Introduction
Ultrasmall superparamagnetic iron-oxide
nanoparticles (USPIOs) have been used as negative contrast agents.
They exert T2- and T1-shortening effects, and their potential as an
angiographic contrast agent has been investigated (1,2). Its
long blood half-life makes it possible to use USPIO as a
blood-pooling agent during the early phase of magnetic resonance
angiography (MRA), however, USPIO is not suitable for the late
phase of MRA, due to the presence of phagocytic Kupffer cells
(3). Gadofosveset, a gadolinium
(Gd)-based blood-pooling agent, has been approved by the U.S. Food
and Drug Administration for aortoiliac MRA in certain patients
(4). A single dose can be injected
for first-pass imaging; a post-injection interval of 10 to 20 min
has been suggested for optimal steady-state imaging (5). However, the use of gadofosveset
trisodium may increase the risk for nephrogenic systemic fibrosis
(NSF) in patients with renal impairment (6).
Xiao et al (7) and Nitta et al (8–10)
reported a novel, long-circulating blood-pooling contrast agent,
which involved iron-based substances. Nitta et al
synthesized carboxymethyl-diethylaminoethyl dextran magnetite
(CMEADM) particles (8–10) and used this Gd-free contrast agent
in experimental magnetic resonance imaging (MRI) studies (8,9). As
with superparamagnetic iron-oxide nanoparticles (SPIOs) and USPIOs,
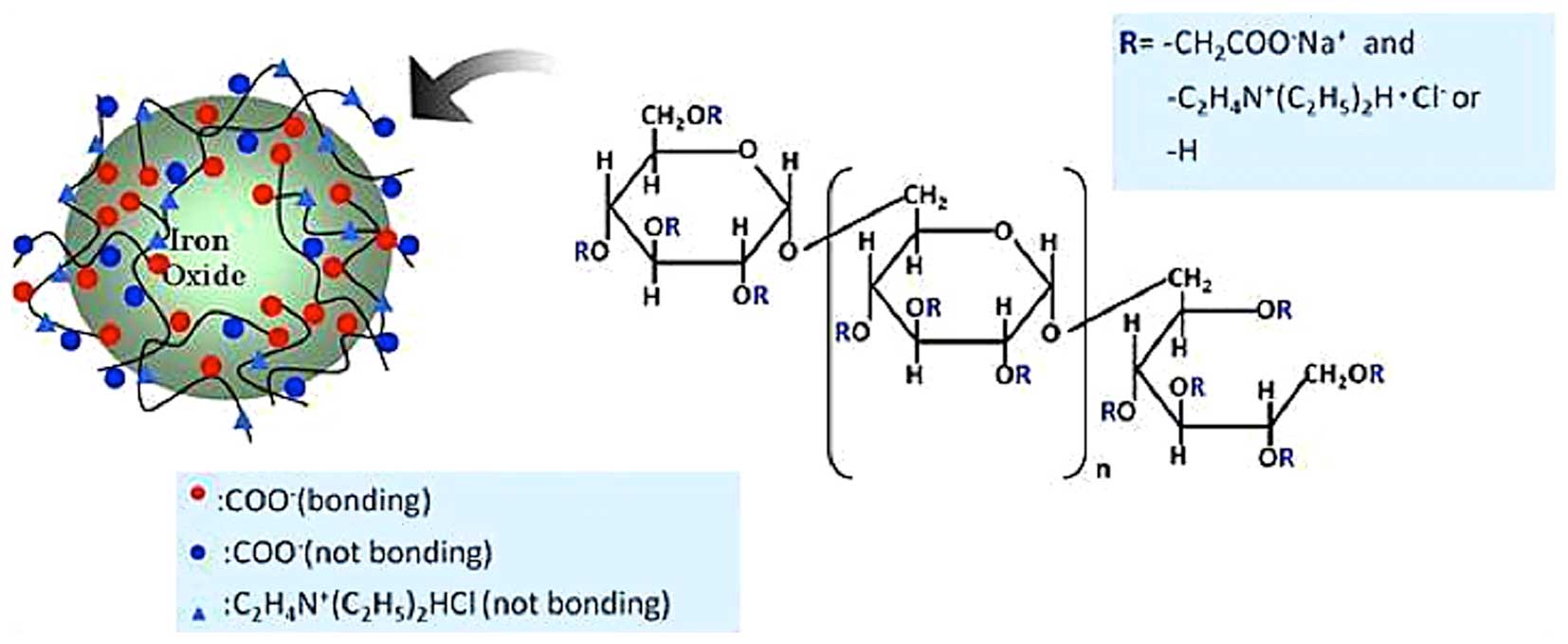
CMEADM is based on iron-oxide. As CMEADM is coated with polymers,
including dextran, to prevent aggregation, its pooling time in
blood vessels is longer, compared with USPIO (11–13),
and its surface carries a negative or a positive charge due to the
binding of amino and carboxyl groups to the hydroxyl group of
dextran (Fig. 1). The present
study evaluated whether the degree of charge on CMEADM altered the
blood pooling time in rabbits.
Materials and methods
Animals
All experiments were approved in advance by the
ethical animal experiments committee of Shiga University of Medical
Science (Otsu, Japan), and performed according to the Animal Care
Guidelines of Shiga University of Medical Science. Female Japanese
white rabbits (3.0 kg) were purchased from Japan SLC, Inc. (Tokyo,
Japan). Prior to each MRI session, the rabbits were anesthetized
with intramuscular injections of a mixture of ketaminehydrochloride
(25 mg/kg Ketalar 50; Sankyo Yell Yakuhin Co., Ltd., Tokyo, Japan)
and medetomidine hydrochloride (0.1 mg/kg; Domitor; Meiji Seika
Co., Ltd., Tokyo, Japan). All animals were housed in a
temperature-controlled room (24±1°C) on a 12-h light/12-h dark
cycle. They had access to standard laboratory chow ad
libitum.
A total of 12 Japanese white rabbits were randomly
divided into four groups of three rabbits. The animals received an
intravenous injection of 40 µmol Fe/kg of differently-charged
CMEADM or USPIO (Table I).
 | Table I.Properties of the injected differently
charged CMEADM or USPIO contrast agents. |
Table I.
Properties of the injected differently
charged CMEADM or USPIO contrast agents.
| Contrast agent | Particle size
(nm) | Iron concentration
(mg/ml) | T2 relaxivity (r2:
mM/s) | T1 relaxivity (r1:
mM/s) | Surface charge
(mV) |
|---|
|
CMEADM− | 32.0 | 15.0 | 114.0 | 34.0 | −10.4 |
|
CMEADM2− | 29.0 | 15.0 | 91.0 | 34.0 | −41.0 |
|
CMEADM+ | 32.0 | 15.0 | 106.0 | 33.0 | 9.6 |
| USPIO | 28.0 | 15.0 | 95.0 | 34.0 | −11.5 |
Following pre-scanning, one of four types of iron
nanoparticles, CMEADM−, CMEADM2− and
CMEADM+ with surface charges of −10.4, −41.0, and +9.6
mV, respectively, or USPIO (−11.5 mV), was injected intravenously
into the auricular vein. All contrast agents were obtained from
Meito Sangyo Co., Ltd. (Tokyo, Japan). MRA images were obtained
immediately following injection, and at 30, 60, 180 and 300 min
post-injection.
MRI
MRI scanning was performed on a 1.5T MRI
instrument (SIGNA Excite HDx; GE Healthcare Life Sciences,
Shanghai, China) with a circularly polarized head coil and the
following scanning parameters: 3D fast-spoiled gradient echo;
repetition time, 9.0 ms; echo time, 1.4 ms; flip angle, 25°C;
field-of-view, 320×240 mm; slice thickness, 1 mm; matrix,
256×256.
The relative signal enhancement, or relative signal
intensity (SIrel), was measured at the abdominal aorta
and the inferior vena cava (IVC) on 1-mm-thick coronal images.
SIrel was calculated using the following formula:
SIrel = (SI post-contrast - SI
pre-contrast / SI pre-contrast) × 100
These values were calculated by dividing the signal
of the aorta or IVC by the background SI observed over time.
Subsequent to the experiments, rabbits were sacrificed by injecting
the heart with an overdose of pentobarbital (Dainippon Sumitomo
Pharma Co., Ltd., Tokyo, Japan).
Statistical analysis
The results were analyzed by performing analysis of
variance followed by Tukey's HSD test using IBM SPSS 20 statistical
software (IBM SPSS, Armonk, NY, USA) for Windows. P<0.05 was
considered to indicate a statistically significant difference.
Results
Visibility of vasculature
The vessels were clearly visualized. Compared with
the group injected with USPIO, the thoracic and abdominal aorta,
and the IVC manifested significantly higher SI values following the
injection of the three types of CMEADM. Even at 300 min-post
injection, the thoracic and abdominal aorta, and the IVC were
clearly visible (Fig. 2).
Irrespective of their charge, with all three CMEADMs
used in the present study, the vascular enhancing effect persisted
for up to 300 min on the MRA images. By contrast, with the
conventional USPIO, the intravascular SI was markedly decreased and
had almost disappeared at 180 min.
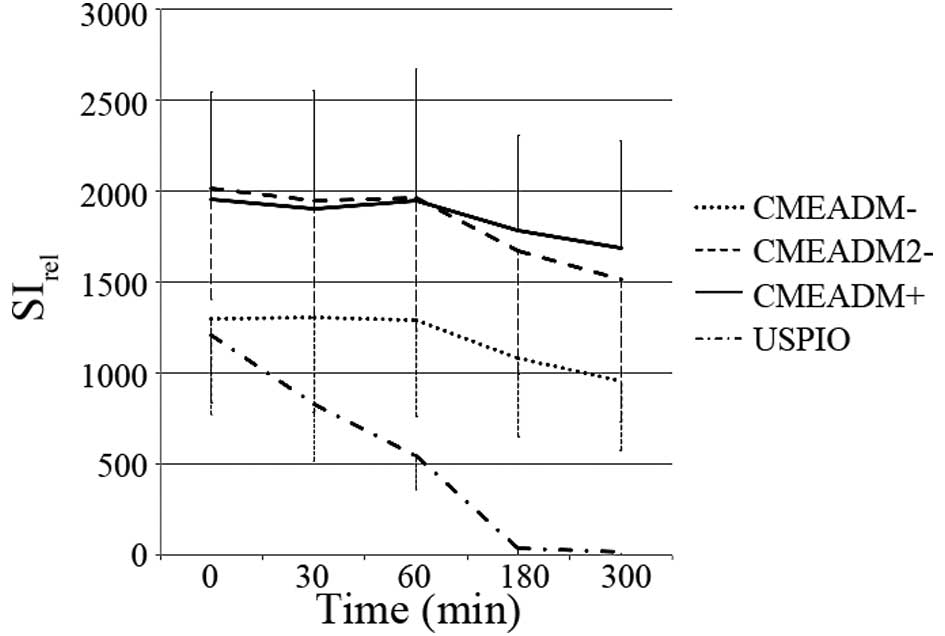
Relative signal enhancement
SIrel continued to be present in the abdominal aorta
and IVC 30, 60, 180 and 300 min following the delivery of
CMEADM−, CMEADM2− or CMEADM+
(Figs. 3 and 4). At 180 and 300 min, the elevation of
SIrel persisted on all the CMEADM− images, but not on
the USPIO images. No significant differences were found in the
enhancing abilities of CMEADM−, CMEADM2− or
CMEADM+ at 30 min post-injection.
Discussion
The present study compared standard USPIO and
CMEADMs for their efficacy as blood-pooling MR contrast agents. All
three differently-charged CMEADMs (CMEADMU−,
CMEADMU2− and CMEADMU+) elicited a
sufficiently prolonged vascular enhancing effect in the rabbits.
Investigations are underway to determine whether these contrast
agents qualify as novel blood-pooling MR contrast agents in the
clinical setting.
The present study documented that, with the newly
synthesized iron nanoparticles, CMEADM, the retention of
SIrel in the blood vessels of the rabbits was prolonged.
As these agents are Gd-free, they can be used in patients with
renal impairment, including stage 4 or 5 chronic kidney disease
(glomerular filtration rate <30 ml/min per 1.73 m2),
who may be at risk for NSF when Gd agents are used. Although the
injection of iron oxide nanoparticles may elicit cellular
disorders, they may represent an alternative contrast agent for use
in patients with renal impairment (14).
Conventional USPIOs have been used as negative
contrast agents. However, as documented in the present study, their
pooling time was shorter, compared with the durations observed in
the CMEADM groups. Smaller particles are less likely to be
phagocytized, compared with large particles (11,13,15),
however, the sizes of CMEADM and USPIO particles are similar.
According to Jo et al (16). iron oxide nanoparticles with
positive surface potential interact ionically with the cell surface
as it is negatively charged. Consequently, the cell internalization
of nanoparticles is increased.
The present study hypothesized that the negatively
charged surface may affect the pooling time as a result of the
decreased likelihood of phagocytosis. The charge of the USPIO in
the present study was −11.5 mV. The present study synthesized three
types of CMEADM with a similar charge of −10.4 mV
(CMEADM−), a lower charge of 9.6 mV (CMEADM+)
and a higher charge of −41.0 mV (CMEADM2+), compared
with that of USPIO, and calculated their SIrel values.
It was found that with all three types of CMEADM, irrespective of
their charge, the blood-pooling time was prolonged. This suggested
that their surface charge, whether positive or negative, did not
affect the pooling time.
Iron nanoparticles, including USPIOs, are
phagocytized in the liver and spleen, and metabolized via the same
metabolic pathway as hemoglobin iron. However, although CMEADM may
be metabolized via a similar pathway, these particles are different
from conventional USPIOs, as the iron in CMEADM is covered by an
increased quantity of dextran. It may be possible to retard their
clearance from vessels by rendering their surface hydrophilic with
poloxamers and poloxamines, including dextran (17). Covering CMEADM particles with
dextran may reduce their recognition by phagocytes and/or their
entrapment by Kupffer cells (8,18).
The superior pooling time of CMEADMs in the blood stream may
reflect a hydrophilic effect, and investigations are underway to
determine whether this is the case.
CMEADM particles are considered to have a longer
half-life, compared with USPIOs, and may be more useful for
long-term angiographic investigations in humans. Due to the long
pooling time of CMEAD in vessels, a single administration of the
agent may suffice for the pre- and post-procedure angiographic
evaluation of patients undergoing percutaneous transluminal
angioplasty of coronary arteries, stent placement (percutaneous
coronary intervention) or aortic stent grafting (8). In addition, MRI examinations using
CMEADM agents may be used for the diagnosis of patients with
intermittent gastrointestinal bleeding. Although Gd contrast agents
may elicit complications, including NSF, to the best of our
knowledge, there have been no reports of patients with serious
adverse effects elicited by the administration of iron
nanoparticles to date.
The present study had a number of limitations.
First, the number of rabbits included in the investigation was
small. However, it was of a sufficient size to enable assessment of
statistical significance, and the prolonged enhancement effect of
CMEADM 180 min post-injection was clearly demonstrated. Therefore,
the number of rabbits used was deemed sufficient to support the
conclusions. Second, it was not possible to compare the CMEADM with
emerging non-contrast MRA techniques, including quiescent-interval
single-shot MRA, with respect to vessel visualization (19). Third, the present study found that
visually confirmed vascular enhancement persisted for up to 300
min. This may raise concerns regarding the wash-out kinetics of
these agents. Although these agents do not elicit NSF or other
renal complications, for example long elimination time, this is a
significant limitation. Fourth, CMEADM particles are covered with
an increased quantity of dextran, compared with USPIO. The dextran
used for particle covering is the same substance used for
ferucarbotran. Although the present study suggested that the use of
CMEADM is safe, in rare instances, dextran has been reported to
elicit severe anaphylactic reactions (20). Consequently, the safety of CMEADM
requires further elucidation. Investigations are underway to
determine the elimination routes and kinetics, and to obtain
quantitative data.
References
|
1
|
Allkemper T, Bremer C, Matuszewski L,
Ebert W and Reimer P: Contrast-enhanced blood-pool MR angiography
with optimized iron oxides: Effect of size and dose on vascular
contrast enhancement in rabbits. Radiology. 223:432–438. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bremerich J, Bilecen D and Reimer P: MR
angiography with blood pool contrast agents. Eur Radiol.
17:3017–3024. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leung K: Ultrasmall superparamagnetic iron
oxide nanoparticles conjugated with
Ile-Pro-Leu-Pro-Phe-Tyr-AsnMolecular imaging and contrast agent
database (MICAD). Bethesda (MD): Fed. 23–2010
|
|
4
|
Bryson J, Reineke JW and Reineke TM:
Macromolecular imaging agents containing lanthanides: Can
conceptual promise lead to clinical potential? Macromolecules.
45:8939–8952. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grist TM, Korosec FR, Peters DC, Witte S,
Walovitch RC, Dolan RP, Bridson WE, Yucel EK and Mistretta CA:
Steady-state and dynamic MR angiography with MS-325: Initial
experience in humans. Radiology. 207:539–544. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weller A, Barber JL and Olsen OE:
Gadolinium and nephrogenic systemic fibrosis: An update. Pediatr
Nephrol. 29:1927–1937. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiao W, Lin J, Li M, Ma Y, Chen Y, Zhang
C, Li D and Gu H: Prolonged in vivo circulation time by
zwitterionic modification of magnetite nanoparticles for blood pool
contrast agents. Contrast Media Mol Imaging. 7:320–327. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nitta N, Tsuchiya K, Sonoda A, Ota S,
Ushio N, Takahashi M, Murata K and Nohara S: Negatively charged
superparamagnetic iron oxide nanoparticles: A new blood-pooling
magnetic resonance contrast agent. Jpn J Radiol. 30:832–839. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsuchiya K, Nitta N, Sonoda A, Nitta-Seko
A, Ohta S, Takahashi M, Murata K, Mukaisho K, Shiomi M, Tabata Y
and Nohara S: Evaluation of atherosclerotic lesions using dextran-
and mannan-dextran-coated USPIO: MRI analysis and pathological
findings. Int J Nanomedicine. 7:2271–2280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsuchiya K, Nitta N, Sonoda A, Otani H,
Takahashi M, Murata K, Shiomi M, Tabata Y and Nohara S:
Atherosclerotic imaging using 4 types of superparamagnetic iron
oxides: New possibilities for mannan-coated particles. Eur J
Radiol. 82:1919–1925. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arbab AS, Liu W and Frank JA: Cellular
magnetic resonance imaging: Current status and future prospects.
Expert Rev Med Devices. 3:427–439. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawaguchi T, Hanaichi T, Hasegawa M and
Maruno S: Dextran-magnetite complex: Conformation of dextran chains
and stability of solution. J Mater Sci Mater Med. 12:121–127. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raynal I, Prigent P, Peyramaure S, Najid
A, Rebuzzi C and Corot C: Macrophage endocytosis of
superparamagnetic iron oxide nanoparticles: Mechanisms and
comparison of ferumoxides and ferumoxtran-10. Invest Radiol.
39:56–63. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Neuwelt EA, Hamilton BE, Varallyay CG,
Rooney WR, Edelman RD, Jacobs PM and Watnick SG: Ultrasmall
superparamagnetic iron oxides (USPIOs): A future alternative
magnetic resonance (MR) contrast agent for patients at risk for
nephrogenic systemic fibrosis (NSF)? Kidney Int. 75:465–474. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Corot C, Port M, Guibert I, Robert P,
Raynal I, Robic C, Raynaud JS, Prigent P, Dencausse A, et al:
Superparamagnetic contrast agents. 1st. London: CRC Press; pp.
59–84. 2007
|
|
16
|
Jo J, Aoki I and Tabata Y: Design of iron
oxide nanoparticles with different sizes and surface charges for
simple and efficient labeling of mesenchymal stem cells. J Control
Release. 142:465–473. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gaur U, Sahoo SK, De TK, Ghosh PC, Maitra
A and Ghosh PK: Biodistribution of fluoresceinated dextran using
novel nanoparticles evading reticuloendothelial system. Int J
Pharm. 202:1–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bulte JW and Kraitchman DL: Iron oxide MR
contrast agents for molecular and cellular imaging. NMR Biomed.
17:484–499. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Klasen J, Blondin D, Schmitt P, Bi X,
Sansone R, Wittsack HJ, Kröpil P, Quentin M, Kuhlemann J, Miese F,
et al: Nonenhanced ECG-gated quiescent-interval single-shot MRA
(QISS-MRA) of the lower extremities: Comparison with
contrast-enhanced MRA. Clin Radiol. 67:441–446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vaage-Nilsen O: Acute, severe and
anaphylactoid reactions are very rare with low-molecular-weight
iron dextran, CosmoFer. Nephrol Dial Transplant. 23:33722008.
View Article : Google Scholar : PubMed/NCBI
|