPARγ overexpression decreased
the expression of iNOS, TNF-α and IFN-γ, and increased the
expression of CD206 at the protein level compared with model cells.
PPARγ knockdown had the opposite effects. GAPDH served as a loading
control. PPARγ; peroxisome proliferator activated receptor-γ; OGD,
oxygen-glucose deprivation; iNOS, inducible nitric oxide synthase;
TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ; CD206, cluster
of differentiation 206; Ctrl, control; shRNA, short hairpin
RNA. |
Furthermore, immunofluorescence was performed to
evaluate the effect of PPARγ on OGD/reperfusion-induced alterations
in iNOS, CD206, TNF-α and IFN-γ expression levels. As presented in
Fig. 4, overexpression of PPARγ
reversed OGD/reperfusion-induced expression of iNOS, TNF-α and
IFN-γ, and downregulation of CD206. By contrast, knockdown of PPARγ
expression enhanced OGD/reperfusion-induced upregulation of iNOS,
TNF-α and IFN-γ, and downregulated the expression of CD206.
Collectively, these data suggest that overexpression of PPARγ
reversed OGD/reperfusion-induced effects on iNOS, CD206, TNF-α and
IFN-γ. Conversely, knockdown of PPARγ expression enhanced the
OGD/reperfusion-induced effects on iNOS, CD206, TNF-α and
IFN-γ.
 | Figure 4.Immunofluorescence staining detected
effects of overexpression and knockdown of PPARγ on
OGD/reperfusion-induced expression of (A) iNOS, (B) TNF-α, (C)
IFN-γ and (D) CD206 in RAW264.7 murine macrophages. Cells were
cultured under normal conditions (control), and in model (OGD/R
treated), PPARγ overexpressing and PPARγ knockdown cells that had
undergone OGD/reperfusion. Cells were stained with antibodies
recognizing iNOS, TNF-α, IFN-γ and CD206 and nuclei were stained
with DAPI. Representative images from at least three independent
experiments are presented (magnification, ×400). PPARγ; peroxisome
proliferator activated receptor-γ; OGD, oxygen-glucose deprivation;
iNOS, inducible nitric oxide synthase; TNF-α, tumor necrosis
factor-α; IFN-γ, interferon-γ; CD206, cluster of differentiation
206; Ctrl, control; shRNA, short hairpin RNA. |
Effects of atorvastatin on the mRNA
expression levels of iNOS and CD206 in cells that had undergone
OGD/reperfusion
A previous study suggested that PPARγ is a target of
atorvastatin (29). In the present
study, PPARγ was demonstrated to be important for
OGD/reperfusion-induced alterations in the expression of iNOS,
TNF-α, IFN-γ and CD206. Thus, it was investigated whether
atorvastatin may regulate the OGD/reperfusion-induced alterations
in PPARγ, iNOS and CD206 expression. OGD/reperfusion was
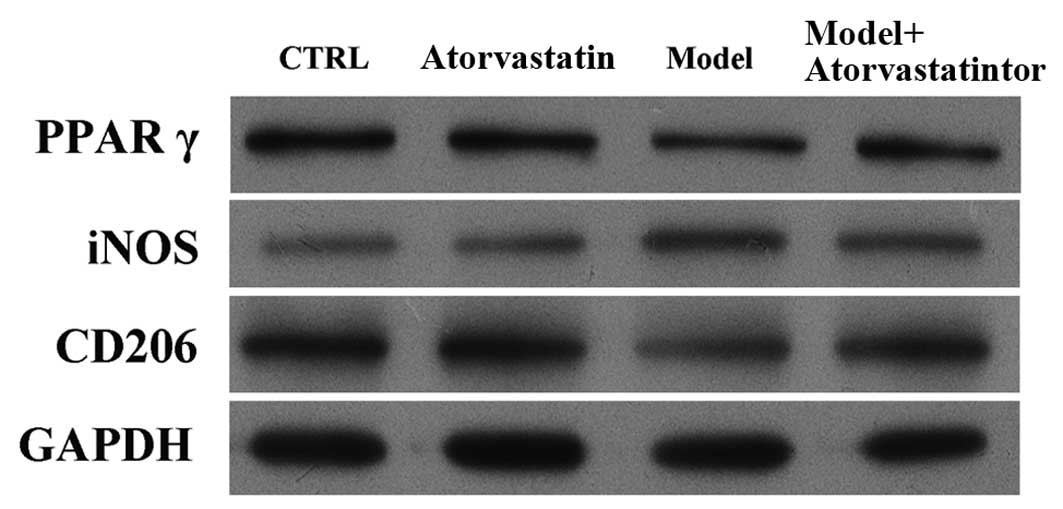
demonstrated to downregulate PPARγ mRNA expression levels;
atorvastatin reversed this effect (Fig. 5A), and the difference between the
model group and the model + atorvastatin group was significant
(P<0.001). In addition, atorvastatin significantly inhibited
OGD/reperfusion-induced upregulation of the mRNA expression levels
of iNOS (Fig. 5B; P<0.001) and
reversed OGD/reperfusion-induced downregulation of the mRNA
expression levels of CD206 (Fig.
5C; P=0.007).
Effects of atorvastatin on the protein
expression of iNOS and CD206 in cells that had undergone
OGD/reperfusion
It was investigated whether atorvastatin may
regulate the OGD/reperfusion-induced alterations in iNOS and CD206
protein expression. Consistent with the results of the RT-qPCR,
western blotting indicated that atorvastatin reversed
OGD/reperfusion-induced downregulation of PPARγ expression at the
protein level, blocked OGD/reperfusion-induced upregulation of iNOS
protein expression and reversed OGD/reperfusion-induced
downregulation of CD206 protein expression (Fig. 6). These results were confirmed by
immunofluorescence staining (Fig.
7). Taken together, these results suggest that atorvastatin is
an important regulator of OGD/reperfusion-induced alterations in
iNOS and CD206 expression at the mRNA and protein levels, and that
the underlying mechanism may involve PPARγ.
Discussion
The results of the present study demonstrate that
overexpression of PPARγ attenuates the OGD/reperfusion-induced
upregulation of iNOS, TNF-α and IFN-γ expression, and the
downregulation of CD206 expression in RAW264.7 murine macrophages.
Conversely, knockdown of PPARγ expression enhanced
OGD/reperfusion-induced alterations in iNOS, TNF-α, IFN-γ and CD206
expression. Notably, atorvastatin reversed OGD/reperfusion-induced
alterations in iNOS and CD206 expression at the mRNA and protein
levels; the underlying mechanism may be via the regulation of PPARγ
expression.
Macrophages are dynamically regulated by their
microenvironment, and may differentiate into the pro-inflammatory
M1 subtype or the anti-inflammatory M2 subtype depending on the
stimuli (16). These two types
play important roles in the production of cytokines and chemokines
and are key for the regulation of I/R injury in various types of
disease, including stroke (30),
ARI (17) and cardiovascular
disease (31). PPARγ belongs to
the nuclear receptor superfamily, and is central to the regulation
of lipid and glucose metabolism, adipogenesis, glucose homeostasis,
cellular differentiation, apoptosis and inflammation (32,33).
Notably, PPARγ is important for regulating macrophage phenotype and
the production of cytokines (34,35).
However, the roles of I/R injury in the induction of cytokine
production by macrophages and PPARγ in regulating I/R-induced
cytokine production and macrophage phenotype remain to be fully
elucidated. In the present study, OGD/reperfusion-stimulated
RAW264.7 murine macrophages were used as a model of I/R injury.
OGD/reperfusion upregulated the expression of iNOS, TNF-α, IFN-γ
and downregulated the expression of CD206, a typical M2-type
macrophage marker. Overexpression of PPARγ abrogated the
OGD/reperfusion-induced alterations of iNOS, TNF-α, IFN-γ and CD206
expression levels. Conversely, knockdown of PPARγ expression
enhanced the OGD/reperfusion-induced alterations of iNOS, TNF-α,
IFN-γ and CD206 expression levels. These results suggest that PPARγ
may be essential for regulating OGD/reperfusion-induced release of
pro-inflammatory cytokines in RAW264.7 cells. Notably, PPARγ
regulates the OGD/reperfusion-induced alterations in the expression
of the M1 macrophage marker, iNOS and the M2 macrophage marker,
CD206. However, whether OGD/reperfusion induced the phenotype
changes of macrophages and whether PPARγ has a role in macrophage
phenotype regulation requires further investigation.
Recent studies have suggested that atorvastatin may
have a beneficial effect in kidney-associated diseases, including
ARI (21,22). However, the protective effects of
atorvastatin in I/R injury-associated diseases and the underlying
mechanisms remain to be fully elucidated. Our previous study in an
experimental rat I/R model indicated that atorvastatin attenuates
renal injury partially via its anti-inflammatory effects (24). However, the effect of atorvastatin
on the OGD/reperfusion-induced production of inflammatory
cytokines, and the underlying mechanism, remain unclear. It is
well-established that atorvastatin activates PPARγ in various cell
types. Grip et al (36)
observed that atorvastatin activates PPAR-γ and attenuates the
inflammatory response in human monocytes. Additionally, Planavila
et al (37) demonstrated
that atorvastatin improved peroxisome proliferator-activated
receptor signaling in cardiac hypertrophy. In the present study,
PPARγ was demonstrated to regulate OGD/reperfusion-induced
alterations in the expression levels of iNOS, CD206, TNFα and IFNγ.
Thus, the roles and underlying molecular mechanisms of atorvastatin
in the regulation of OGD/reperfusion-induced alterations in iNOS
and CD206 expression were investigated. OGD/reperfusion was
revealed to significantly downregulate PPARγ expression at the mRNA
and protein levels, an effect that was reversed by atorvastatin.
Notably, atorvastatin significantly inhibited the
OGD/reperfusion-induced upregulation of iNOS expression and
downregulation of CD206 expression. Together these results
demonstrated that atorvastatin regulates OGD/reperfusion-induced
inflammation, and that the underlying mechanisms may involve PPARγ.
In conclusion, the findings of the present study may reveal a novel
mechanism underlying the protective effects of atorvastatin in I/R
injury-associated diseases by the targeting of
OGD/reperfusion-induced stimulation of macrophages. The present
study is a supplement to the understanding of the mechanism driving
OGD/R injuries and also provides potential for the development of
therapies for the treatment of OGD/reperfusion-induced inflammation
in the clinic.
Acknowledgements
The present study was supported by Guangzhou Medical
Key Subject Construction Project (grant no., XM203190,
2013–2015).
References
|
1
|
Thom T, Haase N, Rosamond W, Howard VJ,
Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O'Donnell C, Kittner S,
et al: Heart disease and stroke statistics-2006 update: A report
from the American heart association statistics committee and stroke
statistics subcommittee. Circulation. 113:e85–e151. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ricci Z, Cruz D and Ronco C: The RIFLE
criteria and mortality in acute kidney injury: A systematic review.
Kidney Int. 73:538–546. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kalogeris T, Baines CP, Krenz M and
Korthuis RJ: Cell biology of ischemia/reperfusion injury. Int Rev
Cell Mol Biol. 298:229–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Groot H and Rauen U:
Ischemia-reperfusion injury: Processes in pathogenetic networks: A
review. Transplant Proc. 39:481–484. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Devarajan P: Update on mechanisms of
ischemic acute kidney injury. J Am Soc Nephrol. 17:1503–1520. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bellomo R, Kellum JA and Ronco C: Acute
kidney injury. Lancet. 380:756–766. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Varol C, Mildner A and Jung S:
Macrophages: Development and tissue specialization. Annu Rev
Immunol. 33:643–675. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao Q, Harris DC and Wang Y: Macrophages
in kidney injury, inflammation, and fibrosis. Physiology
(Bethesda). 30:183–194. 2015.PubMed/NCBI
|
|
9
|
Bonventre JV and Zuk A: Ischemic acute
renal failure: An inflammatory disease? Kidney Int. 66:480–485.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bonventre JV and Yang L: Cellular
pathophysiology of ischemic acute kidney injury. J Clin Invest.
121:4210–4221. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Simmons EM, Himmelfarb J, Sezer MT,
Chertow GM, Mehta RL, Paganini EP, Soroko S, Freedman S, Becker K,
Spratt D, et al: Plasma cytokine levels predict mortality in
patients with acute renal failure. Kidney Int. 65:1357–1365. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jaber BL, Rao M, Guo D, Balakrishnan VS,
Perianayagam MC, Freeman RB and Pereira BJ: Cytokine gene promoter
polymorphisms and mortality in acute renal failure. Cytokine.
25:212–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mantovani A, Sica A, Sozzani S, Allavena
P, Vecchi A and Locati M: The chemokine system in diverse forms of
macrophage activation and polarization. Trends Immunol. 25:677–686.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawanishi N, Yano H, Yokogawa Y and Suzuki
K: Exercise training inhibits inflammation in adipose tissue via
both suppression of macrophage infiltration and acceleration of
phenotypic switching from M1 to M2 macrophages in
high-fat-diet-induced obese mice. Exerc Immunol Rev. 16:105–118.
2010.PubMed/NCBI
|
|
15
|
Mantovani A, Sica A and Locati M: New
vistas on macrophage differentiation and activation. Eur J Immunol.
37:14–16. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goerdt S, Politz O, Schledzewski K, Birk
R, Gratchev A, Guillot P, Hakiy N, Klemke CD, Dippel E, Kodelja V
and Orfanos CE: Alternative versus classical activation of
macrophages. Pathobiology. 67:222–226. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee S, Huen S, Nishio H, Nishio S, Lee HK,
Choi BS, Ruhrberg C and Cantley LG: Distinct macrophage phenotypes
contribute to kidney injury and repair. J Am Soc Nephrol.
22:317–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Jiang ZP, Su N, Fan JJ, Ruan YP,
Peng WX, Li YF and Yu XQ: The role of peritoneal alternatively
activated macrophages in the process of peritoneal fibrosis related
to peritoneal dialysis. Int J Mol Sci. 14:10369–10382. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jo SK, Sung SA, Cho WY, Go KJ and Kim HK:
Macrophages contribute to the initiation of ischaemic acute renal
failure in rats. Nephrol Dial Transplant. 21:1231–1239. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meier CR, Schlienger RG, Kraenzlin ME,
Schlegel B and Jick H: HMG-CoA reductase inhibitors and the risk of
fractures. JAMA. 283:3205–3210. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lewicki M, Ng I and Schneider AG: HMG CoA
reductase inhibitors (statins) for preventing acute kidney injury
after surgical procedures requiring cardiac bypass. Cochrane
Database Syst Rev. CD0104802015.PubMed/NCBI
|
|
22
|
Zamorskiĭ II and Zeleniuk VG:
Renoprotective effects of statins under the conditions of acute
renal failure, caused by rhabdomyolysis. Biofizika. 59:1027–1030.
2014.(In Russian). PubMed/NCBI
|
|
23
|
Wang Y, Zhang MX, Meng X, Liu FQ, Yu GS,
Zhang C, Sun T, Wang XP, Li L, Wang YY, et al: Atorvastatin
suppresses LPS-induced rapid upregulation of Toll-like receptor 4
and its signaling pathway in endothelial cells. Am J Physiol Heart
Circ Physiol. 300:H1743–H1752. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu K, Lei W, Tian J and Li H: Atorvastatin
treatment attenuates renal injury in an experimental model of
ischemia-reperfusion in rats. BMC Nephrol. 15:142014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tontonoz P, Hu E and Spiegelman BM:
Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a
lipid-activated transcription factor. Cell. 79:1147–1156. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen CA and Okayama H: Calcium
phosphate-mediated gene transfer: A highly efficient transfection
system for stably transforming cells with plasmid DNA.
Biotechniques. 6:632–638. 1988.PubMed/NCBI
|
|
27
|
Guo J, Krause DN, Horne J, Weiss JH, Li X
and Duckles SP: Estrogen-receptor-mediated protection of cerebral
endothelial cell viability and mitochondrial function after
ischemic insult in vitro. J Cereb Blood Flow Metab. 30:545–554.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brault M, Ray J, Gomez YH, Mantzoros CS
and Daskalopoulou SS: Statin treatment and new-onset diabetes: A
review of proposed mechanisms. Metabolism. 63:735–745. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu X, Li P, Guo Y, Wang H, Leak RK, Chen
S, Gao Y and Chen J: Microglia/macrophage polarization dynamics
reveal novel mechanism of injury expansion after focal cerebral
ischemia. Stroke. 43:3063–3070. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leitinger N and Schulman IG: Phenotypic
polarization of macrophages in atherosclerosis. Arterioscler Thromb
Vasc Biol. 33:1120–1126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tontonoz P and Spiegelman BM: Fat and
beyond: The diverse biology of PPARgamma. Annu Rev Biochem.
77:289–312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lamers C, Schubert-Zsilavecz M and Merk D:
Therapeutic modulators of peroxisome proliferator-activated
receptors (PPAR): A patent review (2008-present). Expert Opin Ther
Pat. 22:803–841. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lawrence T and Natoli G: Transcriptional
regulation of macrophage polarization: Enabling diversity with
identity. Nat Rev Immunol. 11:750–761. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wahli W and Michalik L: PPARs at the
crossroads of lipid signaling and inflammation. Trends Endocrinol
Metab. 23:351–363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grip O, Janciauskiene S and Lindgren S:
Atorvastatin activates PPAR-γ and attenuates the inflammatory
response in human monocytes. Inflammation Research. 51:58–62. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Planavila A, Laguna JC and Vázquez-Carrera
M: Atorvastatin improves peroxisome proliferator-activated receptor
signaling in cardiac hypertrophy by preventing nuclear factor-κB
activation. Biochimica et Biophysica Acta (BBA)-Molecular and Cell
Biology of Lipids. 1687:76–83. 2005.
|