Introduction
Human epidermal growth factor receptor 2
(HER2)-overexpressing breast cancer constitutes ~20% of breast
cancer cases (1). Overexpression
of the HER2 protein is caused by amplification of the HER2 gene.
HER2-overexpressing cancer often has a highly aggressive phenotype
and is associated with metastasis to the lymph nodes and distant
organs; among breast cancer types the prognosis for this type of
cancer is the worst (2–4). Using anti-HER2 antibodies as
molecular target-based therapy may ameliorate the prognosis of
HER2-overexpressing breast cancer (5). However, the molecular mechanism
underlying the aggressive behavior of HER2-overexpressing breast
cancer remains to be fully elucidated.
Delta-HER2 is a splice variant of HER2, which lacks
the 16 amino acids (coded by 48 nucleotides) of exon 16 in the
extracellular domain (Fig. 1)
(6,7). The deletion of these 16 amino acids
in the cysteine-rich region generates unpaired cysteine residues,
which cause chemical dimerization of the molecules and constitutive
stimulation of cell proliferation (8,9). It
has been reported in vitro that overexpression of delta-HER2
in cultured cells results in the generation of highly proliferative
cells (8–12). Furthermore, in transgenic mice
overexpressing delta-HER2, mammary carcinoma frequently developed,
whereas overexpression of wild type (wt)-HER2 did not induce tumor
formation (13). The findings of
these in vitro experiments and animal models indicated that
delta-HER2 is oncogenic.
The expression levels of delta-HER2 have been
examined in cell lines and in human breast cancer tissues (8,9,11,12,14);
however, in some studies the number of patients is limited and the
expression was examined by semi-quantitative methods (9,14).
The expression of delta-HER2 and its association with the
clinicopathological factors of human breast cancer remain to be
fully elucidated. Furthermore, to the best of our knowledge, the
expression levels of delta-HER2 in Japanese patients with
HER2-overexpressing breast cancer remain unknown.
The present study established a quantitative method
to detect delta-HER2 mRNA expression in formalin-fixed
paraffin-embedded cancer specimens. The aim of the present study
was to explore the expression of delta-HER2 and its
clinicopathological correlation in Japanese cases of
HER2-overexpressing breast cancer.
Patients and methods
HER2-overexpressing breast cancer and
histological evaluation
A total of 40 cases of breast cancer, which were
given a score of 3 by HER2 immunostaining, were retrieved from the
archival files. The present study was performed using
formalin-fixed paraffin-embedded specimens of primary breast cancer
in all cases except case #8. In this case, the specimen was
obtained from the metastatic lymph nodes, and information regarding
the primary tumor was not obtained. The present study was approved
by the Ethical Committee of Hirosaki University (Hirosaki, Japan).
The histology of the breast cancers was classified according to the
World Health Organization Classification of Breast Tumors (15). The HER2 immunostaining score was
determined according to the guidelines recommended by American
Society of Clinical Oncology/College of American Pathologists
(16). No patients received
preoperative neoadjuvant therapy.
Total RNA extraction and synthesis of
cDNA
Total RNA was extracted from the paraffin-embedded
sections using RNeasy FFPE kit (Qiagen K.K., Tokyo, Japan).
Briefly, five slices from the 4 µM-thick paraffin-embedded sections
were deparaffinized using xylene and ethanol, and dried.
Subsequently, the sections were digested with proteinase K solution
until the tissues were completely dissolved. Total RNA was purified
using spin columns, according to the manufacturer's protocol. As a
standardization control, cDNA reverse-transcribed from a mixture of
total RNA extracted from the normal breast tissues of five women
(age, 34, 45, 47, 59 and 78 years) (BioChain Institute Inc.,
Newark, CA, USA) was used. cDNA was synthesized from 2 µg total RNA
by reverse transcription using the SuperScript III First Strand
cDNA Synthesis system (Thermo Fisher Scientific, K.K., Tokyo,
Japan). The cDNA synthesis was primed by random hexamers.
Quantitative polymerase chain reaction
(qPCR) of wt-HER2 and delta-HER2
The delta-HER2 lacks exon 16, from which 16 amino
acids are coded. Exon 16 is positioned at the extracellular domain
close to the transmembrane region. The PCR primers used in the
present study were designed to specifically amplify each transcript
of wt-HER2 and delta-HER2. The primer sequences were as follows:
wt-HER2, forward 5′-TCCTGTGTGGACCTGGATGA-3′; delta-HER2, forward
5′-GCACCCACTCCCCTCTGA-3′; and common reverse primer,
5′-CGCTTGATGAGGATCCCAAA-3′ (FASMAC Co. Ltd., Kanagawa, Japan)
(Fig. 1). qPCR was conducted using
SsoFast™ EvaGreen® Supermix with Low ROX (Bio-Rad
Laboratories, Inc., Tokyo, Japan). A 20 µl reaction solution
contained 300 nM forward and reverse primers and cDNA transcribed
from 40 ng total RNA as a template. The reaction program was
initiated by denaturation at 96°C for 5 min, followed by 40 cycles
at 96°C for 5 sec and 67°C for 30 sec. The quantification of 18S
rRNA was conducted using TaqMan Master Mix and 18S rRNA Primer and
Probe kit (Thermo Fisher Scientific, K.K.) with cDNA transcribed
from 40 ng total RNA. The reaction program was initiated by 50°C
for 2 min and 95°C for 10 min, followed by 40 cycles at 95°C for 15
sec and 60°C for 1 min. The changes in fluorescence were monitored
using the ABI Prism 7000 Sequence Detection system (Thermo Fisher
Scientific, K.K.).
Quantification cycle (Cq) of wt-HER2
(Cqwt-HER2), delta-HER2 (Cqdelta-HER2) and
18S rRNA (Cq18S rRNA) was determined as the cycle where
the linear increase in fluorescence reached the threshold level.
Firstly, wt-HER2 and delta-HER2 were standardized by 18S rRNA, and
ΔCq was calculated as ΔCqwt-HER2 = Cqwt-HER2
- Cq18S rRNA and ΔCqdelta-HER2 =
Cqdelta-HER2 - Cq18S rRNA, respectively. The
relative expression levels were then calculated using the
2−ΔΔCq formula (17),
where ΔΔCq was calculated by subtracting ΔCq of normal breast
tissues from ΔCq of breast cancer tissues. The mRNA expression
levels of wt-HER2 and delta-HER2 in breast cancer tissues were
calculated as a fold increase or decrease relative to normal breast
tissues.
The specificity of PCR was determined by the
amplification of wt-HER2 and delta-HER2 from the mixture of wt-HER2
and delta-HER2 templates. The templates were mixtures of purified
PCR products of wt-HER2 and delta-HER2 at concentration ratios of
100:0.1, 10:1, 1:10, 0.1:100 fg/tube, respectively. PCR was
conducted by the same method used for qPCR. After the reaction, the
PCR products were electrophoresed on 3% agarose gel.
Immunostaining and scoring
Paraffin-embedded sections (4 µm) were immunostained
with antibodies against estrogen receptor (ER; clone SP1; cat. no.
790-4324; 100 µl of prediluted antibody; Roche Diagnostics K.K.,
Tokyo, Japan), progesterone receptor (PgR; clone 1E2; cat. no.
790-2223; 100 µl of prediluted antibody; Roche Diagnostics K.K.),
Ki-67 (clone MIB-1; cat. no. M7240; 1:100 dilution; DAKO Japan,
Tokyo, Japan) and activated form of phosphorylated SRC (pSRC;
Tyr416; cat. no. 2101; 1:100 dilution; Cell Signaling Technology
Japan, K.K., Tokyo, Japan). Immunostaining was conducted using the
Ventana BenchMark GX (Roche Diagnostics K.K.) automated staining
system, and the peroxidase reaction was visualized by
diaminobenzidine.
Nuclear staining was considered positive for ER, PgR
and Ki-67. The expression of ER and PgR was scored according to the
method reported by Allred et al (18), and a total score ≥3 was considered
positive. The Ki-67 index was calculated as a ratio (%) of the
number of positive nuclei per 1,000 neoplastic cells in the area of
the highest labeling, which is also known as the ‘hot spot.’
Cytoplasmic staining close to the cell membrane was considered
positive for pSRC, and the expression was scored semiquantitatively
as follows. When there was no staining, the tumor was scored as 0.
If the cells were stained, the staining was scored between 1 and 3.
A score of 1 was defined as weak staining, the same as some normal
epithelial cells; a score of 3 was defined as strong membranous and
cytoplasmic staining. A score of 2 indicated staining intensity
between scores 1 and 3.
Hierarchical clustering and
statistical analysis
Hierarchical clustering and statistical analysis
were performed using JMP 10 (SAS Institute Japan, Tokyo, Japan).
The comparison between two groups was conducted using Mann-Whitney
U test, and the comparison among three groups was performed using
the Steel-Dwass method. The regressions between wt-HER2 and
delta-HER2 mRNA levels and between wt-HER2 mRNA level and delta/wt
ratio was examined by regression analysis. P<0.05 was considered
to indicate a statistically significant difference.
Results
Clinicopathological features of
HER2-overexpressing breast cancer
A total of 40 cases of HER2-overexpressing breast
cancer were studied in the present study, all of which were women,
aged between 35 and 85 years (Table
I). Breast cancer histology (Fig.
2) observed sheet-like, cribriform and trabecular proliferation
of atypical cells, which possessed irregular-shaped nuclei with
prominent nucleoli and frequent mitotic figures (Fig. 2A). All cases were diagnosed as
invasive carcinoma and histological grades were high. Strong
expression of HER2 was identified on the cell membrane (Fig. 2B). A total of 20 cases were
classified as hormone receptor-positive luminal B subtype (Fig. 2C), and the other 20 cases were
classified as hormone receptor-negative HER2-positive subtype. The
Ki-67 index ranged between 19.0 and 92.3% (Fig. 2D). A total of 23 cases had lymph
node metastasis at the time of operation, and recurrence was noted
in 10 cases.
 | Table I.Clinicopathological features of
patients with HER2-overexpressing breast cancer. |
Table I.
Clinicopathological features of
patients with HER2-overexpressing breast cancer.
| No. | Age (yr) | Intrinsic
subtype | pT | Ki-67(%) | LN | Metastasis | wt-HER2 (fold) | delta-HER2
(fold) | delta/wt(%) | pSRC |
|---|
| 1 | 35 | LumB | 2 | 49.6 | − |
| 46.2 | 32.3 | 1.5 | 2 |
| 2 | 38 | LumB | 3 | 19.0 | + | Br | 54.8 | 49.5 | 3.9 | 1 |
| 3 | 42 | HER2+ | 2 | 82.0 | + | Ms | 16.6 | 25.7 | 3.4 | 1 |
| 4 | 42 | LumB | 1 | 16.7 | − | Br, Li | 4.0 | 3.1 | 1.7 | 1 |
| 5 | 42 | LumB | 2 | 38.5 | + | Br | 1.1 | 1.1 | 2.1 | 0 |
| 6 | 42 | LumB | 1 | 69.3 | + |
| 23.3 | 46.4 | 3.4 | 0 |
| 7 | 43 | HER2+ | 1 | 52.7 | + |
| 3.6 | 5.4 | 3.4 | 2 |
| 8 | 43 | HER2+ | − | 87.5 | + | Li, Lg, Bn, LN | 41.5 | 50.9 | 2.1 | 3 |
| 9 | 43 | LumB | 1 | 42.1 | − |
| 10.2 | 8.7 | 1.9 | 3 |
| 10 | 45 |
HER2+ | 1 | 44.3 | + | Br | 7.5 | 21.0 | 6.2 | 1 |
| 11 | 46 | LumB | 2 | 55.5 | + | Br, Skin | 5.7 | 7.2 | 2.8 | 0 |
| 12 | 47 | LumB | 1 | 29.4 | + |
| 0.5 | 0.3 | 1.4 | 1 |
| 13 | 48 |
HER2+ | 1 | 44.9 | − | Br | 6.6 | 8.1 | 2.7 | 1 |
| 14 | 48 | LumB | 1 | 79.4 | − |
| 94.4 | 222.1 | 3.0 | 2 |
| 15 | 49 | LumB | 2 | 44.5 | − | Br, Bn | 598.4 | 19.3 | 0.1 | 2 |
| 16 | 50 | LumB | 2 | 75.2 | − |
| 169.5 | 150.1 | 2.0 | 3 |
| 17 | 50 | LumB | 4 | 43.7 | + |
| 25.4 | 15.7 | 1.5 | 2 |
| 18 | 50 | LumB | 2 | 69.4 | − |
| 39.7 | 40.4 | 1.8 | 3 |
| 19 | 51 |
HER2+ | 2 | 74.8 | + |
| 113.4 | 83.6 | 1.3 | 3 |
| 20 | 52 | LumB | 1 | 58.4 | − |
| 4.1 | 4.0 | 2.2 | 3 |
| 21 | 53 |
HER2+ | 1 | 78.2 | − |
| 24.9 | 20.6 | 1.9 | 3 |
| 22 | 55 |
HER2+ | 2 | 63.4 | − |
| 177.3 | 156.0 | 1.5 | 0 |
| 23 | 56 |
HER2+ | 4 | 69.7 | + |
| 432 | 499.7 | 2.6 | 2 |
| 24 | 57 |
HER2+ | 2 | 92.3 | + |
| 87.7 | 114.2 | 2.3 | 2 |
| 25 | 57 | LumB | 1 | 67.3 | − |
| 0.8 | 0.6 | 1.6 | 2 |
| 26 | 59 |
HER2+ | 1 | 64.7 | + |
| 9.3 | 7.8 | 1.9 | 2 |
| 27 | 60 |
HER2+ | 1 | 68.5 | − |
| 70.0 | 56.1 | 1.4 | 3 |
| 28 | 61 |
HER2+ | 1 | 51.2 | − |
| 2.0 | 1.8 | 2.1 | 1 |
| 29 | 64 |
HER2+ | 2 | 68.2 | + |
| 6.7 | 3.8 | 1.3 | 1 |
| 30 | 64 |
HER2+ | 1 | 43.7 | + |
| 1.7 | 2.4 | 3.2 | 0 |
| 31 | 64 |
HER2+ | 1 | 31.8 | − |
| 100.8 | 177.3 | 3.0 | 1 |
| 32 | 65 | LumB | 1 | 56.4 | − |
| 315.2 | 128.9 | 0.7 | 3 |
| 33 | 68 |
HER2+ | 1 | 88.0 | + |
| 15.1 | 20.2 | 2.3 | 1 |
| 34 | 69 |
HER2+ | 4 | 92.3 | + | LN | 30.6 | 32.9 | 2.4 | 3 |
| 35 | 72 |
HER2+ | 2 | 72.0 | − |
| 4.1 | 3.9 | 1.7 | 0 |
| 36 | 73 | LumB | 1 | 28.5 | + |
| 10.9 | 9.8 | 2.0 | 3 |
| 37 | 73 | LumB | 1 | 52.7 | − |
| 11.8 | 6.7 | 1.0 | 3 |
| 38 | 78 | LumB | 2 | 74.4 | + |
| 55.7 | 64.9 | 2.0 | 3 |
| 39 | 81 |
HER2+ | 2 | 34.6 | + |
| 3.9 | 3.6 | 2.1 | 3 |
| 40 | 85 | LumB | 2 | 80.1 | − |
| 203.7 | 370.9 | 2.4 | 3 |
mRNA expression levels of wt-HER2 and
delta-HER2
The specificity of qPCR was confirmed by amplifying
a mixture of sequentially diluted wt-HER2 and delta-HER2 mRNA
(Fig. 3A). The amount of each
amplified product correlated with the amount of target in the
mixture, and no cross-reaction was detected.
The expression levels of wt-HER2 and delta-HER2
varied largely, from 0.5 to 598.4-fold and from 0.3 to 499.7-fold
compared with normal breast tissues, respectively (Table I). There was a power regression
between wt-HER2 and delta-HER2 mRNA levels (r2=0.86,
P<0.0001; Fig. 3B). The
delta/wt ratio ranged between 0.1 and 6.2% (Fig. 3C); the average was 2.2±1.0%. There
was a weak inverse correlation between delta/wt ratio and the mRNA
expression levels of wt-HER2 (r2=0.1, Fig. 3C).
Immunostaining of pSRC
Immunostaining of the activated form of pSRC was
negative in six cases (15%). Weak and moderate positive
immunostaining, scored as 1 and 2, was detected in 10 and nine
cases, respectively (25 and 23%) (Fig.
2E). Strong immunostaining of pSRC, scored as 3, was noted in
15 cases (38%; Fig. 2F). The
strong positive reaction was detected on the cell membrane and in
the cytoplasm close to the cell membrane.
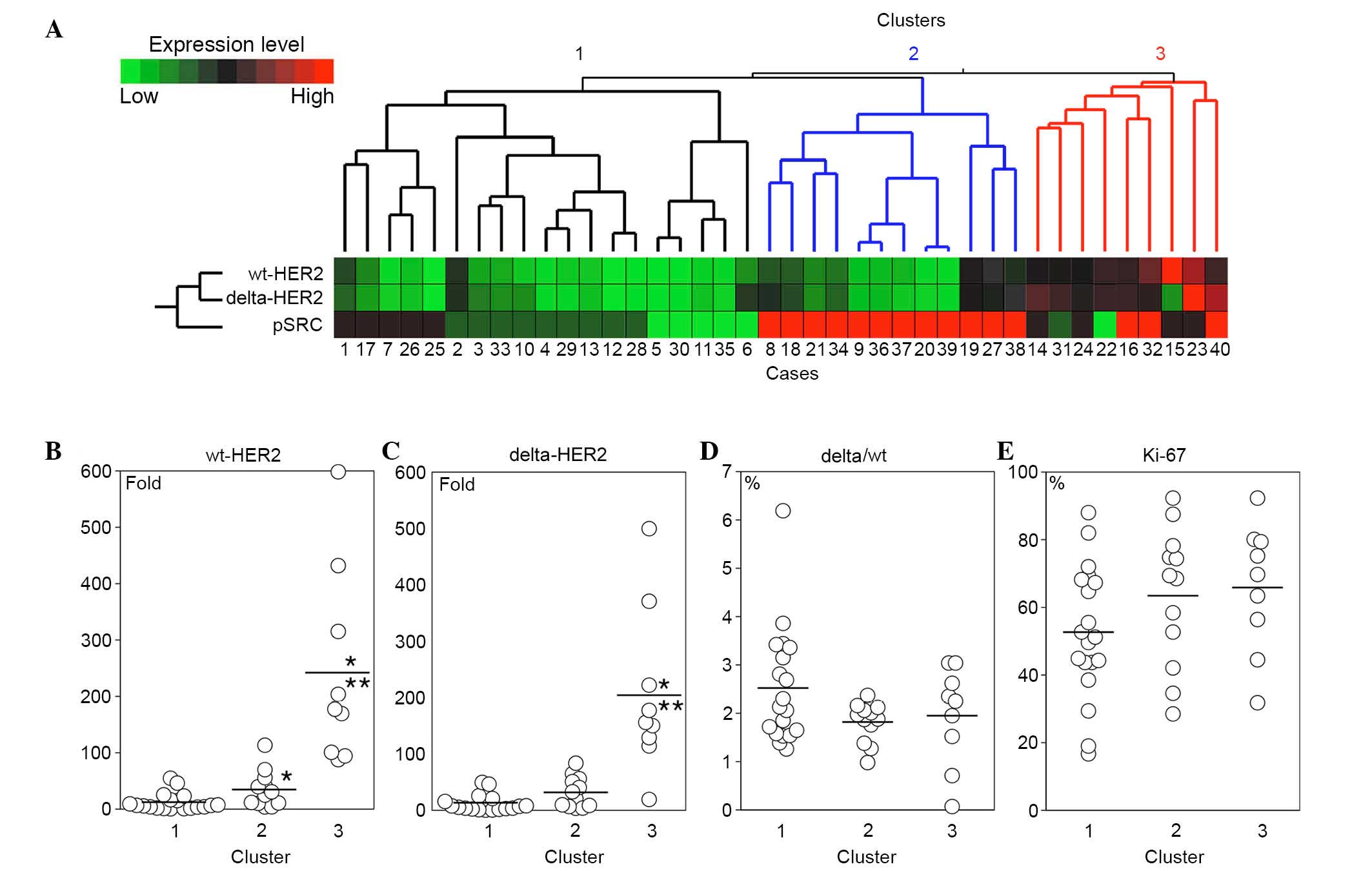
Hierarchical clustering and
clinicopathological features
Hierarchical clustering with wt-HER2, delta-HER2 and
pSRC separated the HER2-overexpressing breast cancer cases into
three major clusters (Fig. 4A). In
cluster 1, the expression levels of wt-HER2 and delta-HER2 were
lower than those in the other clusters (Fig. 4B and C). The mRNA expression levels
of wt-HER2 and delta-HER2 were elevated in clusters 2 and 3, and
the pSRC score was high in the majority of cases in these clusters
(Fig. 4A-C). Delta/wt ratio
appeared to be slightly higher in cluster 1 compared with those in
the other clusters; however, the difference did not reach
statistical significance (Fig.
4D). The Ki-67 index was high in all clusters; however, a trend
was observed that the index was lower in cluster 1 compared with in
clusters 2 and 3 (Fig. 4E).
The clinicopathological characteristics of cases in
the three clusters were analyzed (Table II). There was no difference in the
intrinsic subtypes and primary tumor staging among the clusters.
Lymph node status and recurrence were more frequent in cluster 1;
however, the differences did not reach statistical significance
(Table II).
 | Table II.Comparison of clinicopathological
features among the human epidermal growth factor receptor
2-overexpressing breast cancer clusters. |
Table II.
Comparison of clinicopathological
features among the human epidermal growth factor receptor
2-overexpressing breast cancer clusters.
|
| Clusters |
|
|---|
|
|
|
|
|---|
| Variable | 1 | 2 | 3 | P-value |
|---|
| Intrinsic
subtype |
|
|
| P=0.92 |
| Luminal
B | 9 | 6 | 5 |
|
|
HER2-positive | 10 | 6 | 4 |
|
| pT |
|
|
| P=0.753 |
| 1 | 11 | 6 | 3 |
|
| 2 | 6 | 4 | 5 |
|
| 3 | 1 | 0 | 0 |
|
| 4 | 1 | 1 | 1 |
|
| Lymph node
status |
|
|
| P=0.065 |
| − | 6 | 6 | 7 |
|
| + | 13 | 6 | 2 |
|
| Recurrence |
|
|
| P=0.236 |
| − | 12 | 10 | 8 |
|
| + | 7 | 2 | 1 |
|
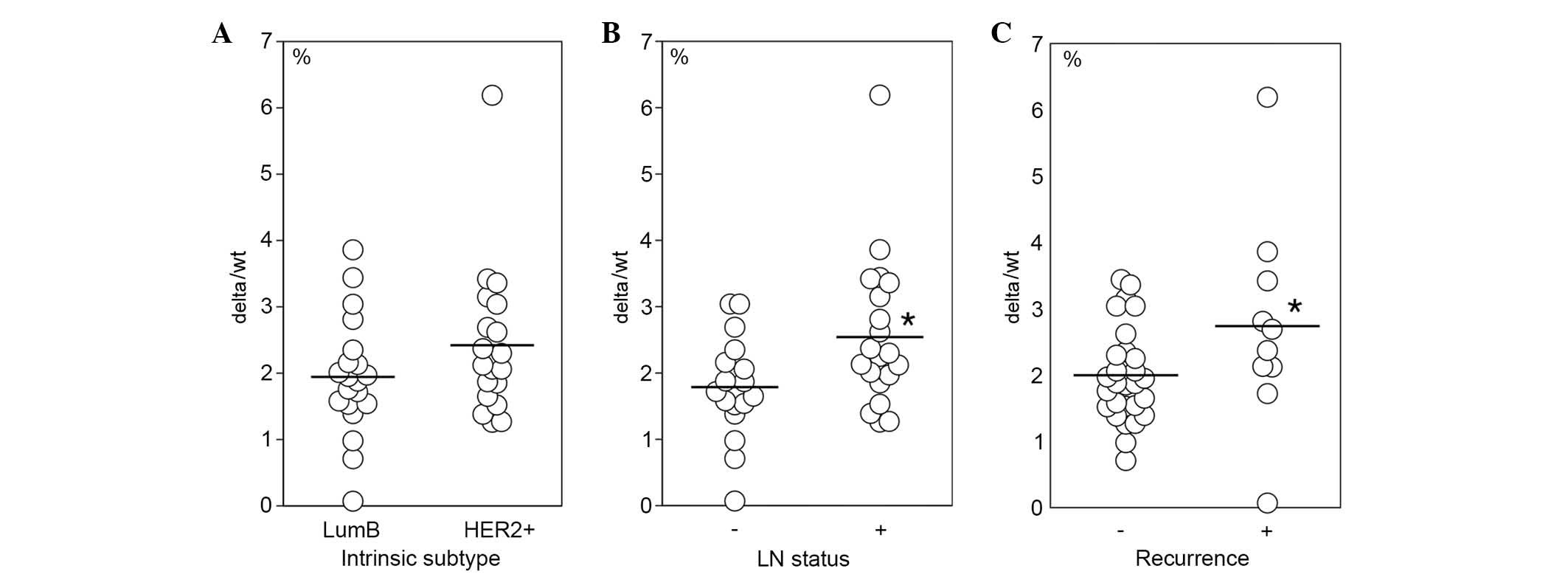
Association between delta/wt ratio and
clinicopathological features
Associations between the delta/wt ratio and
clinicopathological features, such as intrinsic subtypes, lymph
node status and disease recurrence were further analyzed. There was
no significant difference in delta/wt ratio between HER2-positive
and luminal B cases (Fig. 5A).
However, the delta/wt ratio was significantly elevated in lymph
node-positive cases (Fig. 5B) and
in cases with recurrence (Fig.
5C). No significant differences in wt-HER2 and delta-HER2 mRNA
levels were detected between intrinsic subtypes, lymph node status
and recurrence (data not shown).
Discussion
The present study detected delta-HER2 mRNA
expression, a splice variant of HER2, in Japanese patients with
HER2-overexpressing breast cancer. The mRNA expression levels of
delta-HER2 mRNA appeared to be correlated with those of wt-HER2
mRNA. The HER2-overexpressing breast cancer were separated into
cancer with elevated delta-HER2 and enhanced pSRC, and cancer with
low expression level of delta-HER2, but with elevated delta/wt
ratio. Furthermore, the delta/wt ratio was significantly increased
in breast cancer cases with lymph node metastasis and
recurrence.
In the present study, the breast cancer cases were
separated into three clusters by hierarchical clustering with
wt-HER2, delta-HER2 and pSRC. Tumors with moderate and high mRNA
expression levels of wt-HER2 and delta-HER2 (clusters 2 and 3;
Fig. 4A) presented enhanced
phosphorylation of SRC and an increased Ki-67 index. These results
are consistent with the findings of a previous report, which
indicated that overexpression of delta-HER2 induces phosphorylation
of SRC and stimulates the proliferative activity of breast cancer
cells (9). It is also plausible
that the expression of other HER family molecules, such as
epidermal growth factor receptor and HER3, may affect the
phosphorylation of SRC; however, the expression of these molecules
was not examined in the present study (11,19).
It is conceivable that HER2-overexpressing breast cancer cases are
heterogeneous in the expression of wt-HER2 and delta-HER2, and
intracellular signaling.
In 19 out of 40 cases (48%), delta- and wt-HER2
expression was relatively low, and SRC was only moderately
phosphorylated or remained unphosphorylated (cluster 1; Fig. 4A). In addition, in cluster 1 the
Ki-67 index appeared slightly lower compared with in the other
clusters (Fig. 4D). However, there
was a trend that lymph node metastasis was more frequent in these
patients compared with the patients from the other clusters
(Table II). This finding appears
to be in contrast with that of a previous report, which
demonstrated that high frequency of lymph node metastasis was
present in cases with high expression levels of delta-HER2
(9). Furthermore, cases with a
‘high delta-HER2 signature’, which was identified in a cell line
with delta-HER2 overexpression, had a worse prognosis in distant
metastasis-free survival (12). In
the current study, the follow-up duration may be too short to
determine effects on worse prognostic factors, such as local
recurrence and distant metastasis. In addition, the number of
patients in the present study was limited. There may also be ethnic
differences in the biological behavior of HER2-overexpressing
breast cancer (20).
The delta/wt ratio in cluster 1 exhibited a trend to
be higher than in the other clusters. Therefore, the association
between clinicopathological characteristics and the delta/wt ratio
was further examined. The delta/wt ratio was significantly higher
in cases with lymph node metastasis and recurrence compared with in
the cases without them. The mRNA expression levels of delta-HER2
and wt-HER2 were comparable in the two groups. Delta/wt ratio may
be a good biological marker for the prediction of aggressive
behavior of HER2-overexpressing breast cancer in Japanese patients.
It may be valuable to use formalin-fixed paraffin-embedded
specimens for the quantification of wt-HER2 and delta-HER2 mRNA
expression.
The efficacy of trastuzumab on
delta-HER2-overexpressing breast cancer is controversial.
Delta-HER2 may chemically dimerize through disulfide bond formation
and stimulate the signaling pathway to promote proliferation of
tumor cells (8,11,13).
In a previous study, proliferation was not inhibited by trastuzumab
in tumor cells overexpressing delta-HER2 in vitro (8,9).
Conversely, in vivo, the growth of implanted tumor cells in
nude mice was inhibited by trastuzumab (12,14).
Furthermore, human patients with high levels of delta-HER2 and
enhanced pSRC followed a better clinical course, as compared with
the cases with low levels of delta-HER2 and pSRC, following
treatment with trastuzumab (14).
The discrepancy of the efficacy of trastuzumab between in
vitro and in vivo conditions may, in part, be due to the
presence of immunological machinery against HER2-overexpressing
tumor cells in vivo (21).
Further examination is required to elucidate the effects of
trastuzumab on Japanese patients with HER2-overexpressing breast
cancer.
In conclusion, the current study demonstrated the
expression of delta-HER2 in HER2-overexpressing breast cancer from
Japanese patients. The enhanced expression of delta-HER2 was
associated with phosphorylation of SRC, whereas the increase in the
delta/wt ratio was associated with lymph node metastasis and
recurrence. The delta/wt ratio may be a valuable biomarker to
predict the prognosis of HER2-overexpressing breast cancer. Further
examination is required to elucidate the precise molecular effects
induced by delta-HER2.
Acknowledgements
The authors of the present study would like to thank
Dr Shunji Aizawa and Dr Akimasa Nishimura for their assistance and
invaluable suggestions.
References
|
1
|
Barnard ME, Boeke CE and Tamimi RM:
Established breast cancer risk factors and risk of intrinsic tumor
subtypes. Biochim Biophys Acta. 1856:73–85. 2015.PubMed/NCBI
|
|
2
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lobbezoo DJ, van Kampen RJ, Voogd AC,
Dercksen MW, van den Berkmortel F, Smilde TJ, van de Wouw AJ,
Peters FP, van Riel JM, Peters NA, et al: Prognosis of metastatic
breast cancer subtypes: The hormone receptor/HER2-positive subtype
is associated with the most favorable outcome. Breast Cancer Res
Treat. 141:507–514. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scott GK, Robles R, Park JW, Montgomery
PA, Daniel J, Holmes WE, Lee J, Keller GA, Li WL, Fendly BM, et al:
A truncated intracellular HER2/neu receptor produced by alternative
RNA processing affects growth of human carcinoma cells. Mol Cell
Biol. 13:2247–2257. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jackson C, Browell D, Gautrey H and
Tyson-Capper A: Clinical significance of HER-2 splice variants in
breast cancer progression and drug resistance. Int J Cell Biol.
2013:9735842013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Castiglioni F, Tagliabue E, Campiglio M,
Pupa SM, Balsari A and Ménard S: Role of exon-16-deleted HER2 in
breast carcinomas. Endocr Relat Cancer. 13:221–232. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mitra D, Brumlik MJ, Okamgba SU, Zhu Y,
Duplessis TT, Parvani JG, Lesko SM, Brogi E and Jones FE: An
oncogenic isoform of HER2 associated with locally disseminated
breast cancer and trastuzumab resistance. Mol Cancer Ther.
8:2152–2162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kwong KY and Hung MC: A novel splice
variant of HER2 with increased transformation activity. Mol
Carcinog. 23:62–68. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siegel PM, Ryan ED, Cardiff RD and Muller
WJ: Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3
are involved in the induction of mammary tumors in transgenic mice:
Implications for human breast cancer. EMBO J. 18:2149–2164. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alajati A, Sausgruber N, Aceto N, Duss S,
Sarret S, Voshol H, Bonenfant D and Bentires-Alj M: Mammary tumor
formation and metastasis evoked by a HER2 splice variant. Cancer
Res. 73:5320–5327. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marchini C, Gabrielli F, Iezzi M, Zenobi
S, Montani M, Pietrella L, Kalogris C, Rossini A, Ciravolo V,
Castagnoli L, et al: The human splice variant Δ16HER2 induces rapid
tumor onset in a reporter transgenic mouse. PLoS One. 6:e187272011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Castagnoli L, Iezzi M, Ghedini GC,
Ciravolo V, Marzano G, Lamolinara A, Zappasodi R, Gasparini P,
Campiglio M, Amici A, et al: Activated d16HER2 homodimers and SRC
kinase mediate optimal efficacy for trastuzumab. Cancer Res.
74:6248–6259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lakhani SR, Ellis IO, Schnitt SJ, Tan PH
and van de Vijver MJ: WHO Classification of tumours of the breast.
IARC; Lyon: 2012
|
|
16
|
Wolff AC, Hammond ME, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al: Recommendations for human epidermal growth
factor receptor 2 testing in breast cancer: American society of
clinical oncology/college of American pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Allred DC, Harvey JM, Berardo M and Clark
GM: Prognostic and predictive factors in breast cancer by
immunohistochemical analysis. Mod Pathol. 11:155–168.
1998.PubMed/NCBI
|
|
19
|
Zhang S, Huang WC, Li P, Guo H, Poh SB,
Brady SW, Xiong Y, Tseng LM, Li SH, Ding Z, et al: Combating
trastuzumab resistance by targeting SRC, a common node downstream
of multiple resistance pathways. Nat Med. 17:461–469. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Natori A, Hayashi N, Soejima K, Deshpande
GA, Takahashi O, Cristofanilli M, Ueno NT and Yamauchi H: A
comparison of epidemiology, biology, and prognosis of inflammatory
breast cancer in Japanese and US populations. Clin Breast Cancer.
13:460–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bianchini G and Gianni L: The immune
system and response to HER2-targeted treatment in breast cancer.
Lancet Oncol. 15:e58–e68. 2014. View Article : Google Scholar : PubMed/NCBI
|