Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common cancer in the world accounting for ~600,000 mortalities each
year (1–3). HCC is the third most common cause of
cancer mortality and is more prevalent in developing countries,
particularly those located in Eastern and South-Eastern Asia
compared with the developed world (4,5). In
China, the age-standardized incidence rate of HCC is 37.9 per
100,000 for men and 14.2 per 100,000 for women (6). Tumor resection, liver
transplantation, radiofrequency (thermal) ablation, percutaneous
ethanol injection and transarterial chemoembolization are the
primary treatment options (7–9);
however, these treatments are expensive. Therefore, additional
investigation of the pathogenesis of HCC with the goal of
identifying effective and affordable methods of treatment is
required.
The B7 family of T cell co-stimulatory and
co-inhibitory molecules is vital for the regulation of adaptive
immune responses. B7-H4 (also termed B7x or B7S1) has been
determined to be involved in the downregulation of antigen-specific
immune responses as it inhibited T cell proliferation, cell cycle
progression, and cytokine production (10–12).
In mice, B7-H4 transcripts were ubiquitously expressed; however, no
protein expression was detected (11–13).
A previous study did not detect B7-H4 protein expression in normal
human tissues (14). However,
B7-H4 has been overexpressed in various human tumors, including
esophageal squamous cell carcinoma (15), pancreatic cancer (16), gastric cancer (17,18),
colorectal carcinoma (19) and
lung cancer (20). A previous
study also reported that B7-H4 is expressed in patients with liver
cancer (21); however, whether the
level of B7-H4 may be correlated with HCC pathogenesis remains to
be elucidated.
B7-H4 may inhibit the function of human T cells
(22). Th1 and Th2 CD4+
T cells are crucial for effective immune protection (23). Th1 cells mediate antitumor
reactivity through the secretion of cytokines, including
interferon-γ (IFN-γ) and tumor necrosis factor-α. Th2 cells
downregulate antitumor immunity by secretion of cytokines, such as
interleukin (IL)-4, IL-6 and IL-10 (24,25).
A previous study determined that the Th1/Th2 balance may be
disrupted in patients with HCC (26). Therefore, it is possible that
aberrant expression of B7-H4 may be associated with the Th1/Th2
imbalance and contribute to HCC pathogenesis. The present study
investigated the association between the expression levels of B7-H4
and HCC development.
Materials and methods
Cell culture
The H22 murine hepatoma cell line was obtained from
the Department of Medicine & Pharmacy Research Center of
Binzhou Medical University (Binzhou, China). The HL-7702 normal
human normal liver cell line and the Huh7 human hepatoma cell line
were obtained from Cell Research Institute of the Chinese Academy
of Sciences (Shanghai, China) and were cultured in Dulbecco's
modified Eagle's medium (Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA) supplemented with 20% fetal bovine serum (Hyclone;
GE Healthcare Life Sciences), 2 mM L-glutamine, 2 mM
4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid, 100 mg/ml
streptomycin and 100 U/ml penicillin in 5% CO2 at 37°C.
Establishment of tumor model
C57BL/6 mice were purchased from Weitong Lihua
Experimental Animal Technical Co., Ltd. (Beijing, China) In total
30 mice were tumor-bearing mice and 18 were used as controls.
Female and male mice (6–7 weeks old; 20–22 g) were housed at a
ratio of 1:1 in a cage. All mice were housed six per cage at
20–24°C in a specific pathogen-free environment with a 12 h
light/12 h dark cycle. Water and feed were sterilized by high
pressure steam sterilization. All the animal experiments were
performed following the ethical standards formulated by the
Institutional Animal Experimental Ethics Committee of Binzhou
Medical University.
H22 cells (1×106) were injected into the
abdominal cavities of 6–7 week old C57BL/6 mice (n=5). After 10
days, the mice were euthanized by cervical dislocation and a
syringe was used to extract the ascites. The ascites were diluted
to 5.0×105 cells/ml. Next, 0.2 ml H22 cells were
subcutaneously injected into the right armpit of each mouse in
order to establish tumors. After 24 h, 30 mice were randomly
divided into three groups (n=10 per group). Tumors were established
for 20, 30 or 40 days in the three groups. Six mice were used as
controls for each time point. The healthy control group received
only saline injections. The mice were sacrificed via cervical
dislocation after 20, 30 and 40 days.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total mRNA was extracted from HL-7702 and Huh7 cells
(1×106) using TRIzol (TaKaRa Biotechnology Co., Ltd.,
Dalian, China) according to the manufacturer's protocol. RT was
performed using a PrimeScript RT-PCR kit (TaKaRa Biotechnology Co.,
Ltd.) according to the manufacturer's instructions. The following
primers for B7-H4 and β-actin were obtained from
Sangon Biotech Co., Ltd. (Shanghai, China): B7-H4, F
5′-AGGCTTCTCTGTGTGTCTCTTC-3′, R 5′-CTTGCTCTTGTTTGCTCACTCC-3′;
β-actin, F 5′-TTGTTACAGGAAGTCCCTTGCC-3′, R
5′-ATGCTATCACCTCCCCTGTGTG-3′. All reactions were performed using
DNA polymerase from Transgen Biotech Co., Ltd. (Beijing, China) as
follows: 95°C for 10 min, 95°C for 15 sec and 60°C for 1 min for 35
cycles. PCR products were separated on 1% agarose gels and
visualized by ethidium bromide staining.
Western blot analysis
Mouse tumor tissues were lysed using a lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China) and quantified
using a bicinchoninic acid protein assay. Aliquots containing 20 µg
protein were dissolved in Laemmli buffer and incubated at 95°C for
10 min. Proteins were separated on a 12% Tris-glycine SDS-PAGE
(Beyotime Institute of Biotechnology), transferred onto
polyvinylidene difluoride membranes (BD Pharmingen, San Diego, CA,
USA) and incubated with 5% non-fat dry milk in Tris-buffered
saline/0.2% Tween-20 (TBST) for 2 h at room temperature. The
membranes were then incubated with anti-B7-H4 antibody (1:1,000
dilution; cat. no. ab108336; Abcam, Cambridge, UK) at 4°C
overnight. Membranes were then washed three times with 1X TBST
followed by incubation with horseradish peroxidase-conjugated goat
anti-rabbit IgG secondary antibody (1:5,000; dilution; cat. no.
ZB-2301; BIOSS, Beijing, China) for 1 h at room temperature.
Membranes were washed five times in 1X TBST and proteins were
visualized using an enhanced chemiluminescence kit (Roche
Diagnostics, Basel, Switzerland). GAPDH was detected with mouse
anti-GAPDH antibody (1:1,000 dilution; cat. no. AB-P-R 001; OriGene
Technologies, Inc., Beijing, China) as an internal control.
Cytokine quantification
Fresh mouse tumor tissue supernatants, and mouse and
human peripheral blood cells were lysed and centrifuged at 12,000 ×
g at 4°C for 30 min. Cytokine levels in supernatants were
determined using enzyme-linked immunosorbent assay (ELISA) kits for
IL-4 (human, cat. no. BP-E10142; mouse, cat. no. BP-E20011) IFN-γ
(human, BP-E10162; mouse, cat. no. BP-E11382), and soluble B7-H4
(sB7-H4; human, cat. no. BP-E11382; mouse, BP-E20905) from Shanghai
Langdon Biotechnology Co., Ltd. (Shanghai, China) following the
manufacturer's protocols. Standard curves served as internal
controls for the sensitivity and range of each assay. Each sample
was analyzed in triplicate.
Flow cytometry analysis
The expression level of B7-H4 in the HL-7702 and
Huh7 cell lines was determined by flow cytometry. Cells were fixed
and permeabilized using BD Perm Buffer III (BD Biosciences,
Franklin Lakes, NJ, USA) for 20 mins at 4°C. Samples were washed
and incubated with purified mouse anti-human B7-H4 antibody (2 µl
per sample; cat. no. 562506; BD Biosciences) for 60 min and then
with Alexa Fluor® 488 donkey anti-mouse IgG H&L
(1:500; cat. no. ab150105; Abcam) in the dark for 30 min at 4°C.
Finally, the cells were washed and resuspended in Stain buffer (BD
Biosciences) and analyzed on a BD FACSCalibur. The data were
analyzed with BD FACSDiva 7.0 software (BD Biosciences).
Patients
Patients with HCC were recruited from Yu Huang Ding
Hospital (Yantai, China) from August 2013 to January 2015. The
protocol for the present study was approved by University of
Binzhou Medical College Ethics Committee and informed consent was
obtained from all patients. The present study included patients
with HCC (n=60) and healthy controls (n=20). HCC was diagnosed on
the basis of biochemistry and image findings, including sonography,
computerized tomography scans, or magnetic resonance imaging scans.
All samples were examined histologically and diagnosis was
conducted according to the American Association for the Study of
Liver Diseases guidelines (9).
Immunohistochemical staining
B7-H4 expression was analyzed in human normal liver
tissues, HCC tissues and mouse tumor tissues using
immunohistochemistry staining. The B7-H4 primary antibody used was
obtained from Abcam, the secondary biotin-labeled goat-rabbit
antibody and diaminobenzidine tetrahydrochloride solution were
provided by Boster Biological Technology, Ltd. (Wuhan, China). All
samples were fixed in formalin solution and embedded in paraffin.
Sections (5 µm-thick) were dewaxed in xylene, dehydrated in
ethanol, and incubated in 3% H2O2 for 20 min. Following incubation
in 5% normal bovine serum (Boster Biological Technology, Ltd.) for
20 min, slides were incubated with the primary antibody at 4°C
overnight, and then with the secondary antibody at 37°C for 60 min.
Negative controls were established by replacing the primary
antibody with normal mouse IgG (BD Pharmingen). Slides were
visualized using light microscopy.
Evaluation of immunostaining
Immunostaining was independently examined by two
clinical pathologists. Five high-power fields were randomly
selected per sample. Staining intensity of positive tumor cells was
assessed. The extent of the staining was categorized into five
semiquantitative classes based on the percentages of
membrane-positive tumor cells: i) 0 (<5% positive tumor cells);
ii) 1 (6–25% positive tumor cells); iii) 2 (26–50% positive tumor
cells); iii) 3 (51–75% positive tumor cells); and iv) 4 (>75%
positive tumor cells). The intensity of staining was determined
semiquantitatively on a scale of 0 to 3 as follows: i) 0
(negative); ii) 1 (weakly positive), iii) 2 (moderately positive);
and iv) 3 (strongly positive). Multiplication of the intensity and
the percentage scores was used to obtain the final staining scores,
0 (negative), + (1–2), ++ (3–4), and
+++ (5–7).
Statistical analysis
For all statistical analyses, data were processed
with SPSS version 17.0 statistical software (SPSS, Inc., Chicago,
IL, USA). Correlation of B7-H4 protein expression with Clinical and
pathological features of patients with hepatocellular carcinoma was
evaluated with the Chi-square test. The correlations of the
expression levels of sB7-H4, IL-4 and IFN-γ were analyzed by
Spearman correlation coefficients. Two independent sample t-test
was used to analyze the significance of B7-H4 expression scores
between the HCC group and the control group. For comparison of
three or more groups, one-way analysis of variance was performed.
Data are presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference
Results
B7-H4 expression in HL-7702 and Huh7
cells
The expression of B7-H4 in the cell lines was
analyzed using RT-PCR and flow cytometry. B7-H4 transcripts
were expressed in both HL-7702 and Huh7 cell lines as presented in
Fig. 1A. The B7-H4 protein was not
detected in HL-7702 cells (Fig.
1B). B7-H4 was detected in Huh7 cells, with the expression
levels higher in the cytoplasm compared with the cell surface
(Fig. 1C).
Expression of B7-H4 in normal liver
and HCC tissues
In order to determine whether B7-H4 was
differentially expressed in human normal liver and HCC tissues,
immunohistochemical analysis was performed. B7-H4 protein was not
expressed in human normal liver tissues; however, it was expressed
in HCC tissues. B7-H4 was observed in the cytoplasm and membrane of
HCC cells; however, it was not detected in the nucleus (Fig. 1D and E).
B7-H4 expression and
clinicopathological features
Immunohistochemical analysis was used to examine
B7-H4 expression in patients with HCC (Table I). The patients included 35 males
and 25 females with an age range of 47 to 77 years (median, 61.9
years). A total of 27 patients had well or moderately-well
differentiated cancer cells and in 33 patients cancer cells were
poorly differentiated, 25 patients had lymph node metastasis.
Patients were staged from I to IV based on American Joint Committee
on Cancer standards. There were 28 patients in stages I and II and
32 patients in stages III and IV. Patient characteristics and
clinicopathological features are summarized in Table I.
 | Table I.Clinical and pathological features of
patients with hepatocellular carcinoma. |
Table I.
Clinical and pathological features of
patients with hepatocellular carcinoma.
|
|
| B7-H4
expression |
|
|---|
|
|
|
|
|
|---|
| Clinical
characteristic | Number | Negative | Positive |
P-valuea |
|---|
| Gender |
|
|
| 0.895 |
|
Male | 35 | 19 | 16 |
|
|
Female | 25 | 14 | 11 |
|
| Age |
|
|
| 0.979 |
|
<60 | 29 | 16 | 13 |
|
|
≥60 | 31 | 17 | 14 |
|
| TNM stages |
|
|
| 0.004 |
|
I+II | 28 | 21 | 7 |
|
|
III+IV | 32 | 12 | 20 |
|
| Differentiation
degree |
|
|
| 0.007 |
|
Well/moderate | 27 | 20 | 7 |
|
|
Poor | 33 | 13 | 20 |
|
| Lymph node
metastasis |
|
|
| 0.002 |
|
Yes | 25 | 8 | 17 |
|
| No | 35 | 25 | 10 |
|
| Size of tumor
(cm) |
|
|
| 0.979 |
|
<4 | 29 | 16 | 13 |
|
| ≥4 | 31 | 17 | 14 |
|
| Intravascular
cancer embolus |
|
|
| 0.802 |
|
Yes | 19 | 10 | 9 |
|
| No | 41 | 23 | 18 |
|
| Hepatocellular
carcinoma-associated tumor antigens |
| CEA
(0.5 ng/ml) |
|
|
| 0.714 |
|
<5 | 26 | 15 | 11 |
|
|
≥5 | 34 | 18 | 16 |
|
| CA19-9
(0–27 U/ml) |
|
|
| 0.895 |
|
<27 | 25 | 14 | 11 |
|
|
≥27 | 35 | 19 | 16 |
|
| AFP
(0–27 ng/ml) |
|
|
| 0.176 |
|
<400 | 17 | 7 | 10 |
|
|
≥400 | 43 | 26 | 17 |
|
B7-H4 expression in tumor tissues was significantly
positively correlated with TNM stage, differentiation degree and
lymph node metastasis (P<0.05; Table I). No association between B7-H4
expression and the remaining factors, including gender, age, tumor
size or hepatoma carcinoma-associated tumor antigens was identified
(Table I). Therefore, this
indicated that the expression of B7-H4 is associated with
aggressive HCC.
B7-H4 expression as a function of time
in mouse tumor model
The mouse liver tumor model was established by
subcutaneous injection of H22 cells into mice. B7-H4 in tumor
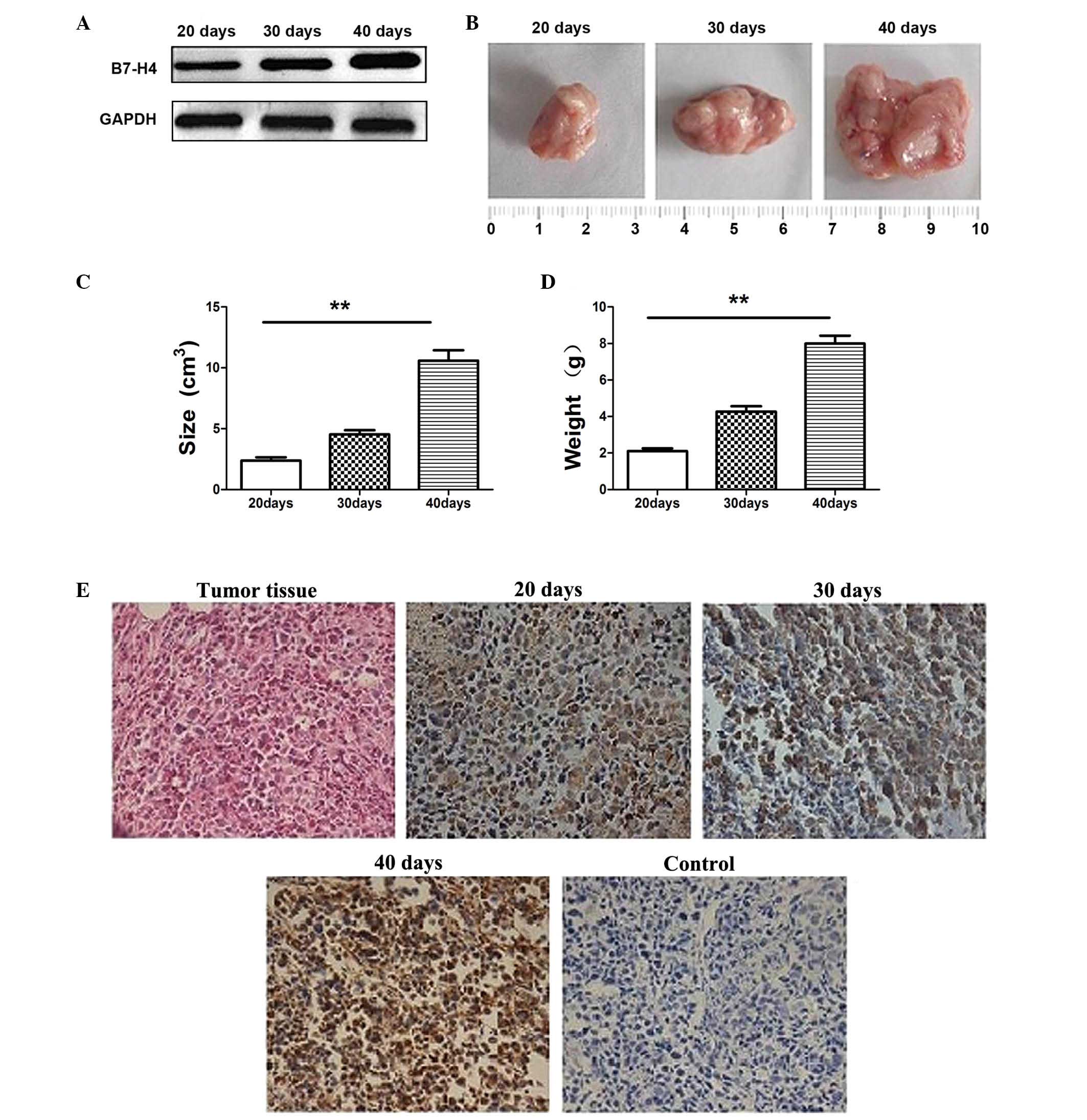
tissues was detected by western blotting (Fig. 2A). The sizes and weights of tumors
were evaluated at 20, 30, and 40 days and were found to
significantly increase with time (P<0.01; Fig. 2B-D). The expression of B7-H4 in
tumor tissues was confirmed by immunohistochemistry (Fig. 2E). At 20 days, B7-H4 levels were
3.28±0.47; at 30 days, levels were 5.36±0.38 and at 40 days, levels
were 6.18±0.32. These results indicated that the expression levels
of B7-H4 increased as tumors increased in size and weight.
Analysis of sB7-H4, IFN-γ and IL-4 in
HCC patients, tumorigenic mice and healthy controls
Cytokine levels in the blood serum were analyzed
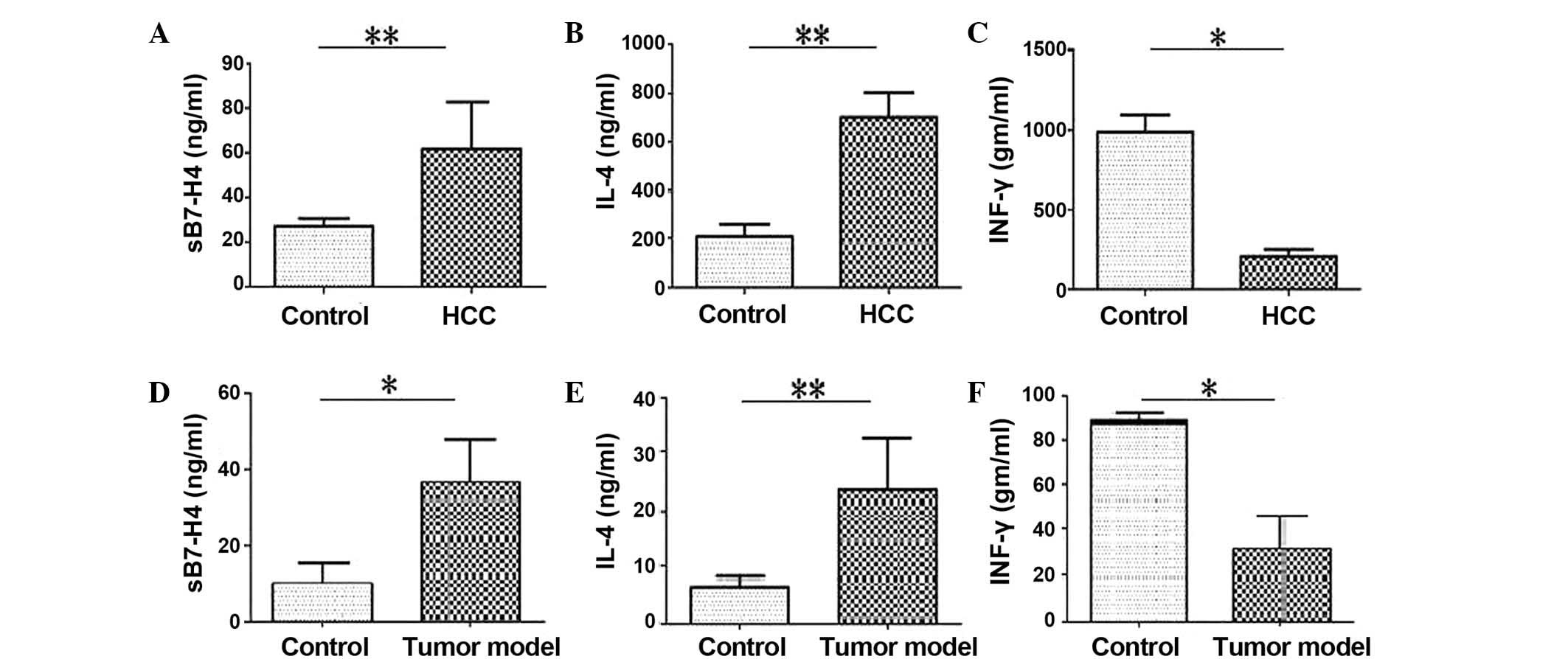
using an ELISA. The results revealed that sB7-H4 and IL-4 levels in
HCC patients were significantly higher compared with healthy
controls (P<0.001; Fig. 3A and
B). IFN-γ levels were significantly reduced in patients with
HCC compared with the healthy control group (P=0.017; Fig. 3C). The results revealed that sB7-H4
(P=0.018; Fig. 3D) and IL-4
(P=0.004; Fig. 3E) levels in the
serum of tumor-carrying mice were significantly higher compared
with healthy controls. IFN-γ levels were significantly reduced in
patients with HCC compared with the healthy control group (P=0.012;
Fig. 3F).
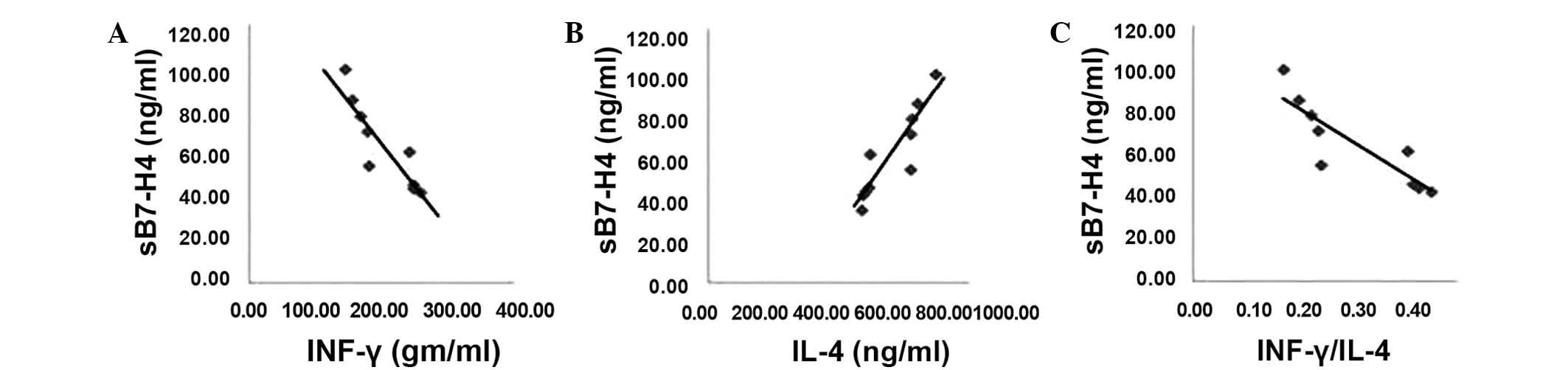
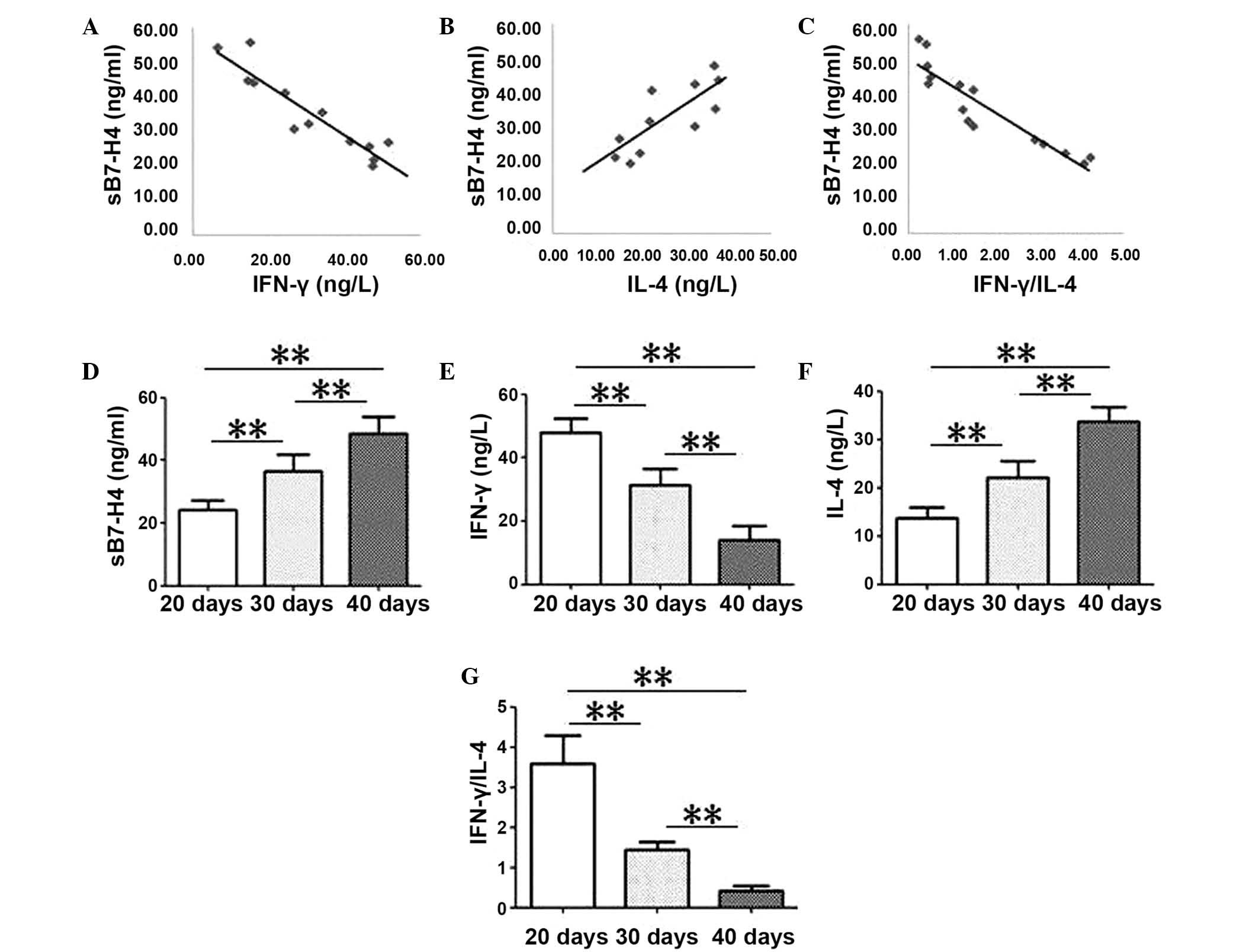
A Spearman's rank correlation analysis was used to
identify the correlations between sB7-H4 levels and IFN-γ, IL-4 and
the ratio between IFN-γ/IL-4 levels in blood serum samples of
patients with HCC (Fig. 4). sB7-H4
levels correlated negatively with IFN-γ and with the ratio of
IFN-γ/IL-4 (R=−0.888; P=0.001 and R=−0.864; P=0.003, Fig. 4A and C). Conversely, sB7-H4 levels
were positively correlated with the levels of IL-4 in the blood
serum samples of patients with HCC (R=0.903, P<0.001; Fig. 4C).
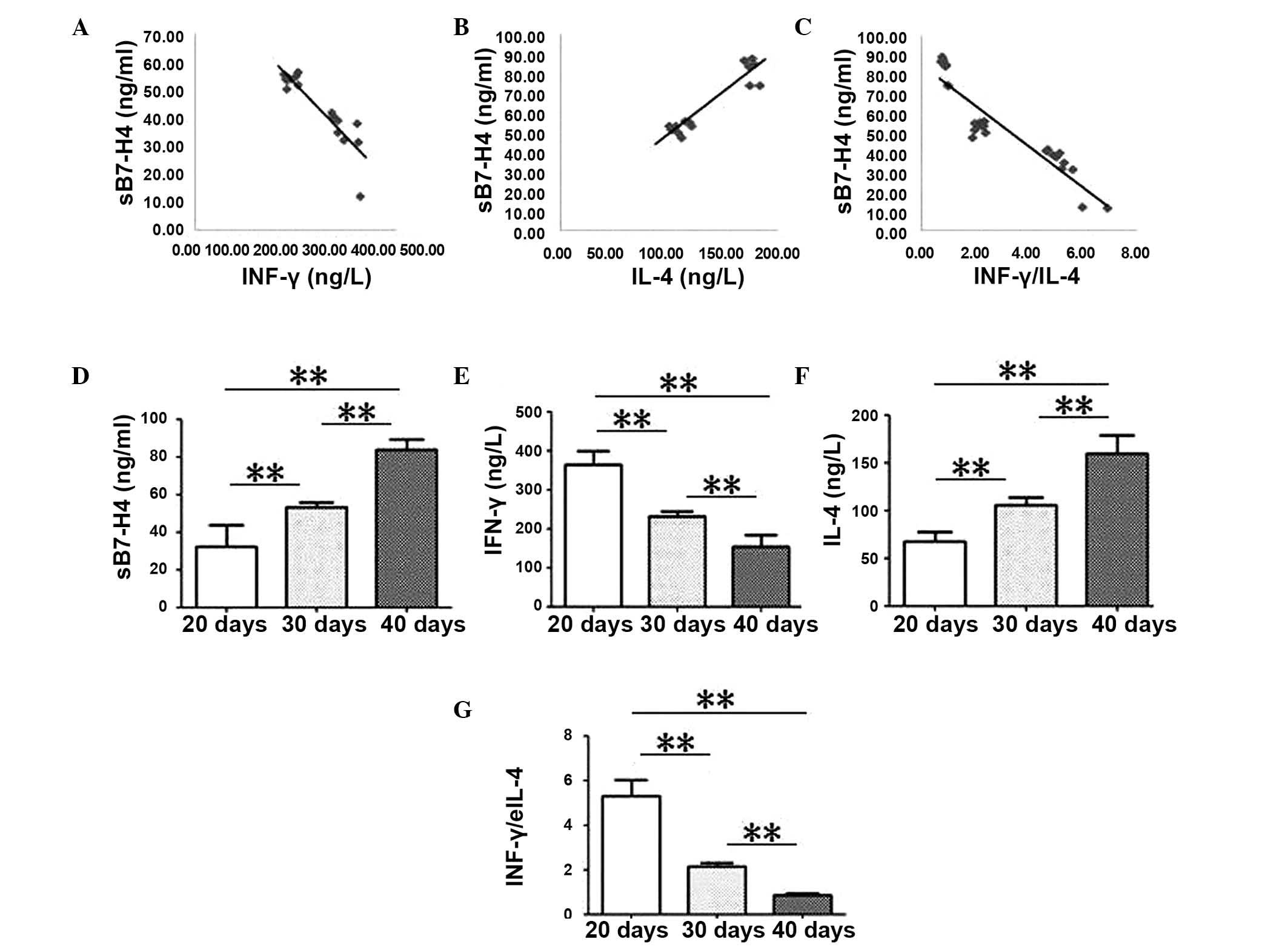
Spearman's rank was also used to determine
correlations between the cytokine levels in tumor samples (Fig. 5) and serum samples (Fig. 6) from the mouse tumor model. In
tumor tissue samples from mice, sB7-H4 levels were negatively
correlated with IFN-γ levels and with the ratio of IFN-γ/IL-4
(R=−0.919, P<0.001; and R=−0.925, P<0.001, respectively;
Fig. 5A and B). Negative
correlations were also observed in serum samples obtained from the
mice (R=−0.942; P<0.001 and R=−0.923; P<0.001; Fig. 6A and C). sB7-H4 levels were
positively correlated with IL-4 levels from mouse tumor tissue
(R=0.951; P<0.001; Fig. 5A) and
serum samples (R=0.917; P<0.001; Fig. 6B).
IFN-γ levels and the ratio of IFN-γ/IL-4
significantly decreased over time in tumor tissue (P<0.001;
Fig. 5E and G) and serum samples
(P<0.001; Fig. 6E and G).
Conversely, sB7-H4 and IL-4 levels in mouse tumor tissues
(P<0.001; Fig. 5D and F) and
serum samples (P<0.001; Fig. 6D and
F) significantly increased with time.
sB7-H4, IFN-γ and IL-4 levels are
altered with the clinical stage of HCC
In order to determine whether the expression levels
of sB7-H4, IFN-γ, and IL-4 are associated with the patient's HCC
clinical stage, ELISA analysis was used (Table II). sB7-H4 and IL-4 levels were
increased in stage III and IV tumors compared with stage I and II
tumors (P<0.001; Table II),
whereas IFN-γ levels were increased in stage III and IV tumors
compared with stage I and II tumors (P<0.001; Table II). Therefore, this indicated that
sB7-H4 may be a potential marker to predict tumor progression in
patients with HCC.
 | Table II.Serum level of sB7-H4, IFN-γ and IL-4
are changed in different clinical stages. |
Table II.
Serum level of sB7-H4, IFN-γ and IL-4
are changed in different clinical stages.
|
|
| sB7-H4 (ng/ml) | IL-4 (ng/ml) | IFN-γ (pg/m) |
|---|
|
|
|
|
|
|
|---|
| Group | n | Mean ± SD |
P-valuea | Mean ± SD |
P-valuea | Mean ± SD |
P-valuea |
|---|
| I–II stages | 28 | 41.94±4.68 | <0.001 | 600.87±13.55 | <0.001 | 247.08±6.48 | <0.001 |
| III–IV stages | 32 | 74.93±16.28 |
| 796.41±42.10 | <0.001 | 163.46±14.94 |
|
Discussion
HCC is a common malignant tumor with high morbidity
and mortality, particularly among patients in China (27). Previous studies have determined
that B7-H4 expression may be important for tumorigenesis (15–20).
B7-H4 is a member of the B7 family of costimulatory
ligands. It has a negative regulatory function in T cell-mediated
immunity as it has been identified to inhibit T cell activation,
proliferation and cytokine production (10–12).
Notably, despite widespread B7-H4 mRNA expression, the
expression levels of the B7-H4 protein have been observed to be
restricted in normal tissues (28). Previous studies have demonstrated
that the B7-H4 molecule is highly expressed in various different
types of human cancers, including pancreatic (16) and gastric cancer (17,18).
The present study revealed that the B7-H4 protein was not expressed
by the HL-7702 normal human liver cell line; however, it was
expressed by the Huh7 human hepatoma cells. Immunohistochemical
staining revealed that B7-H4 was expressed in HCC tissues and not
in normal liver tissues. In addition, in a mouse tumor model was
established using the H22 cell line. B7-H4 protein expression was
observed in the tumor tissues obtained from the mice. These results
indicate an association between B7-H4 expression and HCC.
Previous studies determined that B7-H4 had no
prognostic value for ovarian cancer (29) or breast cancer (30). However, the expression levels of
B7-H4 in renal cell carcinoma (RCC) (31), gastric cancer (17,18)
and colorectal carcinoma (19)
have been identified to be correlated with adverse
clinicopathological features, such as advanced lymph node
metastasis, tumor grade and TNM stage. Prostate carcinoma and
patients with RCC, that have B7-H4-positive tumors have also been
identified to be at a high risk of recurrence and had increased
mortality (32). The present study
determined that higher B7-H4 expression levels were observed in
samples from patients with poor differentiation and lymph node
metastasis and at later stages of progression. In the mouse model,
the expression levels of B7-H4 increased with time. These results
suggested that B7-H4 is important for the progression of HCC, B7-H4
may also be used as a molecular marker of HCC and as a novel target
for HCC therapy.
A previous study determined that serum B7-H4 levels
were significantly increased in patients with gastric cancer
compared with healthy volunteers, additionally high sB7-H4 levels
were significantly correlated with tumor size, lymph node
metastasis and TNM stage in patients with gastric cancer (33). Simon et al (34) determined that B7-H4 expression was
elevated in serum samples from ovarian cancer patients when
compared with healthy controls or women with benign gynecologic
diseases, including endometriosis, enlarged ovaries/edema and
polycystic ovaries. Zhang et al (35) revealed that sB7-H4 levels in
patients with HCC were significantly higher compared with those in
normal controls and that sB7-H4 levels were closely associated with
tumor size, tumor invasion, tumor differentiation and TNM stage
(35). However, they were not
associated with other characteristics, including age, gender and
alanine aminotransferase levels (35). In the present study, elevated
expression levels of sB7-H4 were observed in blood samples from
patients with HCC compared with healthy controls, which was
consistent with previous studies. Additionally, the current study
determined that the levels of IL-4 were higher and those of IFN-γ
lower in serum samples from patients with HCC compared with serum
samples from healthy controls. sB7-H4 levels were negatively
correlated with IFN-γ levels and with the ratio of IFN-γ/IL-4.
However, sB7-H4 levels were positively correlated with IL-4 levels
in serum samples obtained from patients with HCC. Similar results
were observed in serum and tumor tissues samples obtained from the
mouse model. This suggests that the expression levels of sB7-H4 may
be due to an imbalance of Th1 and Th2 cells, that facilitates the
development of HCC. In addition, the present study also determined
that sB7-H4 and IL-4 levels were positively correlated with the TNM
stage in HCC patients, whereas IFN-γ levels were negatively
correlated. Therefore, sB7-H4 may be used as a potential serum
biomarker to facilitate diagnosis of HCC and may be predictive of
tumor progression in patients with HCC.
The present study revealed a possible role for B7-H4
in the development of HCC. Aberrant expression of B7-H4 has been
identified to correlate with the TNM stage, differentiation degree
and lymph node metastasis in patients with HCC. The present study
provided insight into the underlying mechanism that contributed to
the progression of HCC and suggested that B7-H4 may be a promising
target for immunotherapy.
Acknowledgements
The present study was supported by funds from the
Nature Science Foundation of Shandong Province (grant no.
ZR2013HM050) and the Foundation Project in Shandong Province
Department of Education (J02K12).
References
|
1
|
Calle EE, Rodriguez C, Walker-Thurmond K
and Thun MJ: Overweight, obesity, and mortality from cancer in a
prospectively studied cohort of US adults. N Engl J Med.
348:1625–1638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM: Global cancer statistics in the
year 2000. Lancet Oncol. 2:533–543. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schütte K, Bornschein J and Malfertheiner
P: Hepatocellular carcinoma-epidemiological trends and risk
factors. Dig Dis. 27:80–92. 2009. View Article : Google Scholar
|
|
4
|
Hawkins MA and Dawson LA: Radiation
therapy for hepatocellular carcinoma: From palliation to cure.
Cancer. 106:1653–1663. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang JD and Roberts LR: Hepatocellular
carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 7:448–458.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
El-Serag HB, Marrero JA, Rudolph L and
Reddy KR: Diagnosis and treatment of hepatocellular carcinoma.
Gastroenterology. 134:1752–1763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kudo M, Izumi N, Kokudo N, Matsui O,
Sakamoto M, Nakashima O, Kojiro M and Makuuchi M: HCC Expert Panel
of Japan Society of Hepatology: Management of hepatocellular
carcinoma in Japan: Consensus-based clinical practice guidelines
proposed by the Japan Society of Hepatology (JSH) 2010 updated
version. Dig Dis. 29:339–364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bruix J and Sherman M: Practice Guidelines
Committee, American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen L: Co-inhibitory molecules of the
B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol.
4:336–347. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sica GL, Choi IH, Zhu G, Tamada K, Wang
SD, Tamura H, Chapoval AI, Flies DB, Bajorath J and Chen L: B7-H4,
a molecule of the B7 family, negatively regulates T cell immunity.
Immunity. 18:849–861. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zang X, Loke P, Kim J, Murphy K, Waitz R
and Allison JP: B7x: A widely expressed B7 family member that
inhibits T cell activation. Proc Natl Acad Sci USA.
100:10388–10392. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Prasad DV, Richards S, Mai XM and Dong C:
B7S1, a novel B7 family member that negatively regulates T cell
activation. Immunity. 18:863–873. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Flies DB and Chen L: The new B7s: Playing
a pivotal role in tumor immunity. J Immunother. 30:251–260. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen LJ, Sun J, Wu HY, Zhou SM, Tan Y, Tan
M, Shan BE, Lu BF and Zhang XG: B7-H4 expression associates with
cancer progression and predicts patient's survival in human
esophageal squamous cell carcinoma. Cancer Immunol Immunother.
60:1047–1055. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Awadallah NS, Shroyer KR, Langer DA,
Torkko KC, Chen YK, Bentz JS, Papkoff J, Liu W, Nash SR and Shah
RJ: Detection of B7-H4 and p53 in pancreatic cancer: Potential role
as a cytological diagnostic adjunct. Pancreas. 36:200–206. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arigami T, Uenosono Y, Ishigami S,
Hagihara T, Haraguchi N and Natsugoe S: Clinical significance of
the B7-H4 coregulatory molecule as a novel prognostic marker in
gastric cancer. World J Surg. 35:2051–2057. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Geng Y, Wang H, Lu C, Li Q, Xu B, Jiang J
and Wu C: Expression of costimulatory molecules B7-H1, B7-H4 and
Foxp3+ Tregs in gastric cancer and its clinical significance. Int J
Clin Oncol. 20:273–281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao LW, Li C, Zhang RL, Xue HG, Zhang FX,
Zhang F and Gai XD: B7-H1 and B7-H4 expression in colorectal
carcinoma: Correlation with tumor FOXP3(+) regulatory T-cell
infiltration. Acta Histochem. 116:1163–1168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen C, Qu QX, Shen Y, Mu CY, Zhu YB,
Zhang XG and Huang JA: Induced expression of B7-H4 on the surface
of lung cancer cell by the tumor-associated macrophages: A
potential mechanism of immune escape. Cancer Lett. 317:99–105.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jeon H, Vigdorovich V, Garrett-Thomson SC,
Janakiram M, Ramagopal UA, Abadi YM, Lee JS, Scandiuzzi L,
Ohaegbulam KC, Chinai JM, et al: Structure and cancer immunotherapy
of the B7 family member B7x. Cell Rep. 9:1089–1098. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kryczek I, Zou L, Rodriguez P, Zhu G, Wei
S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, et al: B7-H4
expression identifies a novel suppressive macrophage population in
human ovarian carcinoma. J Exp Med. 203:871–881. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu J and Paul WE: CD4 T cells: Fates,
functions, and faults. Blood. 112:1557–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nieters A, Yuan JM, Sun CL, Zhang ZQ,
Stoehlmacher J, Govindarajan S and Yu MC: Effect of cytokine
genotypes on the hepatitis B virus-hepatocellular carcinoma
association. Cancer. 103:740–748. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ognjanovic S, Yuan JM, Chaptman AK, Fan Y
and Yu MC: Genetic polymorphisms in the cytokine genes and risk of
hepatocellular carcinoma in low-risk non-Asians of USA.
Carcinogenesis. 30:758–762. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou D, Gu FM, Gao Q, Li QL, Zhou J and
Miao CH: Effects of anesthetic methods on preserving anti-tumor
T-helper polarization following hepatectomy. World J Gastroenterol.
18:3089–3098. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li W, Huang X, Tong H, Wang Y, Zhang T,
Wang W, Dai L, Li T, Lin S and Wu H: Comparison of the regulation
of β-catenin signaling by type I, type II and type III interferons
in hepatocellular carcinoma cells. PLoS One. 7:e470402012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choi IH, Zhu G, Sica GL, Strome SE,
Cheville JC, Lau JS, Zhu Y, Flies DB, Tamada K and Chen L: Genomic
organization and expression analysis of B7-H4, an immune inhibitory
molecule of the B7 family. J Immunol. 171:4650–4654. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tringler B, Liu W, Corral L, Torkko KC,
Enomoto T, Davidson S, Lucia MS, Heinz DE, Papkoff J and Shroyer
KR: B7-H4 overexpression in ovarian tumors. Gynecol Oncol.
100:44–52. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tringler B, Zhuo S, Pilkington G, Torkko
KC, Singh M, Lucia MS, Heinz DE, Papkoff J and Shroyer KR: B7-H4 is
highly expressed in ductal and lobular breast cancer. Clin Cancer
Res. 11:1842–1848. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Krambeck AE, Thompson RH, Dong H, Lohse
CM, Park ES, Kuntz SM, Leibovich BC, Blute ML, Cheville JC and Kwon
ED: B7-H4 expression in renal cell carcinoma and tumor vasculature:
Associations with cancer progression and survival. Proc Natl Acad
Sci USA. 103:10391–10396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zang X, Thompson RH, Al-Ahmadie HA, Serio
AM, Reuter VE, Eastham JA, Scardino PT, Sharma P and Allison JP:
B7-H3 and B7x are highly expressed in human prostate cancer and
associated with disease spread and poor outcome. Proc Natl Acad Sci
USA. 104:19458–19463. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shi H, Ji M, Wu J, Zhou Q, Li X, Li Z,
Zheng X, Xu B, Zhao W, Wu C and Jiang J: Serum B7-H4 expression is
a significant prognostic indicator for patients with gastric
cancer. World J Surg Oncol. 12:1882014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Simon I, Zhuo S, Corral L, Diamandis EP,
Sarno MJ, Wolfert RL and Kim NW: B7-h4 is a novel membrane-bound
protein and a candidate serum and tissue biomarker for ovarian
cancer. Cancer Res. 66:1570–1575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang C, Li Y and Wang Y: Diagnostic value
of serum B7-H4 for hepatocellular carcinoma. J Surg Res.
197:301–306. 2015. View Article : Google Scholar : PubMed/NCBI
|