Introduction
Hypoxia is a common pathophysiological phenomenon,
which has a profound impact on endothelial cell (EC) properties in
numerous pathological angiogenic diseases, including retinopathy of
prematurity, proliferative diabetic retinopathy, retinal vein
occlusion and age-related macular degeneration (1,2).
These diseases are a major cause of blindness worldwide, however,
there remains a lack of effective medical treatment options.
Therefore, understanding the association between hypoxia and
pathological angiogenesis may be important in characterizing the
mechanisms of disease and assist in the development of novel
treatment strategies.
Cysteine-rich 61 (CCN1), the first cloned member of
the CCN family, mediates cell adhesion, stimulates chemotaxis,
augments growth factor-induced DNA synthesis, fosters cell survival
and enhances angiogenesis (3–5).
Previous studies have demonstrated that hypoxic conditions are able
to induce the expression of CCN1 in several types of cell (6–11).
The phosphatidylinositol 3-kinase (PI3K)/Akt pathway is involved in
multiple cellular processes, including cell survival and
differentiation, and it has been demonstrated to be important in
angiogenesis (12). Previous
studies have demonstrated that CCN1 induces monocyte chemotactic
protein 1 through the activation of PI3K/Akt and nuclear factor-κB
signaling in chorioretinal vascular ECs (13). Additionally, a previous study
indicated that CCN1 can enhance the expression of vascular
endothelial growth factor (VEGF) and promote tumor
neovascularization via the PI3K/Akt signaling pathway (14).
However, the specific mechanisms, which are involved
in CCN1-mediated pathological angiogenesis in ECs remain to be
fully elucidated. The present study hypothesized that the
CCN1/PI3K/AKT/VEGF signaling pathway may be associated with
pathological angiogenesis and comprise possible molecular
therapeutic targets. In order to confirm this hypothesis, the
present study investigated the effect of reducing the expression of
CCN1 in hypoxic ECs, and analyzed the molecular mechanisms involved
in pathological angiogenesis.
Materials and methods
Cell culture
HUVECs were purchased from Cell Systems Corporation
(Kirkland, WA, USA) and were cultured in Dulbecco's modified
Eagle's medium (GE Healthcare Life Sciences, Chalfont, UK) with 10%
fetal bovine serum (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) at 37°C in a humidified atmosphere containing 95% air and 5%
CO2, with subconfluent monolayers passaged 3–10 times
prior to treatment.
Hypoxic treatment
Hypoxic exposure was performed using a tightly
sealed molecular incubator chamber (Billups-Rothenberg, Inc., Del
Mar, CA, USA), which was tightly sealed and flushed with a gas
mixture containing 1% O2, 94% N2 and 5%
CO2, as previously described (15,16),
with the cell culture dishes containing 1×105 cells/well
placed in the chamber and incubated at 37°C for 24 h.
The HUVECs were divided into four groups: A normoxia
group; a hypoxia group; a hypoxia-control group, which was
transiently transfected with scramble small interfering (si)RNA;
and a hypoxia-CCN1 siRNA group, which was transiently transfected
with CCN1 siRNA. The HUVECs were transiently transfected with
plasmids (500 ng/µl) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h.
PI3K/Akt inhibition
The PI3K/Akt inhibitor, LY294002, was used in the
present study to determine the effect of inhibiting the PI3K/Akt
pathway on the normoxic and hypoxic HUVECs. The cells were cultured
under normoxic or hypoxic conditions in six-well plates at a
density of 1×105 cells/well as described above, in the
presence of LY294002 (Sigma-Aldrich, St. Louis, MO, USA). The
solution comprised 40 µmol/l dissolved in dimethyl sulfoxide
(DMSO), with a final concentration of DMSO in the cell culture of
0.1%. The cells were pretreated with LY294002 for 30 min prior to
being placed in the incubator for hypoxic exposure. The mRNA and
protein expression levels of CCN1 were then analyzed using RT-qPCR
and western blotting, respectively, following 24 h normoxia or
hypoxia.
Gene knockdown by siRNA
Four pairs of CCN1 siRNA sequences were designed and
synthesized (Shanghai GenePharma Co., Ltd., Shanghai, China), with
one pair selected based on stability and effectiveness. The
sequences were as follows: CCN1 (Cyr61-homo-553) forward,
5′-GGGAAAGUUUCCAGCCCAACUTT-3′ and reverse,
5′-AGUUGGGCUGGAAACUUUCCCTT-3′; CCN1 (Cyr61-homo-789) forward,
5′-GAGGUGGAGUUGACGAGAAACTT-3′ and reverse,
5′-GUUUCUCGUCAACUCCACCUCTT-3′; CCN1 (Cyr61-homo-1072) forward,
5′-GCAAGAAAUGCAGCAAGACCATT-3′ and reverse,
5′-UGGUCUUGCUGCAUUUCUUGCTT-3′; CCN1 (Cyr61-homo-1268) forward,
5′-GAUGAUCCAGUCCUGCAAAUGTT-3′ and reverse,
5′-CAUUUGCAGGACUGGAUCAUCTT-3′. In addition, a non-silencing siRNA
sequence was selected for use as a negative control (forward
5′-UUCUCCGAACGUGUCACGUTT-3′ and 5′-ACGUGACACGUUCGGAGAATT-3′
reverse). The siRNAs were cloned using a pGPU6/green fluorescent
protein (GFP)/Neomycin resistance screening marker (Neo) siRNA
Expression Vector kit (cat. no. E-07/F-07; Shanghai GenePharma Co.,
Ltd.), according to the manufacturer's protocol, generating the
pGPU6/GFP/Neo-CNN1 siRNA and the pGPU6/GFP/Neo-scramble siRNA
plasmids, which contained Bbs1 and BamH1 restriction
sites. The cells were transfected, according to the manufacturer's
protocol, with the mRNA and protein levels assessed 48 h following
transfection. siRNA was successfully transfected into HUVECs in
six-well culture plates, with each well containing 240 pmol
fluorescent labelled siRNA and 8 µl Lipofectamine® 2000
for 6 h. Transfection efficiency was determined using fluorescence
microscopy (FV1000; Olympus Corp., Tokyo, Japan).
Cell proliferation assay
A Cell Counting Kit-8 (CCK8) assay (Beyotime
Institute of Biotechnology, Jiangsu, China) was used to measure
cell proliferation, according to the manufacturer's protocol.
Briefly, HUVECs were plated in 96-well plates at a density of 2,000
cells/well, and proliferation was measured each day for 4 days
following transfection. A total of 10 µl CCK8 was added to each
well and incubated for 2 h at 37°C. Following incubation, the
samples were vortexed for 10 min and the absorbance of each was
measured in a Sunrise™ microplate reader (Tecan Group, Ltd.,
Männedorf, Switzerland) at 450 nm.
Cellular apoptosis assay
Cellular apoptosis was investigated by flow
cytometry using an Annexin V-Fluorescein Isothiocyanate (FITC)
Apoptosis Detection kit (cat. no. KGA106; Nanjing KeyGen Biotech,
Co., Ltd., Nanjing, China), according to the manufacturer's
protocol. The cells were washed twice in ice-cold
phosphate-buffered saline at pH 7.5 (Zhongshan Jinqiao
Biotechnology Co., Ltd., Beijing, China) and resuspended in 1X
binding buffer (Zhongshan Jinqiao Biotechnology Co., Ltd.) at
1×106 cells/ml. A total of 100 µl cells
(1×105 cells) were gently mixed with 5 µl annexin V-FITC
and 5 µl propidium iodide (PI), and incubated for 15 min in the
dark at room temperature. An additional 400 µl of 1X binding buffer
was added, and cellular apoptosis was detected using a flow
cytometer (FACSCalibur™; BD Biosciences, San Jose, CA, USA). The
apoptotic rates of the cells were calculated as the ratio of early
and late apoptotic cells to the total cells (17).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the HUVECs using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and was
reverse-transcribed into cDNA using a reverse transcription kit
(DRR037S; PrimeScript™ RT Reagent kit-Perfect Real-Time; Takara Bio
Inc., Dalian, China) as previously described (18). Primers were designed using Primer
Express software version 2.0 (Life Technologies; Thermo Fisher
Scientific, Inc.) and are presented in Table I. qPCR was performed using SYBR
Green PCR Master mix (Premix Ex Taq™-Perfect Real Time; cat. no.
DRR041S; Takara Bio, Inc.). The PCR mixture contained 10 µl 2X
TaqMan PCR mix, 0.4 µl PCR forward and 0.4 µl PCR reverse primer,
1.0 µl cDNA and 8.2 µl double-distilled H2O with a total
volume of 20 µl and the reaction was performed in an Applied
Biosystems 7300 Real-Time PCR system (Thermo Fisher Scientific,
Inc.). The cycling conditions were as follows: 95°C for 30 sec, 50
cycles of 95°C for 5 sec and 60°C for 31 sec. β-actin was included
in each reaction as an internal control, and the relative gene
expression levels were calculated using the 2−ΔΔCq
method (19).
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Direction | Primer sequence
(5′-3′) | Product length
(bp) |
|---|
| β-actin | Forward |
CGTGGACATCCGCAAAGAC | 200 |
|
| Reverse |
GGAAGGTGGACAGCGAGGC |
|
| VEGF | Forward |
TGCCCACTGAGGAGTCCAAC | 336 |
|
| Reverse |
TGGTTCCCGAAACGCTGAG |
|
| Akt | Forward |
TTGCTTTCAGGGCTGCTCA | 230 |
|
| Reverse |
TCTTGGTCAGGTGGTGTGATG |
|
| CCN1 | Forward |
CGAGGTGGAGTTGACGAGAA | 211 |
|
| Reverse |
GCACTCAGGGTTGTCATTGGT |
|
Western blot analysis
The cells were lysed in lysis buffer containing 50
mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.5% Nonidet P-40, 0.5% sodium
deoxycholate and phenylmethylsulfonyl fluoride (all from
Sigma-Aldrich), and protein concentration was determined using a
bicinchoninic acid assay (Beyotime Institute of Biotechnology,
Haimen, China). The samples (60 µg) were separated by 8% or 10%
SDS-PAGE (Beyotime Institute of Biotechnology) and transferred onto
a polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA,
USA). Following blocking with 5% bovine serum albumin
(Sigma-Aldrich) in Tris-buffered saline-Tween-20 [20 mM Tris-HCl,
500 mM NaCl and 0.05% Tween-20 (Yesen Biotechnology Co., Ltd.,
Shanghai, China); TBST], membranes were washed four times for 5 min
with TBST, and were then incubated with the following specific
primary antibodies overnight at 4°C: Rabbit anti-CCN1 polyclonal
antibody (1:2,000 dilution; cat. no. ab24448; Abcam, Cambridge,
UK); rabbit anti-phosphorylated (p)AKT1/2/3 (Ser473) polyclonal
antibody (1:2,000 dilution; cat. no. sc-101629); rabbit anti-VEGF
polyclonal antibody (1:2,000 dilution; cat. no. sc-152) and rabbit
anti-mouse β-actin polyclonal antibody (1:2,000 dilution; cat. no.
sc-130656) (all from Santa Cruz Biotechnology, Inc., Dallas, TX,
USA). Subsequently, membranes were incubated for 2 h at 37°C with
horseradish peroxidase-conjugated anti-rabbit-immunoglobulin G
secondary antibodies (1:2,000 dilution; cat. no. ZB-2010; Zhongshan
Jinqiao Biotechnology Co., Ltd.). Protein bands were visualized
using enhanced chemiluminescence reagents (Pierce Biotechnology,
Inc., Rockford, IL, USA) and an MF-ChemiBIS 3.2 (DNR Bio-Imaging
Systems, Ltd., Jerusalem, Israel). Optical density (OD) was
quantified using ImageQuant LAS 4000 software (GE Healthcare Life
Sciences). Protein concentrations were established by calculating
the ratio between the ODs of the protein of interest and
β-actin.
Statistical analysis
SPSS software, version 15.0 (SPSS, Inc., Chicago,
IL, USA) was used for statistical analyses. Data are presented as
the mean ± standard deviation of three independent experiments.
Statistical significance was evaluated using one-way analysis of
variance, with a least significant difference test for post-hoc
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
CCN1 siRNA transfection reduces the
expression of CCN1 in HUVECs
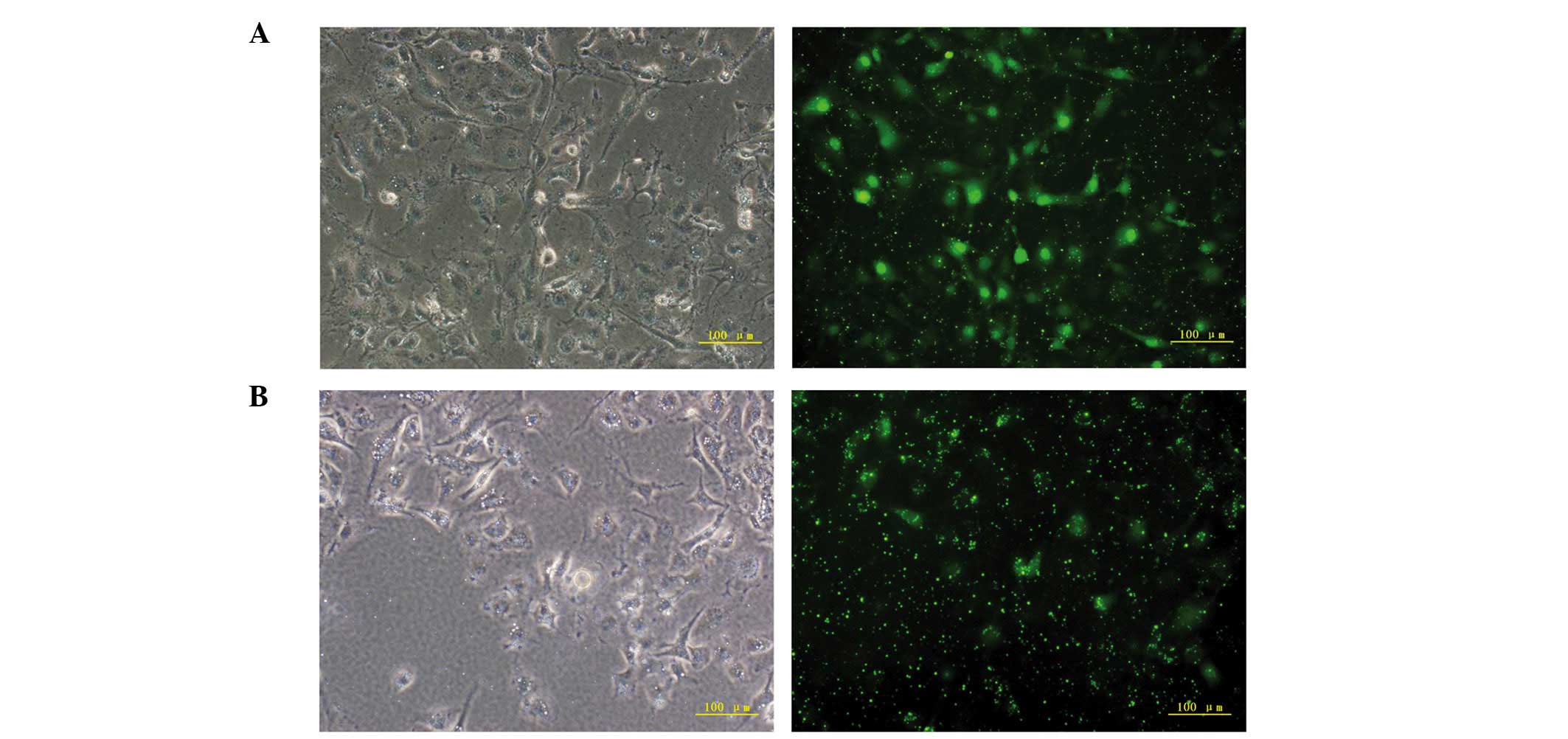
At 6 h post-transfection with CCN1 siRNA, the
percentage of GFP-positive HUVECs was >80% (Fig. 1A and B). RT-qPCR was performed to
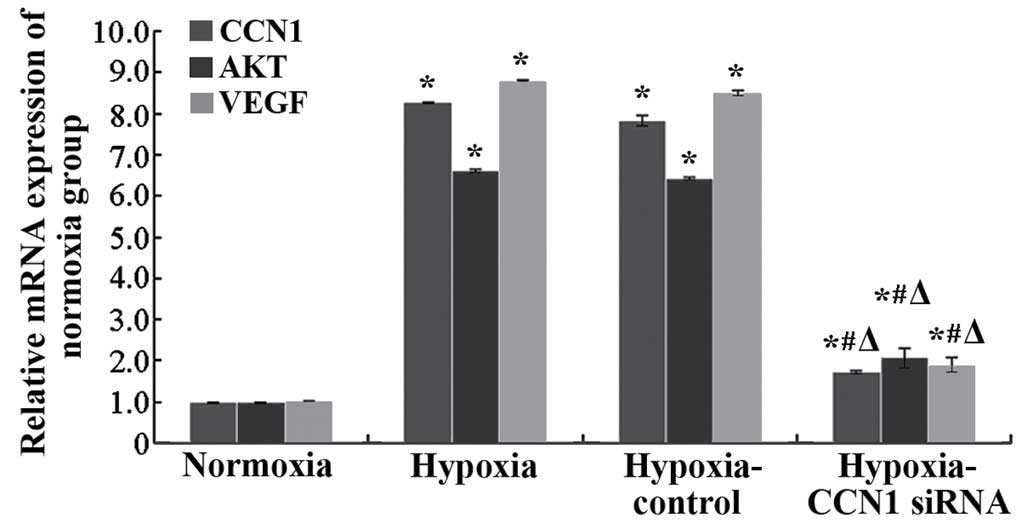
measure the mRNA expression of CCN1. Compared with the
hypoxia-control, mRNA expression of CCN1 in the hypoxia-CCN1 siRNA
group was downregulated by 78.21% (P<0.05; Fig. 2). Western blotting indicated that,
compared with the hypoxia-control group, the protein expression of
CCN1 in the hypoxia-CCN1 siRNA group was downregulated by 32.43%
(P<0.05; Fig. 3).
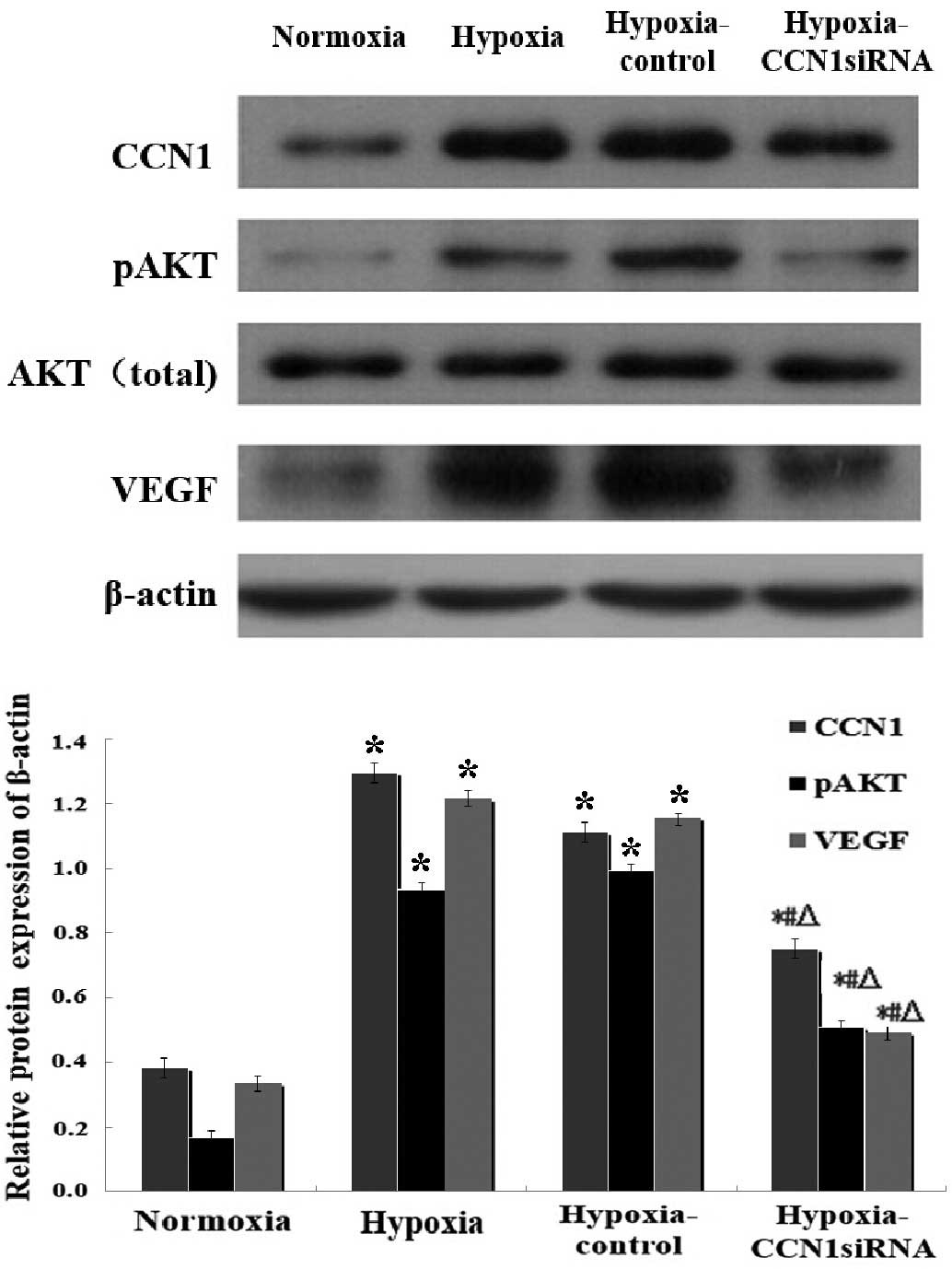 | Figure 3.Western blot analysis of the
CCN1-PI3K/Akt-VEGF pathway under hypoxic conditions. Data are
presented as the mean ± standard deviation of three independent
experiments. The protein expression levels of CCN1, pAkt and VEGF
were determined 2 days post-transfection under hypoxia; *P<0.05,
vs. the normoxia group, #P<0.05, vs. the hypoxia
group, ΔP<0.05, vs. the hypoxia-control group. CCN1,
cysteine-rich 61; PI3K, phosphoinositide 3-kinase; VEGF, vascular
endothelial growth factor; pAkt, phosphorylated Akt; siRNA, small
interfering RNA. |
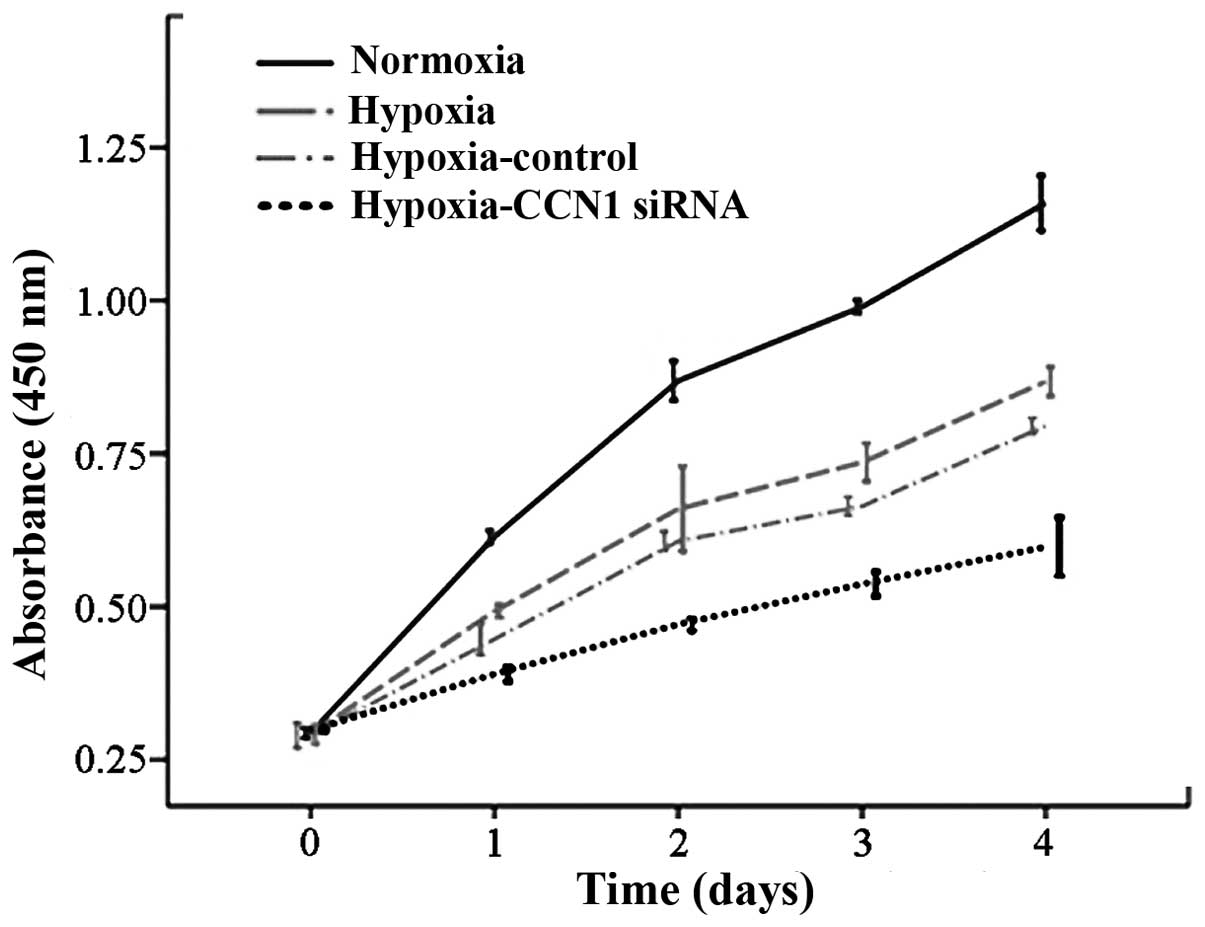
CCN1 siRNA inhibits the growth rate of
HUVECs
The major hallmark of angiogenesis is endothelial
cell proliferation (20);
therefore, HUVEC proliferation was measured using a CCK8 assay. The
proliferation rate was reduced in the hypoxia-CCN1 siRNA group,
compared with the proliferation rates in the hypoxia and normoxia
groups (P<0.05; Fig. 4). These
results indicated that CCN1 siRNA has an anti-proliferative effect
on HUVECs (21), possibly due to
an anti-angiogenic effect.
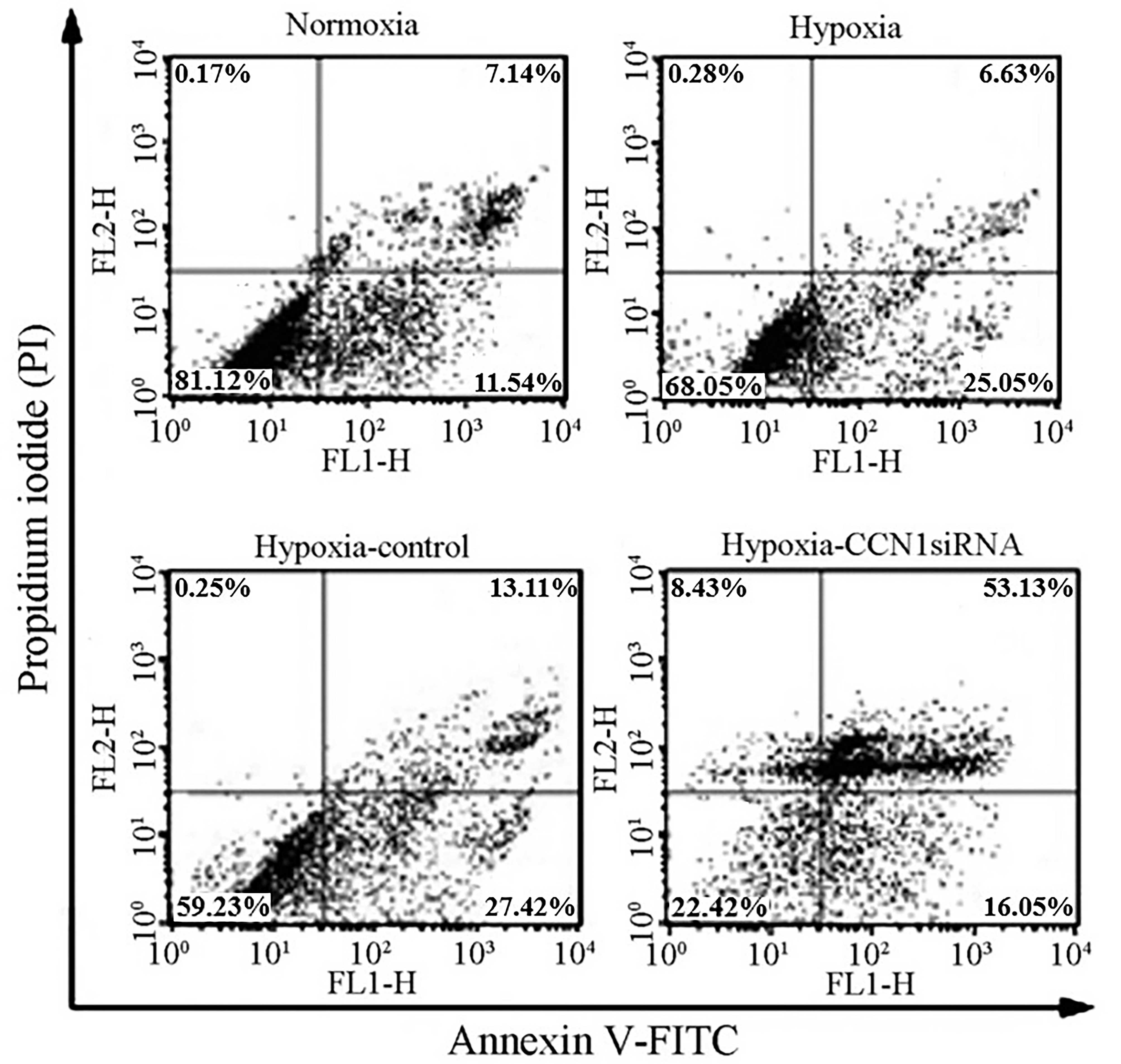
CCN1 siRNA induces apoptosis in
HUVECs
To investigate whether hypoxia can induce apoptosis
in HUVECs, cellular apoptotic ratios were measured using flow
cytometry, in which apoptotic cells determined as annexin V-FITC
positive and PI negative. The results of the flow cytometric
analysis indicated a moderate increase in apoptosis in the HUVECs
transfected with CCN1 siRNA (69.24±0.85%; P<0.05), compared with
the HUVECs transfected with scramble siRNA (40.14±0.78%), under
hypoxic (32.28±0.23%) or normoxic (18.68±0.43%) conditions
(Fig. 5). These results indicated
that CCN1 siRNA had a pro-apoptotic effect on HUVECs (21), possibly due to an anti-angiogenic
effect.
Hypoxia induces the expression of CCN1
through the PI3K/Akt-VEGF signaling pathway
The results of the RT-qPCR (Fig. 2) and western blot analysis
(Fig. 3) indicated that the mRNA
and protein levels of CCN1 and VEGF were increased in the hypoxia
and hypoxia-control groups, compared with the normoxia group
(P<0.05), however, no significant differences were observed
between the hypoxia and hypoxia-control groups (P>0.05). The
mRNA levels of Akt were increased, and western blotting indicated
an increase in the expression levels of p-Akt in the hypoxia and
the hypoxia-control groups, compared with the normoxia group.
Additionally, the mRNA and protein expression levels were reduced
in the hypoxia-CCN1 siRNA group, compared with the hypoxia and
hypoxia-control groups (P<0.05; Figs. 2 and 3). Compared with the hypoxia-control, the
hypoxia-CCN1 siRNA group demonstrated reduced mRNA expression
levels of CCN1, Akt and VEGF, which were reduced by 78.21, 67.19
and 77.65%, respectively (Fig. 2).
The protein levels of CCN1, Akt and VEGF were reduced by 32.43,
48.48 and 57.76%, respectively, in this group (Fig. 3). These results demonstrated that
the hypoxia-induced expression of CCN1 was mediated through the
PI3K/Akt-VEGF signaling pathway.
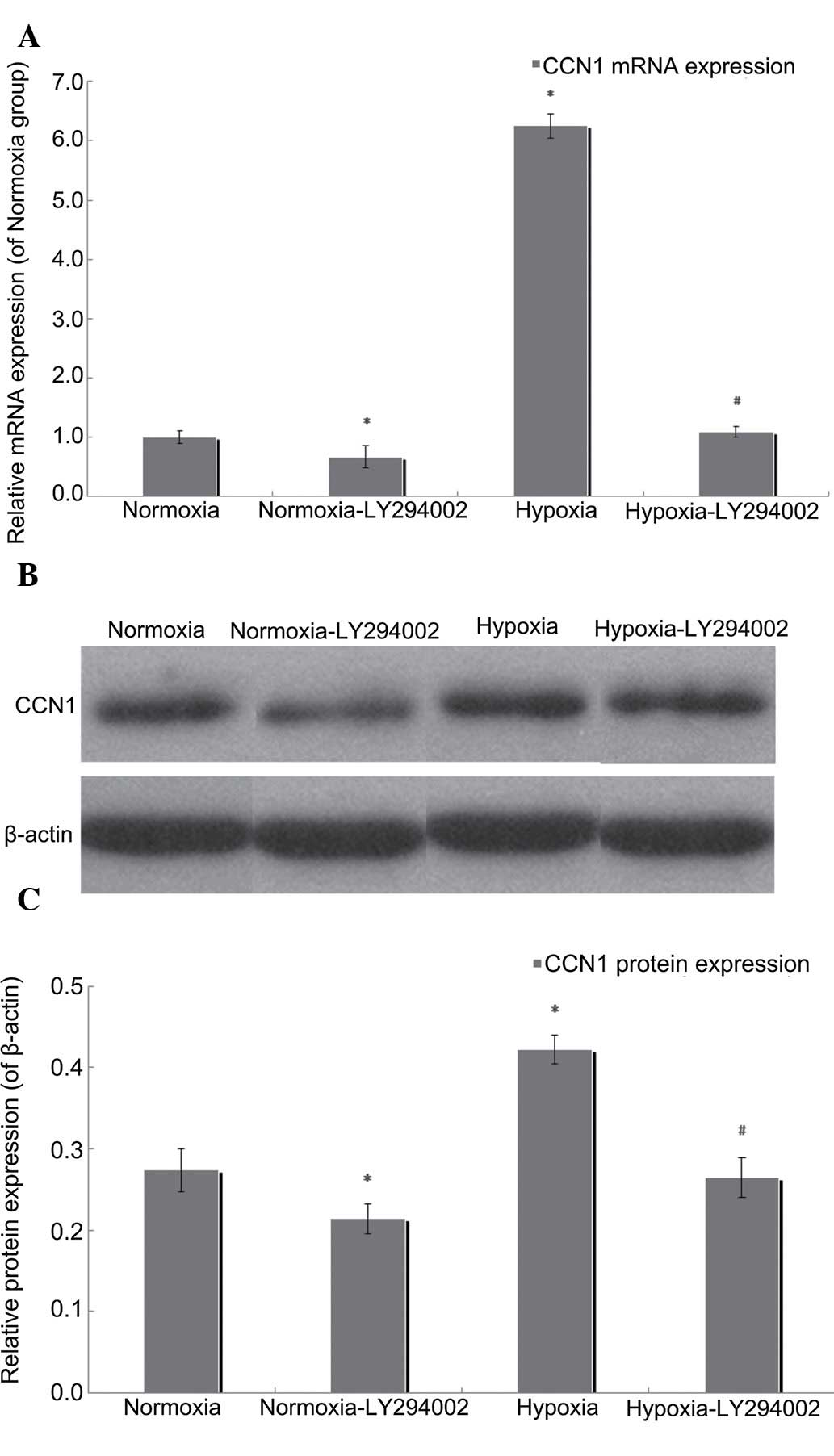
PI3K/Akt inhibition by LY294002
reduces the expression of CCN1
In the present study, RT-qPCR and western blotting
were performed to measure the mRNA and protein expression levels of
CCN1 following exposure of the cells to LY294002 under normoxic or
hypoxic conditions. Compared with the hypoxia group, the mRNA
expression of CCN1 in the LY294002 hypoxia group was downregulated
by 82.38% (P<0.05; Fig. 6A),
and LY294002 treatment reduced the protein expression of CCN1 by
37.32% in the hypoxic cells (P<0.05; Fig. 6B and C). Compared with the normoxia
group, the mRNA expression level of CCN1 in the normoxia-LY294002
group was downregulated by 32.57% (P<0.05; Fig. 6A), as were the protein levels of
CCN2, which were reduced by 21.87% in the normoxic group
(P<0.05; Fig. 6B and C). These
results suggested that the PI3K/Akt inhibitor, LY294002, reduced
the expression levels of CCN1, and that this process involved an
autocrine loop.
Discussion
Hypoxia and ischemia trigger a multitude of
responses, which are designed to compensate for the reduced oxygen
availability (22). In ECs, these
responses increase the expression levels of growth factors and
induce angiogenesis (23). The
growth of blood vessels in angiogenesis is a delicately controlled
process, which involves the activation, proliferation, migration,
differentiation and maturation of ECs (24,25).
Physiological angiogenesis is required for normal vascular
development in addition to vascular homeostasis during adulthood
(26). Pathological angiogenesis,
commonly induced by tissue ischemia, hypoxia or inflammation,
underlies numerous vascular disorders, including retinopathy of
prematurity, which is a leading cause of blindness in childhood
(27).
Previous studies have directly (28,29)
and indirectly (30,31) demonstrated that CCN1 is able to
promote chorioretinal angiogenesis in vitro via the
proliferation and migration of ECs, and the formation of tubular
structures, indicating that CCN1 may be involved in the formation
of angiogenesis in the retina. These processes all begin with EC
proliferation and, mechanistically, CCN1 may promote the
proliferation of ECs by upregulating the PI3K/Akt pathway (10,11,21).
However, the exact role of the CCN1 pathway remains to be
elucidated.
In the present study, examination of the
proliferation of HUVECs following CCN1 siRNA transfection under
hypoxic conditions demonstrated that treatment with CCN1 siRNA
significantly inhibited cell proliferation. Furthermore, it was
demonstrated that CCN1 siRNA promoted apoptosis of the cells, thus
interfering with angiogenesis. However, the aim of the present
study was not to determine whether apoptosis prevented angiogenesis
or whether apoptosis was induced by the inhibition of angiogenesis.
Despite this, these data indicated that the expression of CCN1 was
involved in cell proliferation and apoptosis. These findings are
supported by the findings of previous studies, which demonstrated
that EC proliferation is the initial step in angiogenesis, and is
an essential step prior to both cell migration and tube formation
(30).
In addition, several previous studies have suggested
that VEGF has central role in angiogenesis, therefore,
understanding the interaction between CCN1 and VEGF is important
(32,33). To further investigate the
mechanisms underlying the hypoxia-induced expression of CCN1, the
PI3K/Akt pathway was analyzed in the present study. PI3K/Akt is
downstream effector of insulin signaling (34), in addition to being an important
signaling molecule in the regulation of glycogen metabolism in
myocytes, lipocytes and hepatocytes (12). Furthermore, PI3K/Akt has an
important role in ECs by regulating angiogenesis, proliferation,
microvascular permeability, survival, cellular transformation and
embryonic differentiation (35–37).
It has been reported that CCN1 induces the expression levels of
PI3K/Akt in different types of cell, including breast cancer,
gastric cancer, renal cell carcinoma and glioma cells (10,38–40).
The results of the present study demonstrated that hypoxia
increased the mRNA and protein levels of CCN1 via the PI3K/Akt-VEGF
pathway, and that CCN1 siRNA induced a significant inhibition of
the PI3K/Akt-VEGF pathway. In addition, the data indicated that the
mRNA and protein levels of CCN1 were reduced in the cells treated
with LY294002 prior to hypoxia, compared with hypoxia-exposed cells
without LY294002 treatment. These results supported the hypothesis
that the hypoxia-induced expression of CCN1 acts through the
PI3K/Akt-VEGF pathway.
In addition, the results of the present study
demonstrated that the proliferation and of ECs, and the expression
levels of CCN1, Akt, and VEGF were not completely inhibited by CCN1
siRNA. This may be associated with the actions of other growth
factors, including basic fibroblast growth factor, interleukin-8,
c-Jun and hypoxia-inducible factor-1α (31,41).
Further investigations are required to determine the precise
association between these growth factors and CCN1, and their
involvement in pathological angiogenesis.
Taken together, the present study demonstrated that
CCN1 induced the proliferation of HUVECS, and increased the
secretion of cytokines, including VEGF, which acted through
PI3K/Akt activation. Therefore, CCN1 RNAi may offer a promising
strategy for the treatment of pathological angiogenesis.
Acknowledgements
The authors would like to thank Dr Juanhan Yu
(Department of Pathology, First Affiliated Hospital and College of
Basic Medical Sciences, China Medical University, Shenyang, China),
Dr Rui Zhao (School of Forensic Medicine, China Medical University,
Shenyang, China) and Dr Siyang Zhang (Center of Laboratory
Technology and Experimental Medicine, China Medical University,
Shenyang, China) for their technical assistance and experimental
instructions. The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81371045),
the Liaoning Province Technology Foundation of China (grant no.
2010225034) and the Liaoning Province Natural Science Foundation of
China (grant no. 2010228).
References
|
1
|
Nyengaard JR, Ido Y, Kilo C and Williamson
JR: Interactions between hyperglycemia and hypoxia: Implications
for diabetic retinopathy. Diabetes. 53:2931–2938. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen J and Smith LE: Retinopathy of
prematurity. Angiogenesis. 10:133–140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leask A and Abraham DJ: All in the CCN
family: Essential matricellular signaling modulators emerge from
the bunker. J Cell Sci. 119:4803–4810. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jun JI and Lau LF: The matricellular
protein CCN1 induces fibroblast senescence and restricts fibrosis
in cutaneous wound healing. Nat Cell Biol. 12:676–685. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yan L and Chaqour B: Cysteine-rich protein
61 (CCN1) and connective tissue growth factor (CCN2) at the
crosshairs of ocular neovascular and fibrovascular disease therapy.
J Cell Commun Signal. 7:253–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chintalapudi MR, Markiewicz M, Kose N,
Dammai V, Champion KJ, Hoda RS, Trojanowska M and Hsu T: Cyr61/CCN1
and CTGF/CCN2 mediate the proangiogenic activity of VHL-mutant
renal carcinoma cells. Carcinogenesis. 29:696–703. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meyuhas R, Pikarsky E, Tavor E, Klar A,
Abramovitch R, Hochman J, Lago TG and Honigman A: A Key role for
cyclic AMP-responsive element binding protein in hypoxia-mediated
activation of the angiogenesis factor CCN1 (CYR61) in Tumor cells.
Mol Cancer Res. 6:1397–1409. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmitz P, Gerber U, Schütze N, Jüngel E,
Blaheta R, Naggi A, Torri G and Bendas G: Cyr61 is a target for
heparin in reducing MV3 melanoma cell adhesion and migration via
the integrin VLA-4. Thromb Haemost. 110:1046–1054. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haque I, De A, Majumder M, Mehta S,
McGregor D, Banerjee SK, Van Veldhuizen P and Banerjee S: The
matricellular protein CCN1/Cyr61 is a critical regulator of Sonic
Hedgehog in pancreatic carcinogenesis. J Biol Chem.
287:38569–38579. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Long QZ, Zhou M, Liu XG, Du YF, Fan JH, Li
X and He DL: Interaction of CCN1 with αvβ3 integrin induces
P-glycoprotein and confers vinblastine resistance in renal cell
carcinoma cells. Anticancer Drugs. 24:810–817. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Emre Y and Imhof BA: Matricellular protein
CCN1/CYR61: A new player in inflammation and leukocyte trafficking.
Semin Immunopathol. 36:253–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang XM, Wang YS, Zhang J, Li Y, Xu JF,
Zhu J, Zhao W, Chu DK and Wiedemann P: Role of PI3K/Akt and MEK/ERK
in mediating hypoxia-induced expression of HIF-1alpha and VEGF in
laser-induced rat choroidal neovascularization. Invest Ophthalmol
Vis Sci. 50:1873–1879. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
You JJ, Yang CH, Yang CM and Chen MS:
Cyr61 induces the expression of monocyte chemoattractant protein-1
via the integrin ανβ3, FAK, PI3K/Akt, and NF-κB pathways in retinal
vascular endothelial cells. Cell Signal. 26:133–140. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koon HW, Shih DQ, Hing TC, Chen J, Ho S,
Zhao D, Targan SR and Pothoulakis C: Substance P induces CCN1
expression via histone deacetylase activity in human colonic
epithelial cells. Am J Pathol. 179:2315–2326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shen WG, Peng WX, Shao Y, Xu JF, Dai G,
Zhang Y, Pan FY and Li CJ: Localization and activity of calmodulin
is involved in cell-cell adhesion of tumor cells and endothelial
cells in response to hypoxic stress. Cell Biol Toxicol. 23:323–335.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Zhao T, Yang Z and Li Q: CX3CR1
RNAi inhibits hypoxia-induced microglia activation via p38MAPK/PKC
pathway. Int J Exp Pathol. 95:153–157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aubry JP, Blaecke A, Lecoanet-Henchoz S,
Jeannin P, Herbault N, Caron G, Moine V and Bonnefoy JY: Annexin V
used for measuring apoptosis in the early events of cellular
cytotoxicity. Cytometry. 37:197–204. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee
DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, et al:
Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic
Acids Res. 33:e1792005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zou L, Lai H, Zhou Q and Xiao F: Lasting
controversy on ranibizumab and bevacizumab. Theranostics.
1:395–402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Di Y, Zhang YO, Nie QZ and Chen XL:
CCN1/Cyr61-PI3K/AKT signaling promotes retinal neovascularization
in oxygen-induced retinopathy. Int J Mol Med. 36:1507–1518.
2015.PubMed/NCBI
|
|
22
|
Brunelle JK and Chandel NS: Oxygen
deprivation induced cell death: An update. Apoptosis. 7:475–482.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jin Y, An X, Ye Z, Cully B, Wu J and Li J:
RGS5, a hypoxia-inducible apoptotic stimulator in endothelial
cells. J Biol Chem. 284:23436–23443. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Distler JH, Hirth A, Kurowska-Stolarska M,
Gay RE, Gay S and Distler O: Angiogenic and angiostatic factors in
the molecular control of angiogenesis. Q J Nucl Med. 47:149–161.
2003.PubMed/NCBI
|
|
25
|
Potente M, Gerhardt H and Carmeliet P:
Basic and therapeutic aspects of angiogenesis. Cell. 146:873–887.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Westenskow PD, Kurihara T, Aguilar E, et
al: Ras pathway inhibition prevents neovascularization by
repressing endothelial cell sprouting. J Clin Invest.
123:4900–4908. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gergely K and Gerinec A: Retinopathy of
prematurity - epidemics, incidence, prevalence, blindness. Bratisl
Lek Listy. 111:514–517. 2010.PubMed/NCBI
|
|
28
|
Grote K, Salguero G, Ballmaier M, Dangers
M, Drexler H and Schieffer B: The angiogenic factor CCN1 promotes
adhesion and migration of circulating CD34+ progenitor cells:
Potential role in angiogenesis and endothelial regeneration. Blood.
110:877–885. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu Y, Gao Y, Wang H, Huang L, Qin J, Guo
R, Song M, Yu S, Chen J and Cui B: The matrix protein CCN1 (CYR61)
promotes proliferation, migration and tube formation of endothelial
progenitor cells. Exp Cell Res. 314:3198–3208. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang X, Yu W and Dong F: Cysteine-rich 61
(CYR61) is up-regulated in proliferative diabetic retinopathy.
Graefes Arch Clin Exp Ophthalmol. 250:661–668. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
You JJ, Yang CH, Chen MS and Yang CM:
Cysteine-rich 61, a member of the CCN family, as a factor involved
in the pathogenesis of proliferative diabetic retinopathy. Invest
Ophthalmol Vis Sci. 50:3447–3455. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou D, Herrick DJ, Rosenbloom J and
Chaqour B: Cyr61 mediates the expression of VEGF, alphav-integrin,
and alpha-actin genes through cytoskeletally based
mechanotransduction mechanisms in bladder smooth muscle cells. J
Appl Physiol (1985). 98:2344–2354. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang R, Amir J, Liu H and Chaqour B:
Mechanical strain activates a program of genes functionally
involved in paracrine signaling of angiogenesis. Physiol Genomics.
36:1–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gerszten RE, Friedrich EB, Matsui T, Hung
RR, Li L, Force T and Rosenzweig A: Role of phosphoinositide
3-kinase in monocyte recruitment under flow conditions. J Biol
Chem. 276:26846–26851. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kucharzewska P, Welch JE, Svensson KJ and
Belting M: The polyamines regulate endothelial cell survival during
hypoxic stress through PI3K/AKT and MCL-1. Biochem Biophys Res
Commun. 380:413–418. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hung HS, Wu CC, Chien S and Hsu SH: The
behavior of endothelial cells on polyurethane nanocomposites and
the associated signaling pathways. Biomaterials. 30:1502–1511.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang F, Fang ZF, Hu XQ, Tang L, Zhou SH
and Huang JP: Overexpression of miR-126 promotes the
differentiation of mesenchymal stem cells toward endothelial cells
via activation of PI3K/Akt and MAPK/ERK pathways and release of
paracrine factors. Biol Chem. 394:1223–1233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lin MT, Kuo IH, Chang CC, Chu CY, Chen HY,
Lin BR, Sureshbabu M, Shih HJ and Kuo ML: Involvement of
hypoxia-inducing factor-1alpha-dependent plasminogen activator
inhibitor-1 up-regulation in Cyr61/CCN1-induced gastric cancer cell
invasion. J Biol Chem. 283:15807–15815. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Goodwin CR, Lal B, Zhou X, Ho S, Xia S,
Taeger A, Murray J and Laterra J: Cyr61 mediates hepatocyte growth
factor-dependent tumor cell growth, migration, and Akt activation.
Cancer Res. 70:2932–2941. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin J, Huo R, Wang L, Zhou Z, Sun Y, Shen
B, Wang R and Li N: A novel anti-Cyr61 antibody inhibits breast
cancer growth and metastasis in vivo. Cancer Immunol Immunother.
61:677–687. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
You JJ, Yang CM, Chen MS and Yang CH:
Regulation of Cyr61/CCN1 expression by hypoxia through cooperation
of c-Jun/AP-1 and HIF-1α in retinal vascular endothelial cells. Exp
Eye Res. 91:825–836. 2010. View Article : Google Scholar : PubMed/NCBI
|