Introduction
Ovarian carcinoma is a common female gynecological
cancer, and its incidence ranks third to cervical cancer and
endometrial cancer in China (1).
However, due to the lack of obvious signs and symptoms in the early
stages of ovarian carcinoma, the majority of patients are diagnosed
with ovarian cancer during the treatment of cancer in other organs
(2). Therefore, ovarian carcinoma
is commonly identified only at advanced stages, resulting in the
greatest mortality rate of all types of gynecological cancer and
representing a serious threat to female health (3).
MicroRNAs (miRNAs) are non-coding single-stranded
small RNA molecules, which are highly conserved and exist in
animals and plants. miRNAs consist of a single-chain 21–25 nt in
length (4). miRNAs are produced by
gene transcription, following which miRNAs bind to their target
gene and regulate its expression. miRNA-21 has been observed to be
associated with tumor occurrence and development in numerous types
of cancer, including liver, non-small cell lung, stomach, breast,
and esophageal cancer, and tumors of the nervous system (5,6).
Numerous previous studies have indicated that the
phosphoinositide 3-kinase (PI3K)/AKT signaling pathway serves an
important role in the proliferation, angiogenesis and metastasis of
ovarian carcinoma, and the tumor resistance to radiotherapy and
chemotherapy (7,8). The PI3K/Akt-nuclear factor-κB (NF-κB)
signaling pathway stimulates an increase in the expression levels
of vascular endothelial growth factor (VEGF) to promote
angiogenesis in ovarian carcinoma (9).
Celastrol is a herb found in numerous regions of
China, with extensive pharmacological effects (10). Celastrol is a pentacyclic
triterpene monomer extracted from the root of Tripterygium
wilfordii. In an in vitro study, celastrol was indicated
to exert inhibitory effects on angiogenesis in vascular endothelial
cells and on the proliferation of endothelial cells (11). In the present study, the effects of
celastrol were investigated in OVCAR3 cells and the anticancer
mechanisms of celastrol were explored.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM) and fetal
bovine serum (FBS) were purchased from Gibco, Thermo Fisher
Scientific (Waltham, MA, USA).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and celastrol (Fig. 1) were
purchased from Sigma-Aldrich (St. Louis, MO, USA). The Annexin
V/propidium iodide (PI) staining kit was purchased from BD
Biosciences (San Jose, CA, USA). The bicinchoninic acid (BCA)
protein assay kit was purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA).
Cell culture
OVCAR3 human ovarian carcinoma cells were purchased
from the Shanghai Cell Bank of the Chinese Academy of Sciences
(Shanghai, China) and cultivated in DMEM with 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin (Sangon Biotech Co., Ltd.,
Shanghai, China) at 37°C in a 5% CO2 incubator.
MTT assay
OVCAR3 cells (4,000 cells/well) were seeded and
cultured in 96-well microplates overnight. Subsequently, OVCAR3
cells were treated with varying concentrations of celastrol (0,
0.25, 0.5, 1, 2, 4 and 6 µM) for 0, 1, 2 and 3 days. A total of 10
µl MTT (5 mg/ml) solution was added to each well and incubated at a
temperature of 37°C in a humidified atmosphere of 5%
CO2. Following this, 100 µl of the resolving solution
[10% sodium dodecyl sulfate (SDS) and 0.1 mM HCl] was added to each
well and incubated for 10 min at room temperature whilst the plate
was agitated. The absorbance of the plate was measured at 570 nm
using the Multiskan EX Primary EIA V.2.1–1 spectrophotometer.
Apoptosis levels using Annexin V/PI
staining
Following treatment with celastrol (1, 2 and 4 µM)
for 2 days, OVCAR3 cells were harvested and washed with
phosphate-buffered saline. OVCAR3 cells were re-suspended with 1X
binding buffer prior to staining with Annexin V for 30 min at room
temperature in the dark. OVCAR3 cells were then double-stained with
PI for 30 min at room temperature in the dark. The apoptotic OVCAR3
cells were quantitatively counted by a flow cytometer.
Caspase-3 and −9 activity assay
OVCAR3 cells (1×106 cells/well) were
seeded and cultured in 6-well microplates overnight at 37°C in a 5%
CO2 incubator. Subsequently, the OVCAR3 cells were
treated with varying concentrations of celastrol (1, 2 and 4 µM)
for 2 days. Following treatment with celastrol, the OVCAR3 cells
were prepared in cell lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) for 30–60 min and centrifuged at
12,000 × g for 10 min at 4°C. The protein concentration was
measured using a BCA protein assay kit. An equal quantity of total
protein extract was mixed with the reaction buffer
(acetyl-Leu-Glu-His-Asp-p-nitroanilide for caspase-9 and
acetyl-Asp-Glu-Val-Asp-p-nitroanilide for caspase-3) (Beyotime
Institute of Biotechnology) for 4–6 h. Caspase-9 and −3 activity
was measured at an absorbance of 405 nm.
Quantitative polymerase chain reaction
(qPCR) analysis of miRNA-21 expression
OVCAR3 cells (1×106 cells/well) were
seeded and cultured in 6-well microplates overnight at 37°C.
Subsequently, OVCAR3 cells were treated with varying concentrations
of celastrol (1, 2 and 4 µM) for 2 days. Following treatment with
celastrol, OVCAR3 cells were prepared in cell lysis buffer for
30–60 min and centrifuged at 12,000 × g for 10 min at 4°C.
Total RNA was extracted from the cell lysate using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA (1–2 µg)
was reversed transcribed into to cDNA using PrimeScript RT Master
Mix (Takara Bio, Inc., Otsu, Japan). qPCR was conducted using the
ABI 7500 system (Takara Bio, Inc.). The cycling conditions were as
follows: One cycle at 94°C for 5 min, followed by 30 cycles at 95°C
for 15 sec, 60°C for 30 sec and 72°C for 30 sec. The relative
expression of miRNA-21 was measured using a Bulge-Loop miRNA
qRT-PCR kit (Invitrogen; Thermo Fisher Scientific, Inc.). The
primers used were as follow: miRNA-21, forward
5′-GCCCGCTAGCTTATCAGACTGATG-3′ and reverse
5′-GCCCGCTAGCTTATCAGACTGATG-3′; and U6 forward
5′-GTTGACATCCGTAAAGACC-3′ and reverse 5′-GGAGCCAGGGCAGTAA-3′.
Western blot analysis
OVCAR3 cells (1×106 cells/well) were
seeded and cultured in 6-well microplates overnight at 37°C in a 5%
CO2 incubator. Subsequently, OVCAR3 cells were treated
with varying concentrations of celastrol (1, 2 and 4 µM) for 2
days. Following treatment with celastrol, the OVCAR3 cells were
prepared in radioimmunoprecipitation assay lysis buffer with
protease and phosphatase inhibitors (Beyotime Institute of
Biotechnology) for 30–60 min and centrifuged at 12,000 × g
for 10 min at 4°C. The protein concentration was measured using a
BCA protein assay kit. A total of 30 µg of total protein lysate was
loaded and electrophoresed onto 10% SDS-polyacrylamide gels (Sangon
Biotech Co., Ltd.) and the separated proteins were transferred to
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked with 5% fat-free milk in
Tris-buffered saline with 0.1% Tween-20 (TBST) for 2 h at room
temperature. The membranes were then incubated with anti-PI3K
(sc-67306; 1:500; Santa Cruz Biotechnology, Inc.),
anti-phosphorylated-Akt (p-Akt; sc-7985-R and sc-1618; 1:1,000;
Santa Cruz Biotechnology, Inc.), anti-NF-κB (sc-109; 1:1,000; Santa
Cruz Biotechnology, Inc.) and anti-β-actin (sc-130657; 1:500; Santa
Cruz Biotechnology, Inc.) overnight at 4°C. Following washing with
TBST three times for 2 h at room temperature, secondary fluorescent
antibodies were incubated with the membranes at room temperature
for 2 h. Proteins were visualized using an LI-COR Odyssey scanner
(LI-COR, Inc., Lincoln, NE, USA).
Transfection plasmid
Anti-miRNA-21 plasmids were chemically synthesized
by BeastBio Co., Ltd. (Shanghai, China). OVCAR3 cells
(1×106 cells/well) were seeded and cultured in 6-well
microplates overnight. A total of 100 pmol/l anti-miRNA-21 plasmid
was transfected into the OVCAR3 cells with Lipofectamine 2000 serum
medium (Invitrogen; Thermo Fisher Scientific) for 24 h.
Subsequently, the transfected OVCAR3 cells were treated with
celastrol for 48 h.
Statistical analysis
Statistical analysis was conducted with SPSS
software, version 18.0 (SPSS, Inc., Chicago, IL, USA). Values are
presented as the mean ± standard error. Experiments were conducted
a minimum of three times. Differences between the groups were
assessed by two-way analysis of variance and no post hoc tests were
used. P<0.01 was considered to indicate a statistically
significant difference.
Results
Effects of celastrol on the cellular
proliferation of ovarian carcinoma cells
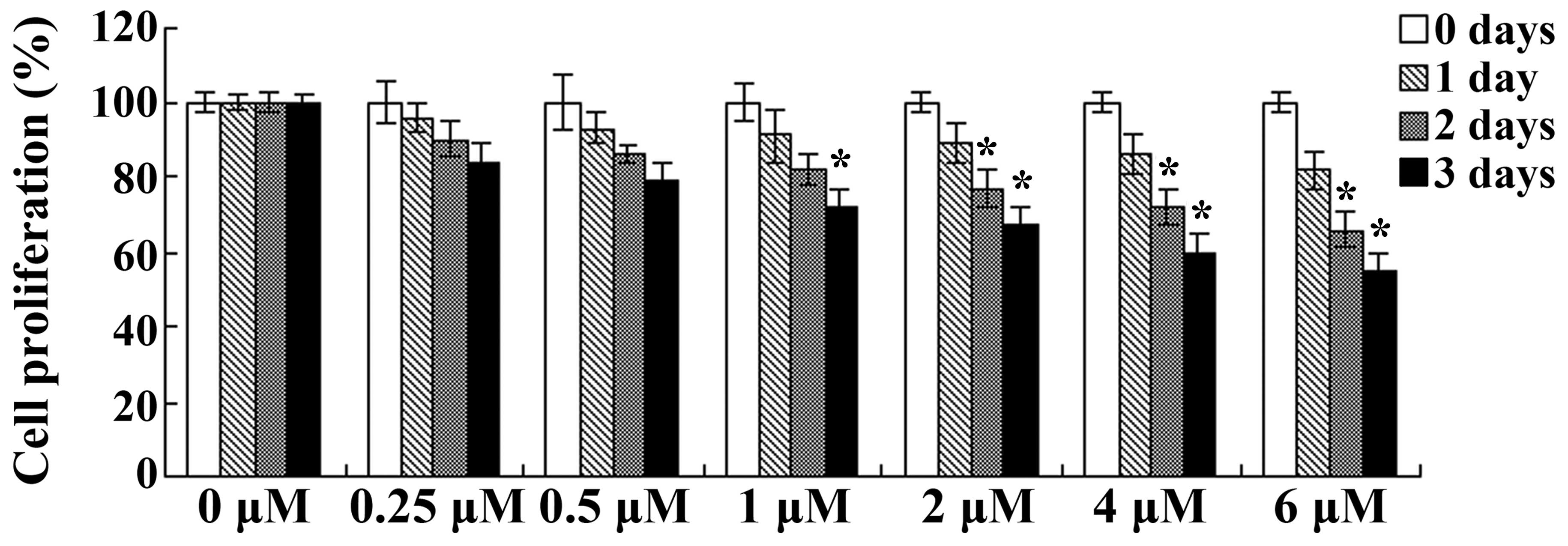
The effects of celastrol on the cellular
proliferation of ovarian carcinoma cells was investigated using an
MTT assay. OVCAR3 cells were treated with a range of celastrol
concentrations (0, 0.25, 0.5, 1, 2, 4 and 6 µM) for 0, 1, 2 and 3
days. As presented in Fig. 2, the
proliferation of OVCAR3 cells was inhibited following treatment
with celastrol in a dose- and time-dependent manner. Treatment with
celastrol (1, 2, 4 and 6 µM) for 3 days or celastrol (2, 4 and 6
µM) for 2 days resulted in significant reductions in cellular
proliferation (P<0.01; Fig. 2).
These results suggest that celastrol exerted clear anticancer
effects on human ovarian carcinoma cells.
Effects of celastrol on apoptosis in
ovarian carcinoma cells
The effects of celastrol on apoptosis in ovarian
carcinoma cells was investigated using Annexin V/PI staining. As
presented in Fig. 3, celastrol
induced apoptosis in ovarian carcinoma cells in a dose-dependent
manner, with a significant increase in the levels of apoptotic
cells following celastrol treatment for 2 days at 2 and 4 µM
(P<0.01; Fig. 3).
Effects of celastrol on caspase-9 and
−3 activity in ovarian carcinoma cells
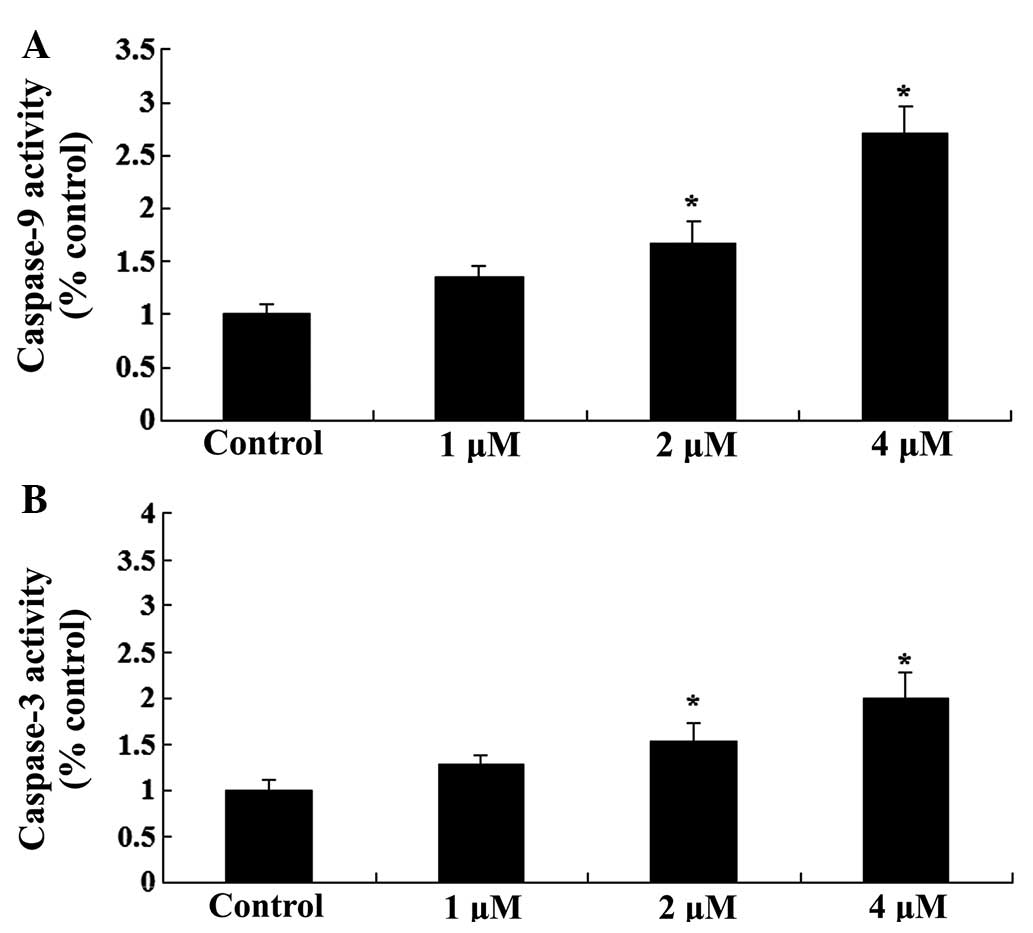
To further investigate the effects of celastrol
treatment on apoptosis in ovarian carcinoma cells, the activity
levels of caspase-9 and −3 was investigated. As presented in
Fig. 4, celastrol increased the
activity levels of caspase-9 and −3 in ovarian carcinoma cells in a
dose-dependent manner. Treatment with celastrol (2 and 4 µM) for 2
days resulted in a significant increase in the activity levels of
caspase-9 and −3 (P<0.01; Fig.
4).
Effects of celastrol on miRNA-21
expression in ovarian carcinoma cells
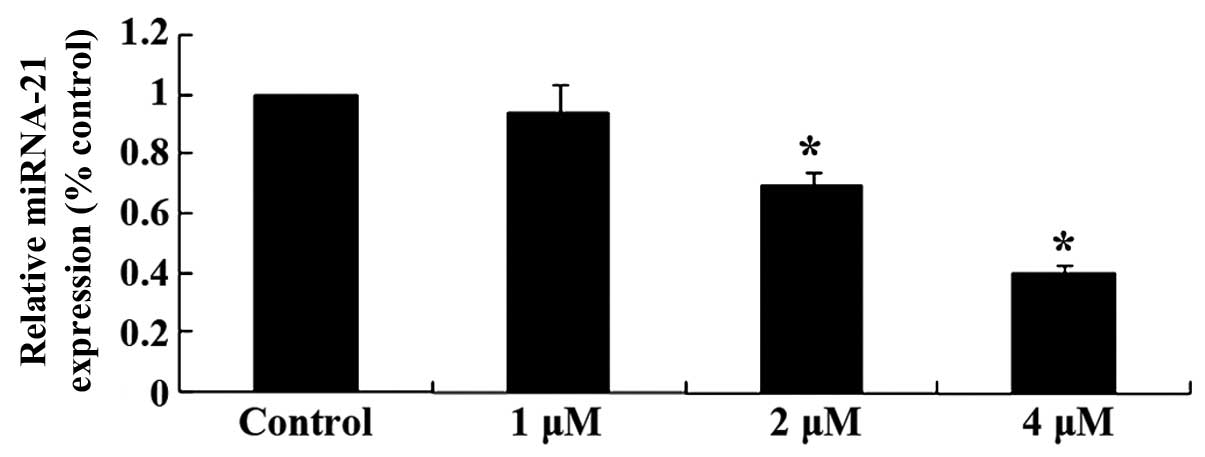
To explore the mechanisms involved in the effect of
celastrol on ovarian carcinoma cells, miRNA-21 expression was
measured using qPCR. The relative expression levels of miRNA-21 in
OVCAR3 cells was reduced following treatment with celastrol (2 and
4 µM) for 2 day (P<0.01; Fig.
5). These results indicated that the anticancer effect of
celastrol may be involved with reducing miRNA-21 levels.
Effects of celastrol on the expression
levels of PI3K/Akt in ovarian carcinoma cells
To investigate the mechanisms involved in the effect
of celastrol treatment on ovarian carcinoma cells, the expression
levels of PI3K/Akt were measured in ovarian carcinoma cells using
western blot analysis. Following treatment with celastrol (2 and 4
µM) for 2 days, the protein expression levels of PI3K and p-Akt
were reduced in OVCAR3 cells (P<0.01; Fig. 6). These results indicate that the
anticancer effects of celastrol may be associated with the
suppression of the PI3K/Akt signaling pathway.
Effects of celastrol on NF-κB protein
expression in ovarian carcinoma cell
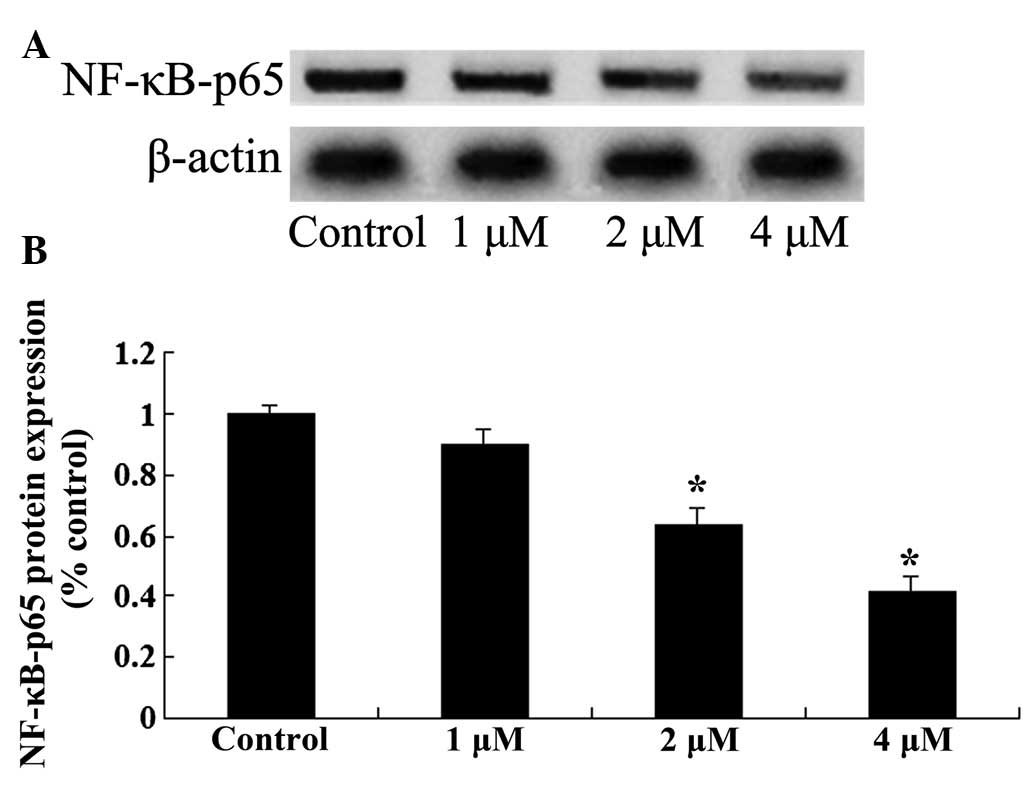
To further investigate the mechanisms involved in
the effect of celastrol treatment on ovarian carcinoma cells, NF-κB
protein expression was measured by western blot analysis. Following
treatment with celastrol (2 and 4 µM) for 2 days, the expression
levels of NF-κB in OVCAR3 cells were significantly reduced
(P<0.01; Fig. 7). These results
indicated that the anticancer effect of celastrol may be associated
with the suppression of the NF-κB signaling pathway.
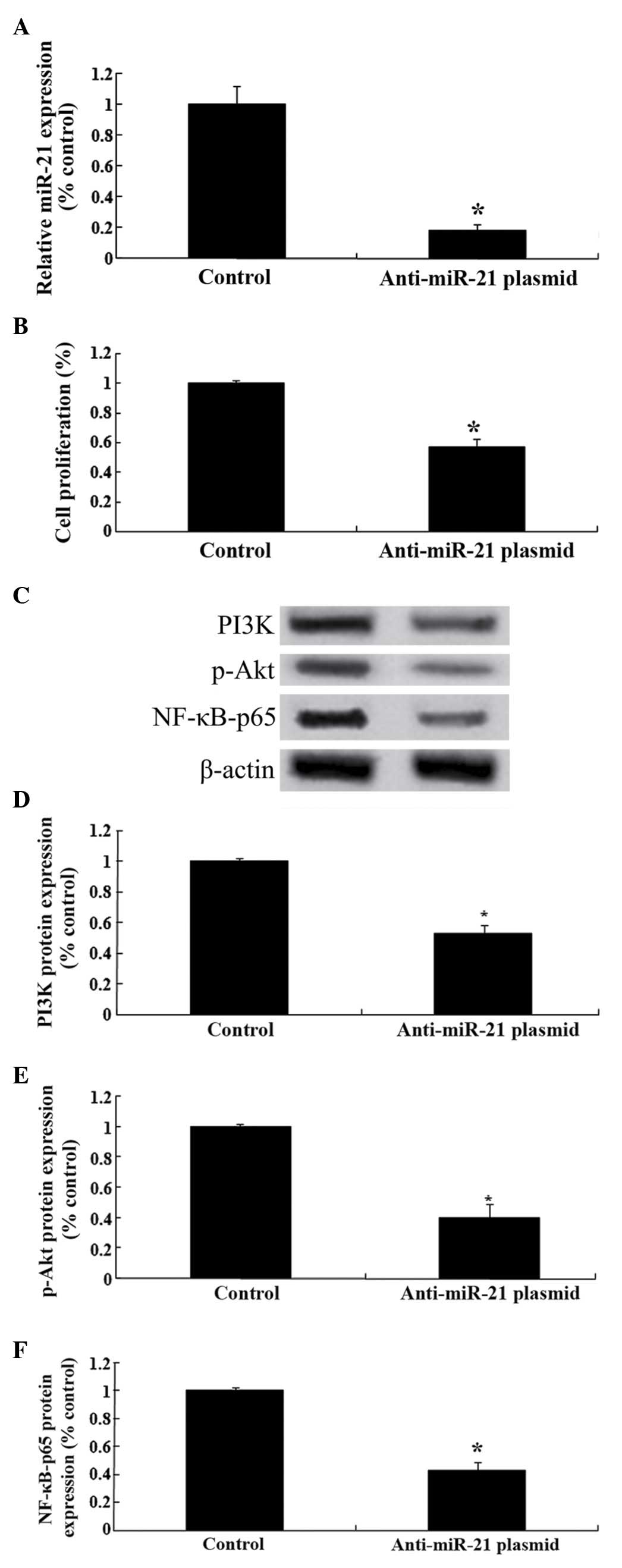
Effect of downregulation of miRNA-21
on PI3K/Akt/NF-κB expression
To further analyze the mechanisms involved in the
effect of celastrol treatment on ovarian carcinoma cells,
anti-miRNA-21 plasmids were transfected into OVCAR3 cells. As
presented in Fig. 8A, the
anti-miRNA-21 plasmids significantly reduced the relative
expression levels of miRNA-21 in OVCAR3 cells. In addition, the
anti-miRNA-21 plasmids were able to reduce the cellular
proliferation of OVCAR3 cells (Fig.
8B). Notably, the anti-miRNA-21 plasmids suppressed the
PI3K/Akt-NF-κB signaling pathway in ovarian carcinoma cells
(Fig. 8C-F).
Discussion
Ovarian carcinoma is the most common ovarian cancer,
and has the greatest associated mortality of all types of female
gynecological cancer (12).
Despite the progress made in the treatments available for ovarian
carcinoma, the prognosis remains poor, with a mortality rate of
22/100,000 and a 5-year survival rate of 25–30% (13). Therefore, ovarian carcinoma is an
important disease in the field of gynecology, of which the
etiology, pathogenesis, biological characteristics and exploration
of novel effective treatments are the focus of research (14). To the best of our knowledge, the
current study is the first to demonstrate that celastrol is an
effective and potent agent in treating ovarian carcinoma cells
in vitro. Celastrol exerted anticancer effects on ovarian
carcinoma cells via a reduction in the cellular proliferation and
the activation of caspase-dependent apoptosis in OVCAR3 cells.
Previous reports have indicted that celastrol significantly
inhibits the cellular proliferation and induces apoptosis in
gastric cancer cells (15,16) and prostate cancer cells (17).
miRNA are a type of non-coding single-stranded small
RNA molecules, which are highly conserved and exist widely in
animals and plants. Previous studies have indicated that there are
significant differences in the expression profile of miRNA between
cancer cells and normal tissues (18,19).
The alterations in the expression of miRNA are associated with
tumorigenesis, and the treatment and prognosis of cancer (20). A previous study indicated that the
expression levels of miRNA-21 were increased in the tissues of
breast, stomach, liver and cervical cancer, indicating that
miRNA-21 may serve a role as an oncogene in tumorigenesis (21). However, reports of miRNA-21
expression in ovarian carcinoma tissue are inconsistent, though the
majority indicate upregulated expression (22). In a previous study, microarrays and
additional methods were used to screen miRNA expression in ovarian
carcinoma tissue, and demonstrated that 12 miRNAs were upregulated,
including miRNA-21 (23). The
current study demonstrated that treatment with celastrol inhibited
the relative expression levels of miRNA-21 in OVCAR3 cells. Sha
et al (16) reported that
celastrol induced apoptosis in gastric cancer cells via the
miRNA-21-mediated inhibition of the PI3K/Akt-NF-κB signaling
pathway.
PI3K is a member of the lipid kinase family and is
involved in the regulation of cellular metabolism, survival and
proliferation. Akt is an important protein kinase downstream of
PI3K, and its continuous activation is closely associated with
tumor development (24,25). In breast and ovarian cancer, as
well as additional malignancies, the PI3K/Akt pathway has been
observed to be resistant to the induction of apoptosis by
chemotherapy and radiotherapy (26,27).
Selective inhibition of PI3K or Akt activity reduces the
phosphorylation levels of Akt, which is able to increase the
sensitivity of the cells to the induction of apoptosis by
chemotherapy and radiotherapy (28). The current study indicates that
celastrol treatment reduced the protein expression levels of PI3K
and p-Akt in OVCAR3 cells. In a previous study, Lee et al
(29) indicated that treatment
with celastrol was able to suppress cell growth and increase
apoptosis in melanoma cells through the suppression of PI3K/AKT
signaling. Sha et al (16)
demonstrated that celastrol induces apoptosis in gastric cancer
cells via the inhibition of the PI3K/Akt-NF-κB signaling pathway by
miRNA-21.
The PI3K/Akt pathway directly or indirectly affects
downstream processes that are associated with cellular
proliferation, protein synthesis and certain apoptosis-associated
factors. NF-κB is a key factor downstream of Akt, and under
physiological conditions is bound to its inhibitor, IκB, and is
localized in the cytoplasm in an inactive form (30). The activation of NF-κB results in
the regulation of gene transcription associated with the promotion
of cellular proliferation and the inhibition of apoptosis (31). The present study indicated that
treatment with celastrol reduced the expression levels of NF-κB in
OVCAR3 cells. Youn et al (32) reported that celastrol treatment
ameliorated human immunodeficiency virus-1 Tat-induced inflammatory
responses via the inhibition of NF-κB (32).
The current study demonstrated that the
downregulation of miRNA-21 expression was able to replicate the
anticancer effect of celastrol on OVCAR3 cells, resulting in a
reduction in the expression levels of PI3K/Akt-NF-κB in OVCAR3
cells. In conclusion, the current study indicates that celastrol is
able to significantly suppress cellular proliferation and induce
apoptosis of OVCAR3 cells. These results demonstrated that
celastrol may represent a potential novel anticancer treatment,
with its mechanisms associated with the downregulation of
microRNA-21 and the suppression the PI3K/Akt-NF-κB signaling
pathway in an in vitro model of ovarian carcinoma.
References
|
1
|
Ren Y, Huang X, Shan B, Wu X, Huang X, Shi
D and Wang H: Adjuvant concurrent chemoradiation followed by
chemotherapy for high-risk endometrial cancer. Gynecol Oncol.
140:58–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bao LJ, Jaramillo MC, Zhang ZB, Zheng YX,
Yao M, Zhang DD and Yi XF: Nrf2 induces cisplatin resistance
through activation of autophagy in ovarian carcinoma. Int J Clin
Exp Pathol. 7:1502–1513. 2014.PubMed/NCBI
|
|
3
|
Krtolica A, Krucher NA and Ludlow JW:
Molecular analysis of selected cell cycle regulatory proteins
during aerobic and hypoxic maintenance of human ovarian carcinoma
cells. Br J Cancer. 80:1875–1883. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li X, Abdel-Mageed AB, Mondal D and Kandil
E: MicroRNA expression profiles in differentiated thyroid cancer, a
review. Int J Clin Exp Med. 6:74–80. 2013.PubMed/NCBI
|
|
5
|
Kumarswamy R, Volkmann I and Thum T:
Regulation and function of miRNA-21 in health and disease. RNA
Biol. 8:706–713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bonci D: MicroRNA-21 as therapeutic target
in cancer and cardiovascular disease. Recent Patents Cardiovasc
Drug Discov. 5:156–161. 2010. View Article : Google Scholar
|
|
7
|
Liu J, Lin B, Hao Y, Qi Y, Zhu L, Li F,
Liu D, Cong J, Zhang S and Iwamori M: Lewis y antigen promotes the
proliferation of ovarian carcinoma-derived RMG-I cells through the
PI3K/Akt signaling pathway. J Exp Clin Cancer Res. 28:1542009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zi D, Zhou ZW, Yang YJ, Huang L, Zhou ZL,
He SM, He ZX and Zhou SF: Danusertib induces apoptosis, cell cycle
arrest, and autophagy but inhibits epithelial to mesenchymal
transition involving PI3k/Akt/mTOR signaling pathway in human
ovarian cancer cells. Int J Mol Sci. 16:27228–27251. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chou CH, Wei LH, Kuo ML, Huang YJ, Lai KP,
Chen CA and Hsieh CY: Up-regulation of interleukin-6 in human
ovarian cancer cell via a Gi/PI3K-Akt/NF-kappaB pathway by
lysophosphatidic acid, an ovarian cancer-activating factor.
Carcinogenesis. 26:45–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Astry B, Venkatesha SH, Laurence A,
Christensen-Quick A, Garzino-Demo A, Frieman MB, O'Shea JJ and
Moudgil KD: Celastrol, a Chinese herbal compound, controls
autoimmune inflammation by altering the balance of pathogenic and
regulatory T cells in the target organ. Clin Immunol. 157:228–238.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kannaiyan R, Shanmugam MK and Sethi G:
Molecular targets of celastrol derived from Thunder of God Vine:
Potential role in the treatment of inflammatory disorders and
cancer. Cancer Lett. 303:9–20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo C, Shibata K, Suzuki S, Kajiyama H,
Senga T, Koya Y, Daimon M, Yamashita M and Kikkawa F: GPC3
expression in mouse ovarian cancer induces GPC3-specific T
cell-mediated immune response through M1 macrophages and suppresses
tumor growth. Oncol Rep. 32:913–921. 2014.PubMed/NCBI
|
|
13
|
Nakanishi T, Aoki D, Watanabe Y, Ando Y,
Tomotsugu N, Sato Y and Saito T: A Phase II clinical trial of
pegylated liposomal doxorubicin and carboplatin in Japanese
patients with platinum-sensitive recurrent ovarian, fallopian tube
or primary peritoneal cancer. Jpn J Clin Oncol. 45:422–426. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fruscio R, Colombo N, Lissoni AA, Garbi A,
Fossati R, Ieda' N, Torri V and Mangioni C: A phase II randomised
clinical trial comparing cisplatin, paclitaxel and ifosfamide with
cisplatin, paclitaxel and epirubicin in newly diagnosed advanced
epithelial ovarian cancer: Long-term survival analysis. Br J
Cancer. 98:720–727. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee HW, Jang KS, Choi HJ, Jo A, Cheong JH
and Chun KH: Celastrol inhibits gastric cancer growth by induction
of apoptosis and autophagy. BMB Rep. 47:697–702. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sha M, Ye J, Zhang LX, Luan ZY, Chen YB
and Huang JX: Celastrol induces apoptosis of gastric cancer cells
by miR-21 inhibiting PI3K/Akt-NF-κB signaling pathway.
Pharmacology. 93:39–46. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wolfram J, Suri K, Huang Y, Molinaro R,
Borsoi C, Scott B, Boom K, Paolino D, Fresta M, Wang J, et al:
Evaluation of anticancer activity of celastrol liposomes in
prostate cancer cells. J Microencapsul. 31:501–507. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kiga K, Fukuda-Yuzawa Y, Tanabe M, Tsuji
S, Sasakawa C and Fukao T: Comprehensive silencing of
target-sharing microRNAs is a mechanism for SIRT1 overexpression in
cancer. RNA Biol. 11:1347–1354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mezzanzanica D, Canevari S, Cecco LD and
Bagnoli M: miRNA control of apoptotic programs: Focus on ovarian
cancer. Expert Rev Mol Diagn. 11:277–286. 2011.PubMed/NCBI
|
|
20
|
Wang J, Yu H, Ye L, Jin L, Yu M and Lv Y:
Integrated regulatory mechanisms of miRNAs and targeted genes
involved in colorectal cancer. Int J Clin Exp Pathol. 8:517–529.
2015.PubMed/NCBI
|
|
21
|
Huang Y, Yang YB, Zhang XH, Yu XL, Wang ZB
and Cheng XC: MicroRNA-21 gene and cancer. Med Oncol. 30:3762013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vaksman O, Tropé C, Davidson B and Reich
R: Exosome-derived miRNAs and ovarian carcinoma progression.
Carcinogenesis. 35:2113–2120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Echevarría-Vargas IM, Valiyeva F and
Vivas-Mejía PE: Upregulation of miR-21 in cisplatin resistant
ovarian cancer via JNK-1/c-Jun pathway. PLoS One. 9:e970942014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bommareddy A, Crisamore K, Fillman S,
Brozena S, Steigerwalt J, Landis T, Vanwert AL and Dwivedi C:
Survivin down-regulation by α-santalol is not mediated through
PI3K-AKT pathway in human breast cancer cells. Anticancer Res.
35:5353–5357. 2015.PubMed/NCBI
|
|
25
|
Hussain A, Qazi AK, Mupparapu N, Kumar A,
Mintoo MJ, Mahajan G, Sharma PR, Singh SK, Bharate SB, Zargar MA,
et al: A novel PI3K axis selective molecule exhibits potent tumor
inhibition in colorectal carcinogenesis. Mol Carcinog. Jan
13–2016.(Epub ahead of print). View
Article : Google Scholar
|
|
26
|
Wang JH, Nao JF, Zhang M and He P:
20(s)-ginsenoside Rg3 promotes apoptosis in human ovarian cancer
HO-8910 cells through PI3K/Akt and XIAP pathways. Tumour Biol.
35:11985–11994. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang CH, Ou TT, Yang MY, Huang CC and
Wang CJ: Nelumbo nucifera Gaertn leaves extract inhibits the
angiogenesis and metastasis of breast cancer cells by
downregulation connective tissue growth factor (CTGF) mediated
PI3K/AKT/ERK signaling. J Ethnopharmacol. May 10–2016.(Epub ahead
of print). View Article : Google Scholar
|
|
28
|
Ye Y, Tang X, Sun Z and Chen S:
Upregulated WDR26 serves as a scaffold to coordinate PI3K/AKT
pathway-driven breast cancer cell growth, migration, and invasion.
Oncotarget. Feb 17–2016.(Epub ahead of print). View Article : Google Scholar
|
|
29
|
Lee JH, Won YS, Park KH, Lee MK, Tachibana
H, Yamada K and Seo KI: Celastrol inhibits growth and induces
apoptotic cell death in melanoma cells via the activation
ROS-dependent mitochondrial pathway and the suppression of PI3K/AKT
signaling. Apoptosis. 17:1275–1286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bai L, Xu S, Chen W, Li Z, Wang X, Tang H
and Lin Y: Blocking NF-κB and Akt by Hsp90 inhibition sensitizes
Smac mimetic compound 3-induced extrinsic apoptosis pathway and
results in synergistic cancer cell death. Apoptosis. 16:45–54.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu T, Liu D, Liu J, Song JT, Gao SL, Li
H, Hu LH and Liu BR: Effect of NF-κB inhibitors on the
chemotherapy-induced apoptosis of the colon cancer cell line HT-29.
Exp Ther Med. 4:716–722. 2012.PubMed/NCBI
|
|
32
|
Youn GS, Kwon DJ, Ju SM, Rhim H, Bae YS,
Choi SY and Park J: Celastrol ameliorates HIV-1 Tat-induced
inflammatory responses via NF-kappaB and AP-1 inhibition and heme
oxygenase-1 induction in astrocytes. Toxicol Appl Pharmacol.
280:42–52. 2014. View Article : Google Scholar : PubMed/NCBI
|