Introduction
An estimated 300 million patients suffer from asthma
worldwide (1). Allergic asthma is
a T-helper 2 (Th2) lymphocyte-mediated inflammatory airway disease,
and airway hyperresponsiveness (AHR) is a key part of the
definition of asthma, and is one of the key features underpinning
periodic bronchoconstriction (2).
AHR is characterized by exaggerated airway narrowing subsequent to
exposure to nonspecific stimuli including methacholine (MCh),
histamine and exercise, or allergens including certain respiratory
viruses, certain organic chemicals or pollutants (3). The degree of AHR is usually in
proportion to the severity of the underlying asthma (4). The requirements for asthma medication
include improving airflow limitations, reducing symptoms and
modifying underlying AHR.
Classical Th2 cell-derived cytokines, particularly,
interleukin (IL)-5 and IL-13 serve a pivotal role in the
pathogenesis of asthma via eotaxin to regulate eosinophilia
(5). IL-5 delays eosinophil
apoptotic death, promotes eosinophil adhesion activity and enhances
eosinophil effector function (6).
Blocking IL-5 has been previously identified to inhibit eosinophil
influx into the airways in addition to suppressing AHR (7). IL-13 shares several biological
responses with IL-4 and may also be important in the development of
allergic airway eosinophilia and AHR (8,9).
IL-13 serves a regulatory role in Th2 activation (10) and is a key mediator in asthma,
including switching plasma cell antibody synthesis from
immunoglobulin (Ig)M to IgE production (11), promoting eosinophil migration into
the lungs (12), upregulating
adhesion molecules (13),
increasing goblet cell hyperplasia and mucus production (14), increasing proliferation of airway
smooth muscle (15), stimulating
airways hyperresponsiveness (16).
IL-13 blockade inhibited airway inflammation, hyperresponsiveness
and remodeling (17).
It is widely accepted that exposure to pathogens can
elicit protective effects, potentially through activation of innate
immune responses. The hygiene hypothesis states that early exposure
to pathogens provides protective effects against asthma and the
prevalence of allergies, which is suggested to be as a result of
alterations in environmental factors including reduced exposure to
microbial antigens during infancy. Therefore, the impact of
microbes and bacterial extracts on respiratory allergies have been
investigated in mouse asthma models and in humans (18–20).
Additional studies have examined the effects of mycobacterial
infection including Mycobacterium tuberculosis and
mycobacteria other than tuberculosis on asthma (21).
Previous studies demonstrated that the Bacillus
Calmette-Guérin (BCG) vaccine can prevent the formation of a rat
asthmatic model and inhalation of inactivated-Mycobacterium
phlei can restore the balance of the immune system and
attenuate airway inflammation in a murine model of asthma (22,23).
However, the effects of inhaled inactivated-Mycobacterium
phlei on AHR in a mice model of asthma remain unclear. In the
current study, the effect of inactivated-Mycobacterium phlei
on airway response to MCh was investigated in a murine model of
ovalbumin (OVA)-induced allergic asthma.
Materials and methods
Animals
Male pathogen-free Balb/c mice (4–6 weeks old;
weight, 20–24 g) were obtained from the Laboratory Animal Center of
Guangxi Medical University (Nanning, China). The mice were housed
under specific-pathogen-free conditions in a facility with an
automatic 12/12 h day and night cycle and fed with standard
laboratory food and water ad libitum. All experimental
animals were used in accordance with the guidelines issued by the
Chinese Council on Animal Care. The study was approved by the
ethics committee of Guangxi Medical University (Nanning,
China).
Sensitization, allergen exposure and
treatment
Mice were randomly categorized into three
experimental groups and each group consisted of 8 mice: The normal
control group (group A), the asthma model group (group B) and the
intervention group (group C). In groups B and C, the mice were
repeatedly sensitized and challenged with OVA (grade V;
Sigma-Aldrich; Merck Millipore; Darmstadt, Germany) to establish a
murine asthma model according to the method described in a previous
study (23). In brief, mice were
sensitized by intraperitoneal injection of 25 µg OVA emulsified in
1 mg Al(OH)3 (Chengdu Kelong Chemical Reagent Factory, Chengdu,
China) suspended in 0.2 ml saline on days 0, 7 and 14. Following
sensitization, the mice were challenged for 20 min with 2% OVA once
daily by an ultrasonic nebulizer (WH-2000; Guangdong YueHua Medical
Instrument Factory Co., Ltd., Guangzhou, China) in a closed chamber
from days 21–28. Mice in group C inhaled the solution of
inactivated Mycobacterium phlei (each ampoule contained 1.72
µg Mycobacterium phlei dissolved in 10 ml saline) using a
nebulizer once daily prior to each challenge. The normal group and
model group were sham-treated with 10 ml saline atomization. Lung
resistance (RL) was assessed 24 h subsequent to the last
inactivated-Mycobacterium phlei treatment or saline
treatment and then the mice were intraperitoneally anesthetized
with 10% chloral hydrate (0.3 ml/100 g; Dalian Meilun Biological
Technology Co., Ltd., Dalian, China) and sacrificed by cervical
dislocation under anesthesia.
Measurement of AHR to MCh
Respiratory resistance (RL, cmH2O.s/ml)
was determined as changes in the airway function subsequent to
inhalation of MCh challenge (24).
Respiratory resistance was assessed in anesthetized and
tracheotomized mice that were mechanically ventilated in response
to an increasing concentration of MCh inhalation using the
pulmonary function equipment from the RC System for Mouse (Buxco
Research Systems, Wilmington, NC, USA).
Preparation of bronchoalveolar lavage
fluid (BALF)
Subsequent to assessment of the respiratory
mechanics, the mice were anesthetized with chloral hydrate (400
mg/kg intraperitoneal injection). The trachea was immediately
exposed and carefully cannulated with a 24-gauge-feeding needle.
The catheter was secured with ligatures. Pre-warmed
phosphate-buffered saline (PBS) solution (at 37°C) was slowly
infused into the lungs and withdrawn gently, and this was repeated
three times. The BALF was collected and maintained at 4°C.
Cell fractionation and differential
cell count in serum and BALF
Blood samples for serum were collected from the
orbital artery. The BALF was centrifuged at 300 × g for 2
min at 4°C. Cell pellets were resuspended in 100 µl PBS. Total cell
numbers in BALF were measured using a haemacytometer. Smears of
blood samples and BALF cells were prepared using a cytospin. A
differential cell count was performed using Wright-Giemsa following
the manufacturer's instructions to classify eosinophils on the
basis of morphological criteria and staining characteristics.
Differential cell counts were made blind on 100 cells/slide under
an oil lens at a magnification of ×100.
Histological analysis of lung
tissue
Lungs were isolated from the mice subsequent to
BALF, fixed with 10% formalin for 24 h, and embedded in paraffin.
Specimens were cut into 4-µm sections. The microsections were
stained with hematoxylin-eosin (HE) and alcian blue/periodic acid
Schiff (AB-PAS) to analyze inflammatory cell infiltration and mucus
production, respectively.
Enzyme-linked immunosorbent assay
(ELISA) for IgE, IL-5 and IL-13 in BALF
Levels of IgE, IL-5 and IL-13 in BALF were measured
by ELISA (R&D Systems, Inc., Minneapolis, MN, USA). The
absorbance was measured at 450 nm using a microplate ELISA reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Reverse transcription-quantative
polymerase chain reaction (RT-qPCR) for IL-5 and IL-13
Total RNA was isolated from lung tissues using
TRIzol reagent according to the manufacturer's protocol
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
was quantified with the Nanodrop 2000 spectrophotometer (Thermo
Fisher Scientific, Inc.). A total of 2 µg target RNA was reverse
transcribed into cDNA using RevertAid™ First Strand cDNA Synthesis
kit (Fermentas; Thermo Fisher Scientific, Inc.) at 42°C for 60 min
and 70°C for 5 min. RT-qPCR was performed for IL-5, IL-13 mRNA
using the ABI Prism 7500 Sequence detector (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The cycling conditions for PCR
were as follows: 94°C for 5 min, followed by 40 cycles of 94°C for
30 sec, 59°C for 30 sec, 72°C for 30 sec, then final elongation at
72°C for 5 min and held at 10°C. The primers were designed by
Takara Bio, Inc. (Otsu, Japan) and were as follows: IL-5, forward
5′-TGAGGCTTCCTGTCCCTACTCATAA-3′ and reverse
5′-TTGGAATAGCATTTCCACAGTACCC-3′; IL-13, forward
5′-CGGCAGCATGGTATGGAG-3′ and reverse
5′-ATTGCAATTGGAGATGTTGGTCAG-3′.
Statistical analysis
Data are expressed as the mean ± standard deviation.
One-way analysis of variance was used for the normal control group,
the asthma model group and the intervention group. P<0.05 was
considered to indicate a statistically significant difference.
Results
Inhalation of inactivated
Mycobacterium phlei attenuates OVA-induced pulmonary pathologies
and inhibits airway and blood eosinophilia
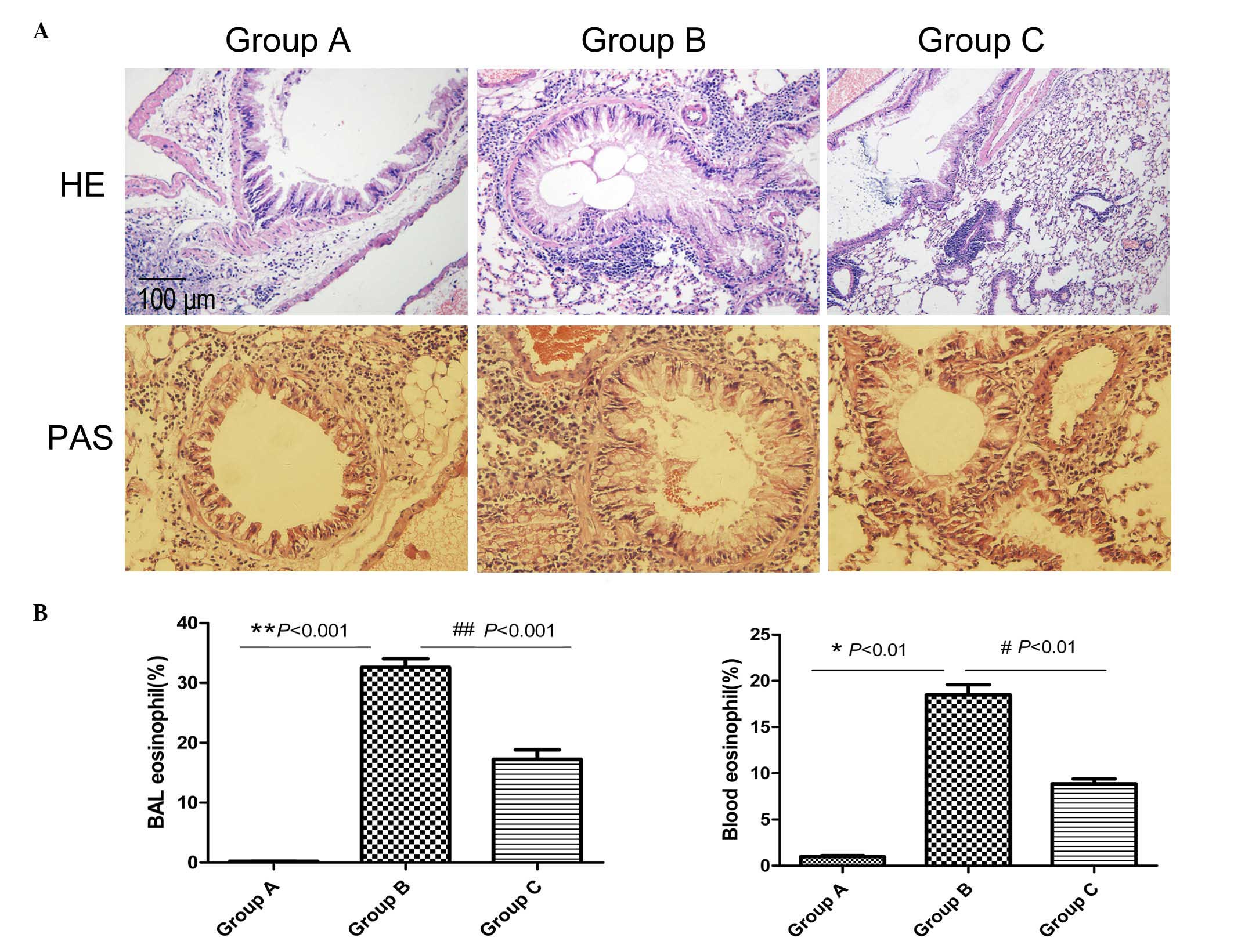
Mice in the normal control group (group A) were
observed with HE staining to exhibit normal lung tissue structures,
with no inflammatory cells (Fig.
1A). In the asthma model group (group B), mice challenged with
OVA exhibited a significant infiltration of inflammatory cells
around the airways and blood vessels (HE; Fig. 1A). In addition, the majority of the
infiltrated inflammatory cells were eosinophils (Fig. 1B). Compared with group A, there was
increased goblet cell hyperplasia in the airway epithelia and
increased thickened basement membranes in group B (PAS; Fig. 1A). The administration of
inactivated Mycobacterium phlei reduced the infiltration of
inflammatory cells (airway and blood eosinophilia) and mucus
production in the peribronchial and perivascular areas compared
with the asthma model mice (Fig.
1).
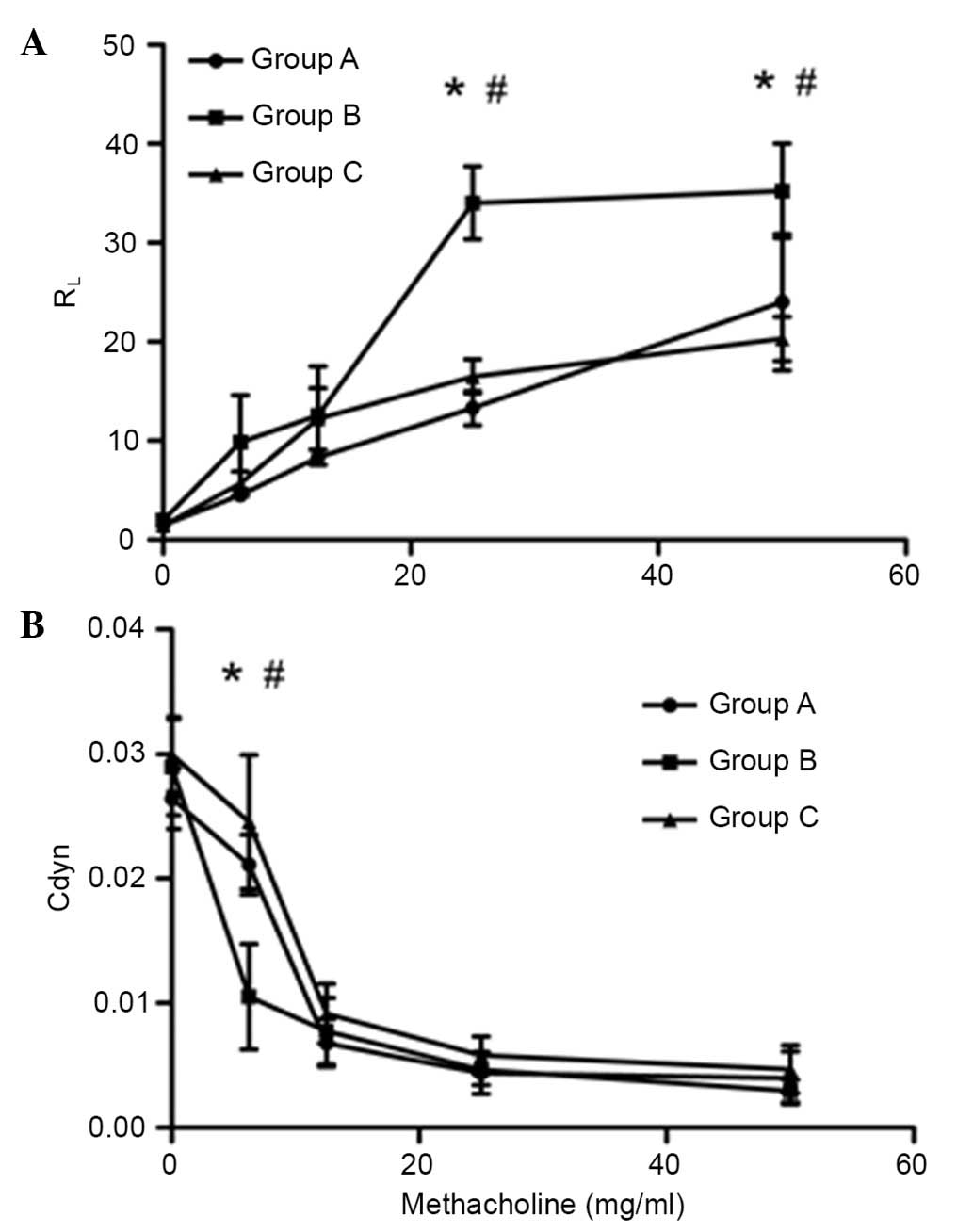
Inactivated Mycobacterium phlei
suppresses AHR
In order to test the effects of inactivated
Mycobacterium phlei on AHR, mice were sensitized and
challenged with OVA. As hypothesized, inhalation of
inactivated-Mycobacterium phlei prior to each challenge
could suppress AHR, or potentially completely prevent AHR. At the
same time, inactivated-Mycobacterium phlei could improve
airway compliance (Fig. 2).
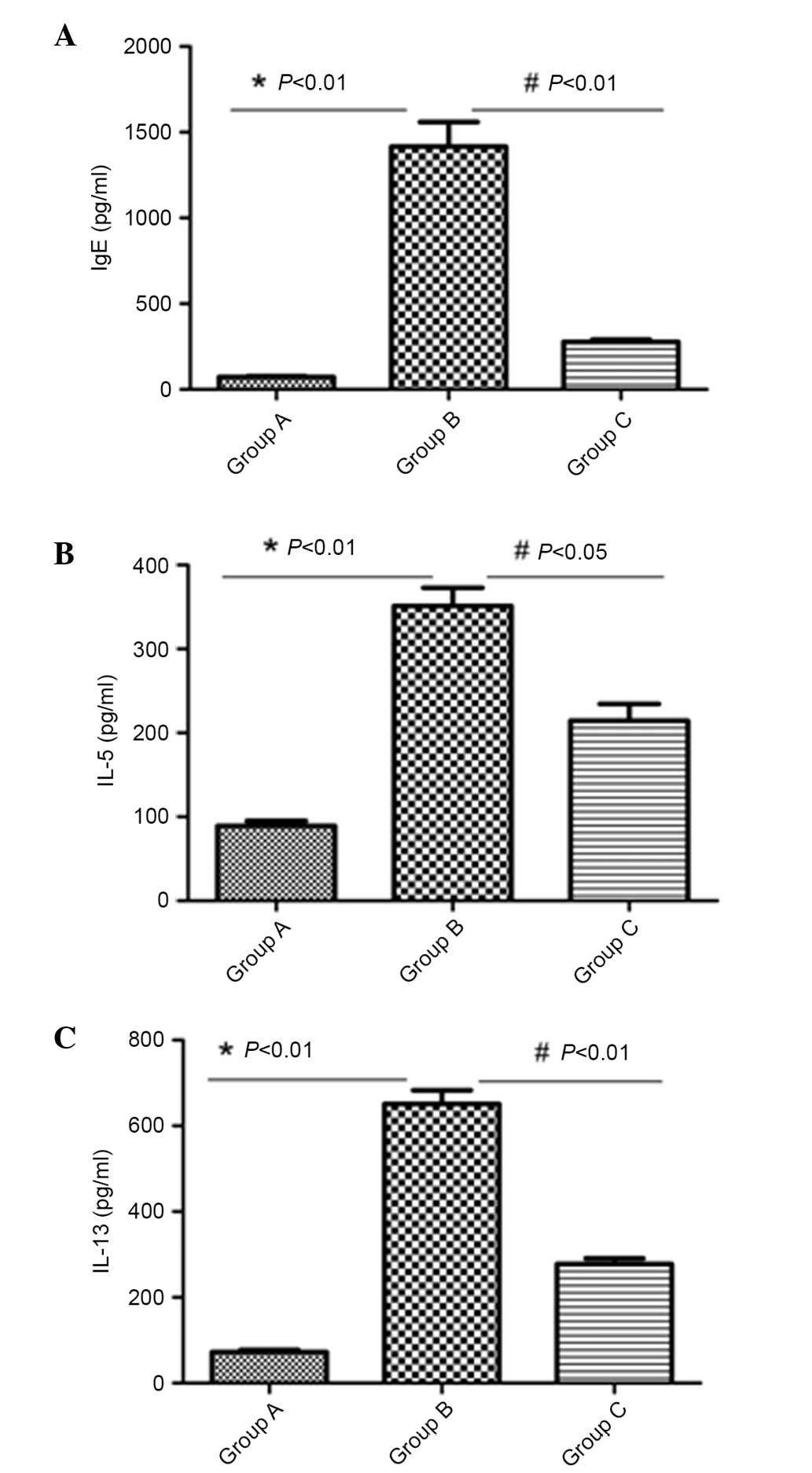
Reduced IgE, IL-5 and IL-13 levels in
BALF following inactivated Mycobacterium phlei administration
The levels of IgE, IL-5 and IL-13 in BALF were
measured. High levels of IgE, IL-5 and IL-13 were present in the
asthma model group. Administration of inactivated-Mycobacterium
phlei significantly (P<0.05) inhibited the levels of IL-5,
IL-13 and IgE in BALF in group C (Fig.
3).
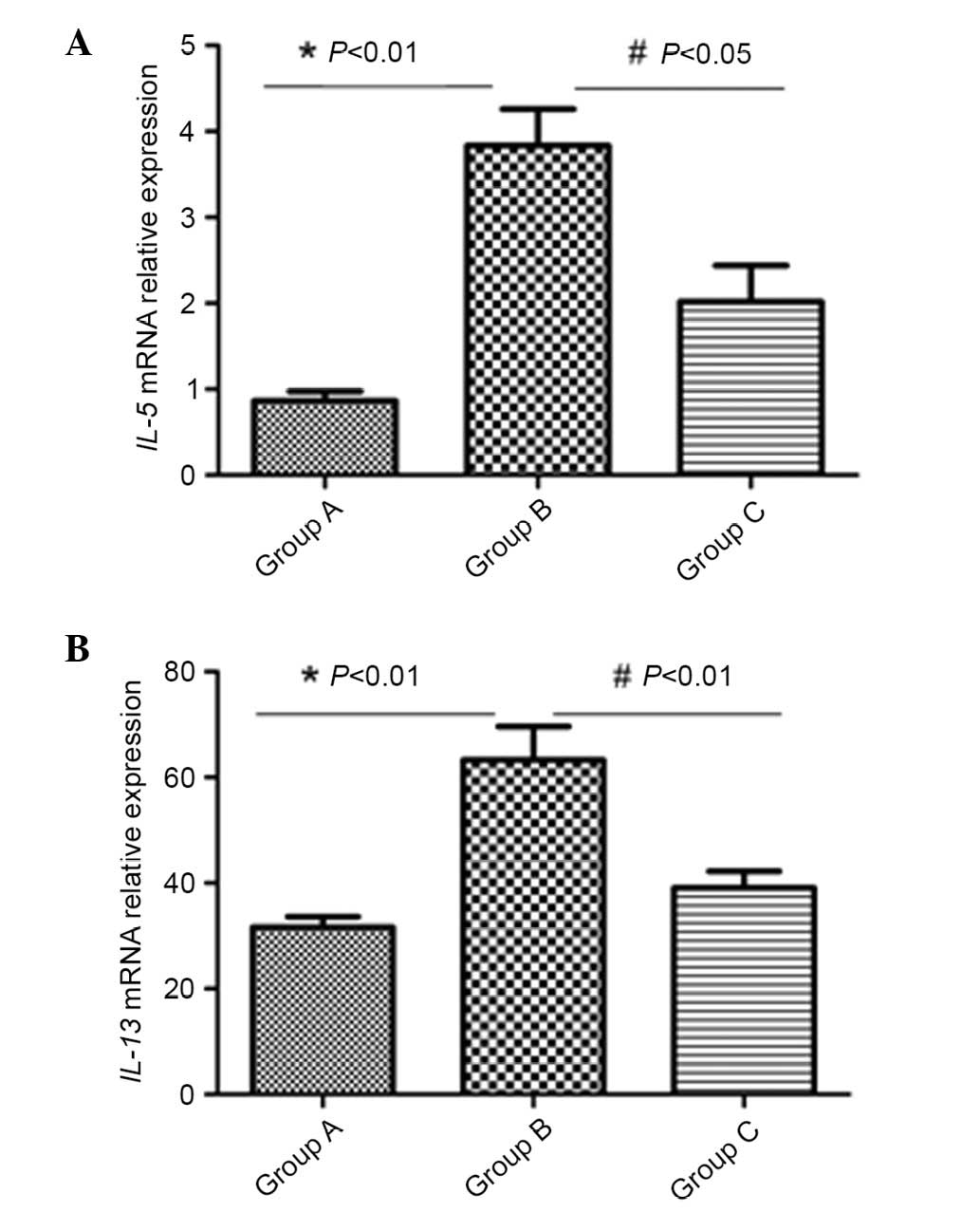
Inactivated-Mycobacterium phlei
reduced IL-5 and IL-13 mRNA expression in the lung
mRNA expression was normalized to the housekeeping
gene (β-actin). mRNA levels of IL-5 and IL-13 in lung tissue were
markedly increased by induction with OVA (P<0.01). However,
subsequent to inhalation administration of inactivated
Mycobacterium phlei, the mRNA levels of the above cytokines
were significantly reduced in group C (P<0.05; Fig. 4).
Discussion
The study examined the effects of inactived
Mycobacterium phlei and the inhalation route of
administration in OVA-sensitized mice. While evidence suggests that
direct, local delivery of mycobacterial antigens is superior to
systemic delivery in suppressing allergic responses in the murine
model (25).
There are numerous potential factors contributing to
the excessive airway response. An association between peripheral
blood eosinophil activation and AHR in asthma has also been
demonstrated (26,27). Asthma is characterized by high
levels of IgE and dominant Th2 cytokines including IL-4, IL-5 and
IL-13. A previous study reported the association between eosinophil
and airway structural alterations including airway remodeling,
impaired forced expiratory volume after one second (FEV1) and
FEV1/forced vital capacity ratio in patients with rhinitis
(28). IL-5 has been previously
demonstrated to serve significant roles in allergic diseases that
affect various organs including the airways, such as asthma
(6,29). IL-5 has been observed to regulate
growth, differentiation, activation and survival of eosinophils and
appears to be essential in the development of allergic airway
eosinophilia and AHR in mice (30–32).
IL-13 has been demonstrated as a key regulator in
IgE synthesis, mucus hypersecretion and AHR (33,34).
It is notable that patients with refractory eosinophilic asthma and
prednisone-dependent asthma with sputum eosinophilia appear to be
particularly sensitive to biological therapy for asthma, including
anti-IL-5, anti-IL-13 and anti-IgE (35–38).
Anti-IL-13 treatment both via the intraperitoneal and intranasal
routes has been previously identified to significantly inhibit AHR
in OVA-allergic animals (39).
Previously, it was reported that humanized
monoclonal antibodies (mAb) against IL-5 (mepolizumab) (35,40),
are effective and can be used to reduce the frequency of severe
exacerbations, and improve the quality of life in patients with
severe refractory eosinophilic asthma. However, there have
additionally been contradictory reports, suggesting that treatment
with mAb against IL-5 had no effect on the reversal of established
AHR (41,42).
The BCG vaccine is used in tuberculosis prevention
and is a strong inducer of a Th1-type immune response (43). The potential benefits of BCG and
mycobacteria other than tuberculosis on asthma have been previously
hypothesized (44). In
experimental allergic asthma, inhaled inactived-Mycobacterium
phlei led to a reduction of inflammatory scores in the airways,
a reduction in the levels of the Th2 cytokine IL-4, and an increase
in the levels of Th1 cytokines IFN-γ and IL-10 in BALF (23).
The results on BALF eosinophilia correlate well with
the observations reported by Hopfenspirger et al (45) who conducted a similar study
indicating a negative correlation between Mycobacterium and
BALF eosinophilia in OVA-sensitized and challenged mice. The
reduction in eosinophil percentages observed in the current study
in group C is similar to the outcome of the effect of anti-IL-5
therapy (mepolizumab and reslizumab) when used in patients with
eosinophilic esophagitis (46,47)
and eosinophilic asthma (48).
While the exact mechanisms involved remain unclear, with further
research required in order to elucidate whether the medication is
able to block eosinophil production in the bone marrow or/and their
removal from the circulation, the effect of
inactived-Mycobacterium phlei on the eosinophils residing in
the target organ is considered notable.
The mechanism of action of inactivated
Mycobacterium phlei remains unclear. It is suggested that
the inhibitory effects of inactivated-Mycobacterium phlei on
eosinophil infiltration are due to the inhibition of
proinflammatory cytokine release, due to the fact that inactivated
Mycobacterium phlei treatments did markedly modify the
increased expression of IL-5 and IL-13 mRNA in lung tissue and the
level of IL-5 and IL-13 in BALF induced by antigens (Figs. 3 and 4). Although the mechanism of action of
inactivated-Mycobacterium phlei remains unclear, its effect
on Toll-like receptors may be involved. Toll-like receptors (TLRs)
are a family of triggers of the innate and adaptive immune
responses against invading pathogens (49). TLR2 has been suggested to be
involved in recognition of Mycobacterium tuberculosis
(50,51) and to be important in the initiation
of innate host defense through its stimulatory effects on tumor
necrosis factor α production (52). TLR2, in particular, has been
implicated in the downregulation or deviation of the immune
response through the induction of IL-10 and Th2 cells or regulatory
T cell responses, IgE production and eosinophil infiltration in the
lung (53,54). A previous study demonstrated that
the level of TLR2 mRNA in the lungs was increased subsequent to
treatment with inhaled inactivated-Mycobacterium phlei compared
with the OVA-induced asthmatic mice (23).
In addition to the current study, previous studies
have demonstrated a suppressive effect on various parameters of
allergic inflammation in mouse models following mycobacteria
pretreatment (23,25,55).
However, excluding one study by Zhang et al (23), there is no information regarding
the atomization route of administration for
inactivated-Mycobacterium phlei in the attenuation of airway
inflammation. Hopfenspirger and Agrawal (25) observed that Mycobacterium
administration via nasal injection was the most effective in
attenuating allergic airway inflammation and AHR. Systemic
immunization, even with adjuvants, has been observed to induce weak
adaptive immune responses in the airways, while intranasal
immunization elicits systemic and mucosal responses (56–58).
Takabayashi et al (59)
reported that intranasal immunotherapy was more effective than
intradermal immunotherapy. Similarly, the results of the current
study suggested that modulation of the airways rather than systemic
immunity may be an important therapeutic target.
In conclusion, the present study demonstrated that
inhalation of inactivated-Mycobacterium phlei prior to each
allergen challenge inhibited AHR, in addition to reducing IL-5 and
IL-13 mNRA expression in lung tissues of asthmatic mice. These
inhibitory effects of inhaled inactivated-Mycobacterium
phlei may be beneficial for the prevention of allergic
bronchial asthma.
Acknowledgements
The current study was funded by the National Natural
Science Foundation of China (grant no. 81360007).
References
|
1
|
Krishnan V, Diette GB, Rand CS, Bilderback
AL, Merriman B, Hansel NN and Krishnan JA: Mortality in patients
hospitalized for asthma exacerbations in the United States. Am J
Respir Crit Care Med. 174:633–638. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bateman ED, Hurd SS, Barnes PJ, Bousquet
J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O'Byrne P, Pedersen
SE, et al: Global strategy for asthma management and prevention:
GINA executive summary. Eur Respir J. 31:143–178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Taube C, Wei X, Swasey CH, Joetham A,
Zarini S, Lively T, Takeda K, Loader J, Miyahara N, Kodama T, et
al: Mast cells, Fc epsilon RI, and IL-13 are required for
development of airway hyperresponsiveness after aerosolized
allergen exposure in the absence of adjuvant. J Immunol.
172:6398–6406. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cockcroft DW and Davis BE: Mechanisms of
airway hyperresponsiveness. J Allergy Clin Immunol. 118:551–559;
quiz 560–561. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asquith KL, Ramshaw HS, Hansbro PM,
Beagley KW, Lopez AF and Foster PS: The IL-3/IL-5/GM-CSF common
receptor plays a pivotal role in the regulation of Th2 immunity and
allergic airway inflammation. J Immunol. 180:1199–1206. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takatsu K and Nakajima H: IL-5 and
eosinophilia. Curr Opin Immunol. 20:288–294. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Busse WW, Ring J, Huss-Marp J and Kahn JE:
A review of treatment with mepolizumab, an anti-IL-5 mAb, in
hypereosinophilic syndromes and asthma. J Allergy Clin Immunol.
125:803–813. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Corren J: Role of interleukin-13 in
asthma. Curr Allergy Asthma Rep. 13:415–420. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Vries JE: The role of IL-13 and its
receptor in allergy and inflammatory responses. J Allergy Clin
Immunol. 102:165–169. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McKenzie GJ, Emson CL, Bell SE, Anderson
S, Fallon P, Zurawski G, Murray R, Grencis R and McKenzie AN:
Impaired development of Th2 cells in IL-13-deficient mice.
Immunity. 9:423–432. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Punnonen J, Aversa G, Cocks BG, McKenzie
AN, Menon S, Zurawski G, de Waal Malefyt R and de Vries JE:
Interleukin 13 induces interleukin 4-independent IgG4 and IgE
synthesis and CD23 expression by human B cells. Proc Natl Acad Sci
USA. 90:3730–3734. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Horie S, Okubo Y, Hossain M, Sato E,
Nomura H, Koyama S, Suzuki J, Isobe M and Sekiguchi M:
Interleukin-13 but not interleukin-4 prolongs eosinophil survival
and induces eosinophil chemotaxis. Intern Med. 36:179–185. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luttmann W, Knoechel B, Foerster M,
Matthys H, Virchow JC Jr and Kroegel C: Activation of human
eosinophils by IL-13. Induction of CD69 surface antigen, its
relationship to messenger RNA expression, and promotion of cellular
viability. J Immunol. 157:1678–1683. 1996.PubMed/NCBI
|
|
14
|
Kondo M, Tamaoki J, Takeyama K, Isono K,
Kawatani K, Izumo T and Nagai A: Elimination of IL-13 reverses
established goblet cell metaplasia into ciliated epithelia in
airway epithelial cell culture. Allergol Int. 55:329–336. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bossé Y, Thompson C, Audette K, Stankova J
and Rola-Pleszczynski M: Interleukin-4 and interleukin-13 enhance
human bronchial smooth muscle cell proliferation. Int Arch Allergy
Immunol. 146:138–148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chiba Y, Nakazawa S, Todoroki M, Shinozaki
K, Sakai H and Misawa M: Interleukin-13 augments bronchial smooth
muscle contractility with an up-regulation of RhoA protein. Am J
Respir Cell Mol Biol. 40:159–167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hacha J, Tomlinson K, Maertens L,
Paulissen G, Rocks N, Foidart JM, Noel A, Palframan R, Gueders M
and Cataldo DD: Nebulized anti-IL-13 monoclonal antibody Fab'
fragment reduces allergen-induced asthma. Am J Respir Cell Mol
Biol. 47:709–717. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dittrich AM, Erbacher A, Specht S, Diesner
F, Krokowski M, Avagyan A, Stock P, Ahrens B, Hoffmann WH, Hoerauf
A and Hamelmann E: Helminth infection with Litomosoides sigmodontis
induces regulatory T cells and inhibits allergic sensitization,
airway inflammation, and hyperreactivity in a murine asthma model.
J Immunol. 180:1792–1799. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Forsythe P, Inman MD and Bienenstock J:
Oral treatment with live Lactobacillus reuteri inhibits the
allergic airway response in mice. Am J Respir Crit Care Med.
175:561–569. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karimi K, Inman MD, Bienenstock J and
Forsythe P: Lactobacillus reuteri-induced regulatory T cells
protect against an allergic airway response in mice. Am J Respir
Crit Care Med. 179:186–193. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J, Tran V, Leung AS, Alexander DC and
Zhu B: BCG vaccines: Their mechanisms of attenuation and impact on
safety and protective efficacy. Hum Vaccin. 5:70–78. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li C, Xu Y, Zhang Z, Yang D, Liu X and
Xiong W: An experimental study of the effect of bacille
calmette-guerin vaccine on the production of a rat asthmatic model
and its relation with gamma delta T cells. Zhonghua Jie He He Hu Xi
Za Zhi. 25:162–165. 2002.(In Chinese). PubMed/NCBI
|
|
23
|
Zhang J, Li C and Guo S: Effects of
inhaled inactivated Mycobacterium phlei on airway inflammation in
mouse asthmatic models. J Aerosol Med Pulm Drug Deliv. 25:96–103.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kanehiro A, Lahn M, Mäkelä MJ, Dakhama A,
Fujita M, Joetham A, Mason RJ, Born W and Gelfand EW: Tumor
necrosis factor-alpha negatively regulates airway
hyperresponsiveness through gamma-delta T cells. Am J Respir Crit
Care Med. 164:2229–2238. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hopfenspirger MT and Agrawal DK: Airway
hyperresponsiveness, late allergic response, and eosinophilia are
reversed with mycobacterial antigens in ovalbumin-presensitized
mice. J Immunol. 168:2516–2522. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bradley BL, Azzawi M, Jacobson M, Assoufi
B, Collins JV, Irani AM, Schwartz LB, Durham SR, Jeffery PK and Kay
AB: Eosinophils, T-lymphocytes, mast cells, neutrophils, and
macrophages in bronchial biopsy specimens from atopic subjects with
asthma: Comparison with biopsy specimens from atopic subjects
without asthma and normal control subjects and relationship to
bronchial hyperresponsiveness. J Allergy Clin Immunol. 88:661–674.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jo EJ, Kim MY, Lee SE, Lee SY, Kim MH,
Song WJ, Kim SH, Kang HR, Chang YS, Cho SH and Min KU: Eosinophilic
airway inflammation and airway hyperresponsiveness according to
aeroallergen sensitization pattern in patients with lower airway
symptoms. Allergy Asthma Immunol Res. 6:39–46. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang MS, Lee HS, Kim MH, Song WJ, Kim TW,
Kwon JW, Kim SH, Park HW, Chang YS, Cho SH and Min KU: Rhinitis
patients with sputum eosinophilia show decreased lung function in
the absence of airway hyperresponsiveness. Allergy Asthma Immunol
Res. 5:232–238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stein ML and Munitz A: Targeting
interleukin (IL) 5 for asthma and hypereosinophilic diseases.
Recent Pat Inflamm Allergy Drug Discov. 4:201–209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Losol P, Kim SH, Hwang EK, Shin YS and
Park HS: IL-5 promoter polymorphism enhances IgE responses to
staphylococcal superantigens in adult asthmatics. Allergy Asthma
Immunol Res. 5:106–109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jayaprakasam B, Yang N, Wen MC, Wang R,
Goldfarb J, Sampson H and Li XM: Constituents of the anti-asthma
herbal formula ASHMI(TM) synergistically inhibit IL-4 and IL-5
secretion by murine Th2 memory cells, and eotaxin by human lung
fibroblasts in vitro. J Integr Med. 11:195–205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Seys SF, Grabowski M, Adriaensen W,
Decraene A, Dilissen E, Vanoirbeek JA, Dupont LJ, Ceuppens JL and
Bullens DM: Sputum cytokine mapping reveals an ‘IL-5, IL-17A,
IL-25-high’ pattern associated with poorly controlled asthma. Clin
Exp Allergy. 43:1009–1017. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hussain S: IL-13 gene polymorphisms and
their association with atopic asthma and rhinitis in Pakistani
patients. Iran J Allergy Asthma Immunol. 13:298–299.
2014.PubMed/NCBI
|
|
34
|
Utsumi Y, Sasaki N, Nagashima H, Suzuki N,
Nakamura Y, Yamashita M, Kobayashi H and Yamauchi K: Association of
IL-13 gene polymorphisms with airway hyperresponsiveness in a
Japanese adult asthmatic population. Respir Investig. 51:147–152.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Haldar P, Brightling CE, Hargadon B, Gupta
S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw
AJ and Pavord ID: Mepolizumab and exacerbations of refractory
eosinophilic asthma. N Engl J Med. 360:973–984. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pavord ID, Korn S, Howarth P, Bleecker ER,
Buhl R, Keene ON, Ortega H and Chanez P: Mepolizumab for severe
eosinophilic asthma (DREAM): A multicentre, double-blind,
placebo-controlled trial. Lancet. 380:651–659. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nair P, Pizzichini MM, Kjarsgaard M, Inman
MD, Efthimiadis A, Pizzichini E, Hargreave FE and O'Byrne PM:
Mepolizumab for prednisone-dependent asthma with sputum
eosinophilia. N Engl J Med. 360:985–993. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Castro M, Mathur S, Hargreave F, Boulet
LP, Xie F, Young J, Wilkins HJ, Henkel T and Nair P: Res-5-0010
Study Group: Reslizumab for poorly controlled, eosinophilic asthma:
A randomized, placebo-controlled study. Am J Respir Crit Care Med.
184:1125–1132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Y and McCusker CT:
Interleukin-13-dependent bronchial hyper-responsiveness following
isolated upper-airway allergen challenge in a murine model of
allergic rhinitis and asthma. Clin Exp Allergy. 35:1104–1111. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hashimoto S and Bel EH: Targeting IL-5 in
severe asthma: A DREAM come true? Lancet. 380:626–627. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mathur M, Herrmann K, Li X, Qin Y,
Weinstock J, Elliott D, Monahan J and Padrid P: TRFK-5 reverses
established airway eosinophilia but not established
hyperresponsiveness in a murine model of chronic asthma. Am J
Respir Crit Care Med. 159:580–587. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Leckie MJ, ten Brinke A, Khan J, Diamant
Z, O'Connor BJ, Walls CM, Mathur AK, Cowley HC, Chung KF,
Djukanovic R, et al: Effects of an interleukin-5 blocking
monoclonal antibody on eosinophils, airway hyper-responsiveness and
the late asthmatic response. Lancet. 356:2144–2148. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
El-Zein M, Parent ME, Benedetti A and
Rousseau MC: Does BCG vaccination protect against the development
of childhood asthma? A systematic review and meta-analysis of
epidemiological studies. Int J Epidemiol. 39:469–486. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Obihara CC, Bollen CW, Beyers N and Kimpen
JL: Mycobacterial infection and atopy in childhood: A systematic
review. Pediatr Allergy Immunol. 18:551–559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hopfenspirger MT, Parr SK, Hopp RJ,
Townley RG and Agrawal DK: Mycobacterial antigens attenuate late
phase response, airway hyperresponsiveness, and bronchoalveolar
lavage eosinophilia in a mouse model of bronchial asthma. Int
Immunopharmacol. 1:1743–1751. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Assa'ad AH, Gupta SK, Collins MH, Thomson
M, Heath AT, Smith DA, Perschy TL, Jurgensen CH, Ortega HG and
Aceves SS: An antibody against IL-5 reduces numbers of esophageal
intraepithelial eosinophils in children with eosinophilic
esophagitis. Gastroenterology. 141:1593–1604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Spergel JM, Rothenberg ME, Collins MH,
Furuta GT, Markowitz JE, Fuchs G III, O'Gorman MA, Abonia JP, Young
J, Henkel T, et al: Reslizumab in children and adolescents with
eosinophilic esophagitis: Results of a double-blind, randomized,
placebo-controlled trial. J Allergy Clin Immunol. 129:456–463,
463.e1-3. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Assa'ad AH and Rothenberg ME: Eosinophilic
asthma: Insights into the effects of reducing IL-5
receptor-positive cell levels. J Allergy Clin Immunol.
132:1097–1098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Akira S: Pathogen recognition by innate
immunity and its signaling. Proc Jpn Acad Ser B Phys Biol Sci.
85:143–156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Reba SM, Li Q, Onwuzulike S, Ding X, Karim
AF, Hernandez Y, Fulton SA, Harding CV, Lancioni CL, Nagy N, et al:
TLR2 engagement on CD4(+) T cells enhances effector functions and
protective responses to Mycobacterium tuberculosis. Eur J Immunol.
44:1410–1421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Almeida PE, Roque NR, Magalhães KG, Mattos
KA, Teixeira L, Maya-Monteiro C, Almeida CJ, Castro-Faria-Neto HC,
Ryffel B, Quesniaux VF and Bozza PT: Differential TLR2 downstream
signaling regulates lipid metabolism and cytokine production
triggered by Mycobacterium bovis BCG infection. Biochim Biophys
Acta. 1841:97–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chávez-Galán L, Ramon-Luing LA,
Torre-Bouscoulet L, Pérez-Padilla R and Sada-Ovalle I: Pre-exposure
of Mycobacterium tuberculosis-infected macrophages to crystalline
silica impairs control of bacterial growth by deregulating the
balance between apoptosis and necrosis. PLoS One. 8:e809712013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Akdis CA, Kussebi F, Pulendran B, Akdis M,
Lauener RP, Schmidt-Weber CB, Klunker S, Isitmangil G, Hansjee N,
Wynn TA, et al: Inhibition of T helper 2-type responses, IgE
production and eosinophilia by synthetic lipopeptides. Eur J
Immunol. 33:2717–2726. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Manicassamy S and Pulendran B: Modulation
of adaptive immunity with Toll-like receptors. Semin Immunol.
21:185–193. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cdos S Samary, Antunes MA, Silva JD, Silva
AL, Araujo CC, Bakker-Abreu I, Diaz BL, Fernezlian S, Parra ER,
Capelozzi VL, et al: Impact of bacillus Calmette-Guerin Moreau
vaccine on lung remodeling in experimental asthma. Respir Physiol
Neurobiol. 189:614–623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Czerkinsky C, Anjuere F, McGhee JR,
George-Chandy A, Holmgren J, Kieny MP, Fujiyashi K, Mestecky JF,
Pierrefite-Carle V, Rask C and Sun JB: Mucosal immunity and
tolerance: Relevance to vaccine development. Immunol Rev.
170:197–222. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Horner AA and Raz E: Immunostimulatory
sequence oligodeoxynucleotide: A novel mucosal adjuvant. Clin
Immunol. 95:(Suppl). S19–S29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Horner AA, Datta SK, Takabayashi K,
Belyakov IM, Hayashi T, Cinman N, Nguyen MD, Van Uden JH, Berzofsky
JA, Richman DD and Raz E: Immunostimulatory DNA-based vaccines
elicit multifaceted immune responses against HIV at systemic and
mucosal sites. J Immunol. 167:1584–1591. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Takabayashi K, Libet L, Chisholm D,
Zubeldia J and Horner AA: Intranasal immunotherapy is more
effective than intradermal immunotherapy for the induction of
airway allergen tolerance in Th2-sensitized mice. J Immunol.
170:3898–3905. 2003. View Article : Google Scholar : PubMed/NCBI
|