Introduction
Following injury, axons in the central nervous
system (CNS) fail to regenerate (1). Conversely, peripheral nervous system
(PNS) axons regenerate following injury and exhibit restored
function. This lack of regeneration in the CNS after injury may be
associated with the aberrant expression of specific molecules in
the CNS myelin and in the glial scar, including Nogo,
oligodendrocyte-myelin glycoprotein and myelin-associated
glycoprotein (2–4). Previous studies have reported that
these molecules induce the activity of the Rho-Rho-associated
coiled-coil containing protein kinase 2 (ROCKII) and glycogen
synthase kinase-3β (GSK-3β) signaling pathways, resulting in
inhibition of axonal regeneration in the CNS (5,6).
Therefore, the Rho-ROCKII and/or GSK-3β signaling pathways may be
targeted in order to recover the regenerative ability of axons.
Rho A and ROCKII belong to the AGC [protein kinase
(PK)A/PKG/PKC] family of serine/threonine kinases, and are involved
in the reorganization of actin cytoskeletal dynamics (7). Two forms of Rho A have been detected
in vivo: Rho-GTP (active form) and Rho-GDP (inactive form).
Activated Rho A enhances the activity of ROCKII, thus inhibiting
axon growth in the CNS (8,9). The upregulation of ROCK activity
leads to phosphorylation of various target proteins, including
collapsin response mediator protein-2 (CRMP-2), a neuronal protein
that serves a role in semaphorin-3A-mediated axon guidance during
the development of the nervous system (10). Therefore, ROCKII is considered an
integration point for regulating actin-myosin contractility and
axonal regeneration. Similar to ROCKII, GSK-3 is an active protein
kinase that acts as a negative regulator in the hormonal control of
glucose homeostasis, and the regulation of transcription factors
and microtubules. As a subtype of GSK-3, GSK-3β is highly expressed
in neurons during neurite remodeling, and is critical for the
establishment of neuronal polarity and development of axons from
mature neurites in CNS neurons (11). Overactivity of GSK-3β affects
several protein substrates, thus inhibiting the polymerization of
microtubules and transport of proteins in the growth cone, and
regulating the dynamic balance of microtubules and microfilaments
(12). As a result, activated
GSK-3β signaling inhibits axonal regeneration.
Previous studies have supported the hypothesis that
Rho-ROCKII or GSK-3β inhibitors may improve axon growth following
spinal cord injury (SCI) (13,14).
Lingor et al reported that inhibition of ROCKII with the
small molecule antagonist Y27632 increased neurite outgrowth on
chondroitin sulfate proteoglycan in vitro and axonal
regeneration in the adult optic nerve in vivo (15). Furthermore, Chan et al
suggested that Y27632 exerts beneficial effects on axonal sprouting
and functional recovery following rat SCI (16). In addition to Y27632, the selective
GSK-3β inhibitor 4-benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione
(TDZD-8) is also regarded as an important protective factor after
SCI. TDZD-8 reduces the development of inflammation and tissue
injury, which is associated with spinal cord trauma (17). However, Y27632 or TDZD-8 alone only
inhibits one of the signaling pathways involved in protection after
SCI. Furthermore, although high doses of Y27632 are beneficial, a
low dose is detrimental (18).
Therefore, it may be hypothesized that the combined application of
Y27632 and TDZD-8 may provide better protection.
The present study investigated the effects of the
combined application of Y27632 and TDZD-8 on neurite outgrowth and
functional recovery in SCI rats. The results indicated that the
combined application of these two inhibitors more effectively
protects against secondary SCI by inhibiting cellular apoptosis,
enhancing growth-associated protein-43 (GAP-43) expression and
promoting neurite outgrowth in SCI rats, compared with Y27632 or
TDZD-8 alone.
Materials and methods
Rats and SCI
A total of 90 female Sprague-Dawley rats (age, 6–8
weeks; weight, 200–250 g) were purchased from the Experimental
Animal Center of Luzhou Medical College (Luzhou, China). The rats
were housed in a temperature (22–25°C)-, humidity (40–60%)- and
light (12-h light/dark cycle)-controlled environment, and were fed
standard rat chow and water, this access was controlled. The rats
were fasted on the day prior to the experiments. After being
anesthetized with pentobarbital sodium (45–60 mg/kg), a surgical
longitudinal incision was made along the midline of the back. The
spinal cord was exposed using a three-level T9-T11 laminectomy, and
SCI was produced by dropping a weight at the T10 level.
Sham-operated rats were subjected to the laminectomy only. All the
animals were anesthetized by an intraperitoneal injection with 2%
sodium pentobarbital. In all animals, the L4 segmental spinal cord
was exposed and a 3 cm long epidural catheter was implanted into
the spinal dura mater at ~5 mm. The catheter was fixed on the
paraspinal muscles and the muscle and skin were sutured. The rats
were then housed individually in a temperature-controlled room
(25°C). Paralysis of the lower limbs in rats was used to confirm
successful establishment of an SCI model. A total of 1 hour after
surgery, the SCI rats began to receive daily doses of Y27632 (1.6
mg/kg/d; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) for 2
weeks and/or TDZD-8 (1 mg/kg/d; Sigma-Aldrich; Merck Millipore) for
3 weeks via a catheter. Rats were sacrificed by cervical
dislocation under anesthesia with 0.2% sodium pentobarbital at
various time points, and the injured spinal cord tissues from each
SCI rat were fixed in 4% paraformaldehyde solution.
In the present study, rats were randomly assigned to
the following groups (n=15): i) SCI + Y27632 group, SCI rats were
treated with Y27632; ii) SCI + TDZD-8 group, SCI rats were treated
with TDZD-8; iii) SCI + TDZD-8 + Y27632 group, SCI rats were
treated with TDZD-8 and Y27632; iv) SCI + PBS: SCI rats were
treated with 0.01% PBS; v) SCI group, untreated SCI rats; and vi)
sham group, rats were subjected to laminectomy only.
The animal study protocols were approved by the
Institutional Animal Care and Use Committee of Luzhou Medical
College.
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay
A TUNEL assay was performed using In Situ
Cell Death Detection kit, POD (Roche Diagnostics, Basel,
Switzerland) according to the manufacturer's protocols. The TUNEL
assay was conducted on 4-µm thick sections, which were dewaxed with
xylene and rehydrated with graded ethanol (100%, 95%, 85%, and 75%)
and distilled water. The sections were incubated with 10–20 µg/ml
proteinase K for 15 min at room temperature, and endogenous
peroxidase was blocked with 3% hydrogen peroxide at room
temperature for 20 min. The sections were then immersed in terminal
deoxynucleotidyl transferase buffer containing deoxynucleotidyl
transferase and biotinylated dUTP in a humidified atmosphere at
37°C for 90 min. Subsequently, the sections were incubated with a
horseradish peroxidase-conjugated antibody (supplied in the TUNEL
detection kit) at room temperature for 30 min. The signals were
visualized using diaminobenzidine (17) and a DM4000 B LED microscope (Leica
Microsystems GmbH, Wetzlar, Germany) and a microscope camera (Leica
Microsystems GmbH).
Immunohistochemical analysis
Frozen sections (4-µm thick) of the injured rat
spinal cords were prepared following dehydration in graded
sucrose-based solution. The sections were then incubated with a
primary antibody targeting GAP-43 (1:500; Epitomics, Burlingame,
CA, USA; cat. no. 2256–1) at 4°C overnight. Subsequently, the
sections were incubated with biotinylated secondary antibody
(1:2,000; OriGene Technologies, Inc., Beijing, China; cat. no.
SP-9001) at 37°C for 30 min and HRP-labeled streptavidin (OriGene
Technologies, Inc.) at 37°C for 30 min. Diaminobenzidine was used
as the chromogen and sections were observed with a DM4000 B LED
microscope and microscope camera. Staining was quantified by
measuring the intensity of signals using Image-Pro Plus (version
6.0; Media Cybernetics, Inc., Rockville, MD, USA). Two independent
investigators semi-quantitatively or quantitatively assessed the
immunohistochemical studies in a blinded manner.
Anterograde tracer
A total of 6 weeks after the SCI operation rats were
anesthetized with 2% sodium pentobarbital and a green fluorescent
tracer (<50 mmol/ml; Sigma-Aldrich; Merck Millipore) was
injected into the cerebral cortex. After 2 weeks, the SCI rats were
sacrificed. Frozen sections (4-µm thick) of the injured spinal cord
were prepared, and the staining was quantified by measuring the
intensity of signals using Image-Pro Plus (Media Cybernetics,
Inc.).
Basso Beattie Bresnahan locomotor
rating scale (BBB) score
Motor functions of the lower limbs of the rats were
evaluated 3, 6 and 8 weeks after the SCI operation, according to a
previous report (19). The motor
functions of the lower limbs can be divided into 22 grades.
According to function, 0 points indicate hind leg paralysis,
whereas 21 indicates completely normal function. BBB scoring was
used to assess recovery following contusion injuries to the spinal
cord in the rat according to a previous study (20). The BBB score observation period
lasted for 4 min, during which the animals should be maintained in
the center of the range area.
Somatosensory evoked potential (SEP)
monitoring
Spinal SEP monitoring (Nihon Kohden Corporation,
Tokyo, Japan) is widely used intraoperatively, due to its ease of
use and reliability. In the present study, SEP values were assessed
3 and 8 weeks after the SCI operation, according to a previous
report (21). The amplitudes and
latent periods of SEP at the C3/C4 position were recorded by
percutaneous electrical stimulation of the contralateral limb of
the median nerve and posterior tibial nerve.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. All statistical analyses were performed using SPSS
(version 16.0; SPSS Inc., Chicago, USA). A one-way analysis of
variance was used when more than two groups were compared followed
by Dunnett's method for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of Rho-ROCKII and GSK-3β
inhibitors on cellular apoptosis after SCI
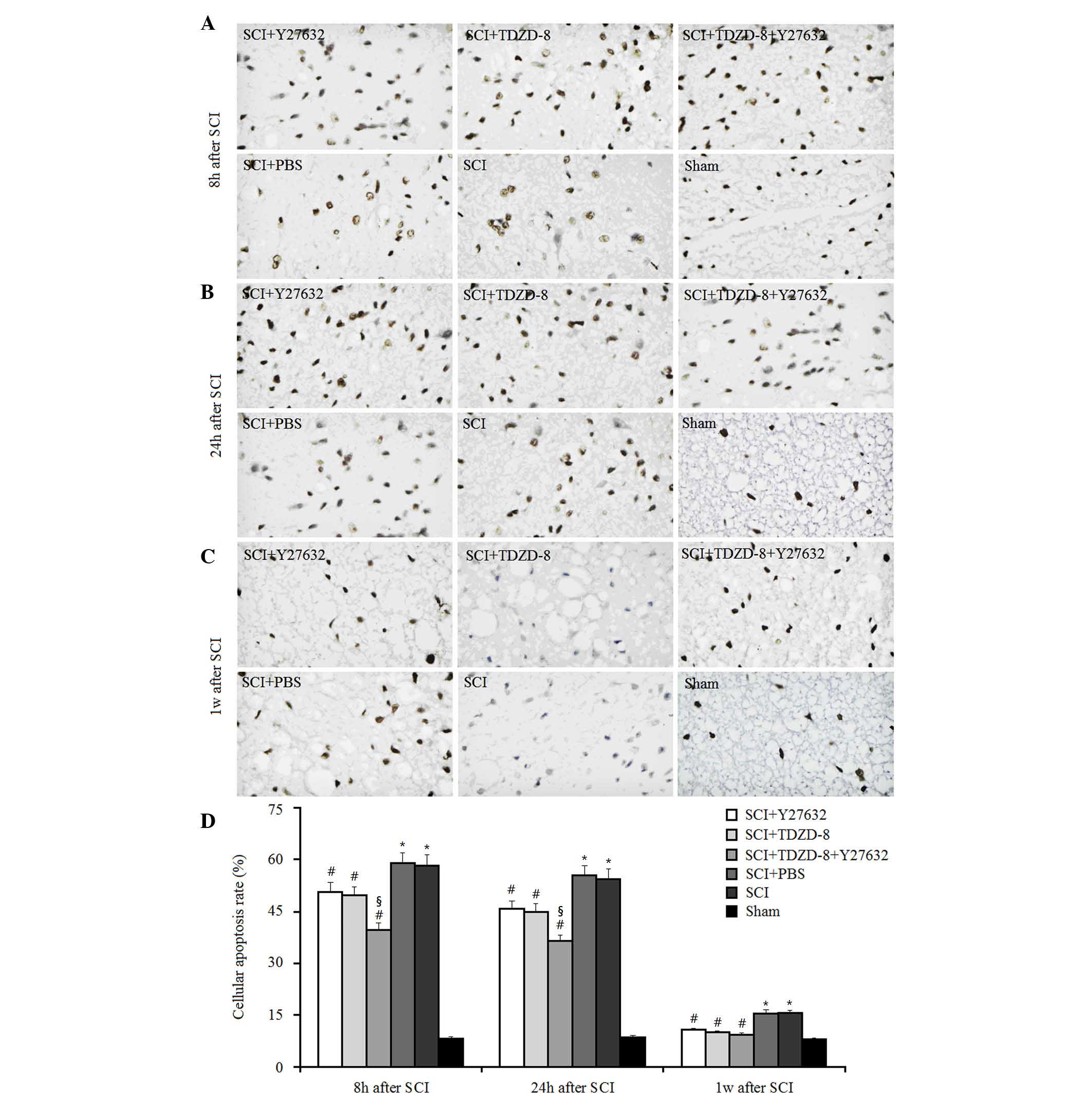
To investigate the combined effects of Rho-ROCKII
and GSK-3β inhibitors on cellular apoptosis after SCI, TUNEL-like
staining was measured in the perilesional spinal cord tissues 8, 24
h and 1 week after surgery (Fig.
1). As expected, the number of apoptotic cells was
significantly higher in SCI rats compared with in the sham group at
these time points. Treatment with Y27632, TDZD-8 or combined
treatment lowered the number of apoptotic cells in SCI rats
compared with in SCI rats treated with or without PBS, thus
suggesting that Rho-ROCKII and GSK-3β inhibitors may protect spinal
cord tissues following SCI in rats. In addition, there was no
significant difference between the Y27632-treated and
TDZD-8-treated groups, which indicated that inhibiting the
Rho-ROCKII or GSK-3β pathway exerts similar effects on cellular
apoptosis. Combined application of these two inhibitors decreased
the number of apoptotic cells compared with Y27632 or TDZD-8
treatment alone 8 and 24 h after surgery. However, there was no
significant difference among these three groups 1 week after SCI.
Therefore, it may be hypothesized that the time-dependent
downregulatory effect of Y27632, TDZD-8 or their combined
application on the number of apoptotic cells in SCI rats may be
associated with the body's own natural healing mechanisms, since
the reduction in the number of apoptotic cells in SCI rats did not
differ among the Y27632-, TDZD-8-, PBS-, Y27632 and TDZD-8-, or
untreated groups after 1 week.
Effects of Rho-ROCKII and GSK-3β
inhibitors on GAP-43 expression
GAP-43 is a rapidly transported growth-associated
protein enriched in elongating axons, which is considered an
important endogenous indicator of nerve regeneration (18,22).
In the present study, it was demonstrated that the expression of
GAP-43 gradually increased in SCI rats over time, as indicated by
immunohistochemical staining (Fig.
2). The expression of GAP-43 in SCI rats was significantly
higher compared with in sham-operated rats 8, 24 h and 1 week after
surgery, thus suggesting that injuries to the spinal cord induced
marked axonal regeneration. In addition, the expression levels of
GAP-43 were higher in SCI rats treated with Y27632, TDZD-8 or
combined treatment, as compared with in untreated or PBS-treated
SCI rats. These results indicate that Rho-ROCKII and GSK-3β
inhibitors may improve axonal regeneration after SCI. Although no
significant difference was detected among these three groups 8 and
24 h after SCI, combined application promoted axonal regeneration
more effectively 1 week after surgery compared with Y27632 or
TDZD-8 alone.
Effects of Rho-ROCKII and GSK-3β
inhibitors on anterograde tracer transmission
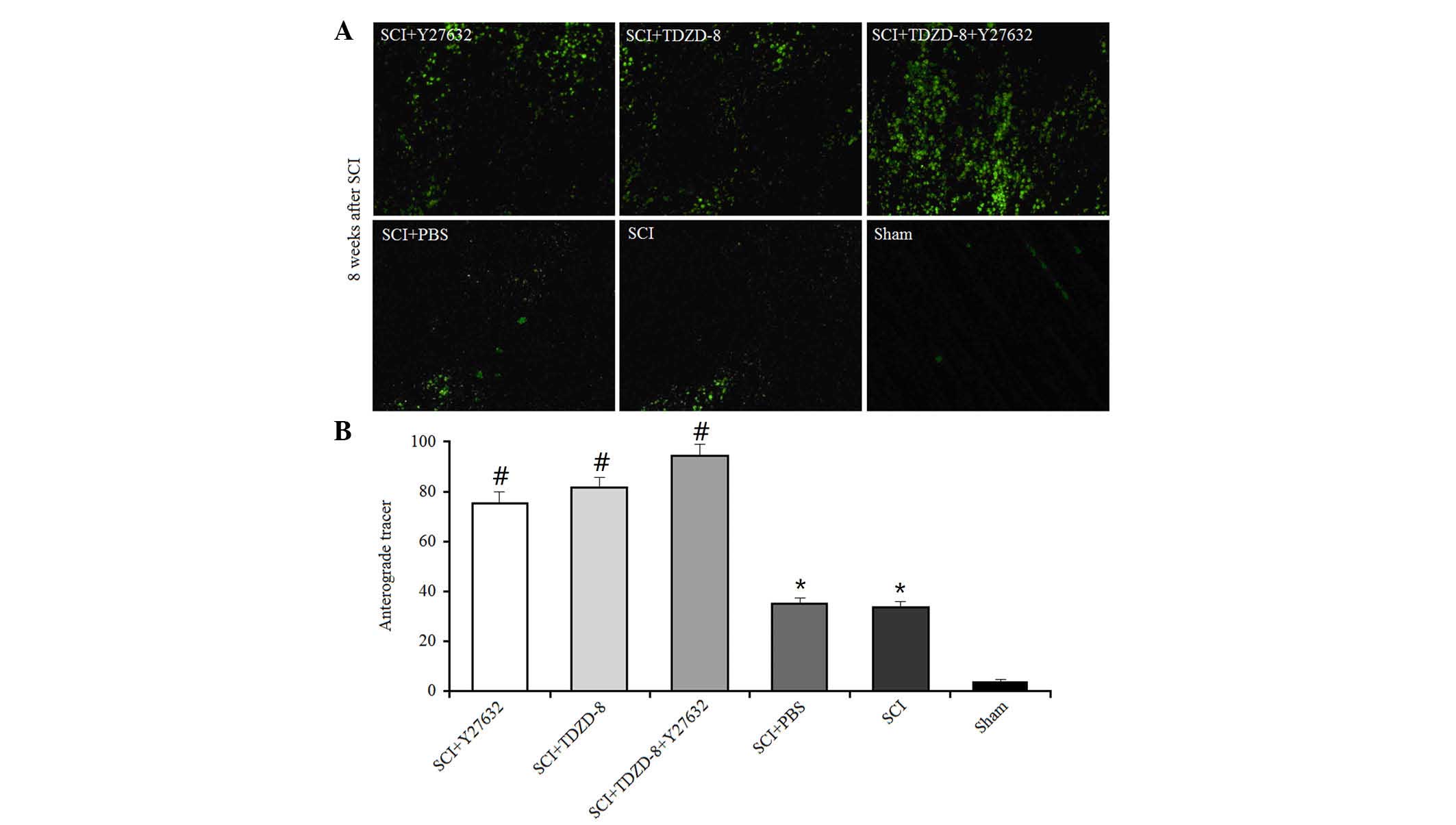
The anterograde tracer method was used to
investigate the effects of Rho-ROCKII and GSK-3β inhibitors on
axonal regeneration. A green fluorescent tracer (<50 mmol/ml)
was injected into the cerebral cortex 6 weeks after SCI operation
in rats; this tracer could be transmitted across the axonal gap.
The areas of fluorescence at the lesion were measured using a
computer-assisted digital image analysis system (Fig. 3). SCI operation promoted axonal
regeneration in rats, as indicated by the larger fluorescent areas
compared with in the sham group. In addition, the fluorescent areas
were larger in drug-treated SCI rats compared with in untreated SCI
rats, thus suggesting that Rho-ROCKII and GSK-3β inhibitors
improved axonal regeneration. There was no difference between the
single drug-treated groups; however, the combined application of
Y27632 and TDZD-8 improved axonal regeneration.
Effects of Rho-ROCKII and GSK-3β
inhibitors on motor function
The BBB scores at weeks 3, 6 and 8 were used to
determine the motor function of the lower limbs of rats. As shown
in Fig. 4, lower limb function was
recovered in SCI rats in a time-dependent manner. The motor
function recovered to half its normal level 8 weeks after SCI.
Despite the lack of significant differences in the functional
recovery of lower limbs among SCI rats with or without drug
treatment, Y27632- or TDZD-8-treated rats appeared to exhibit
improved recovery.
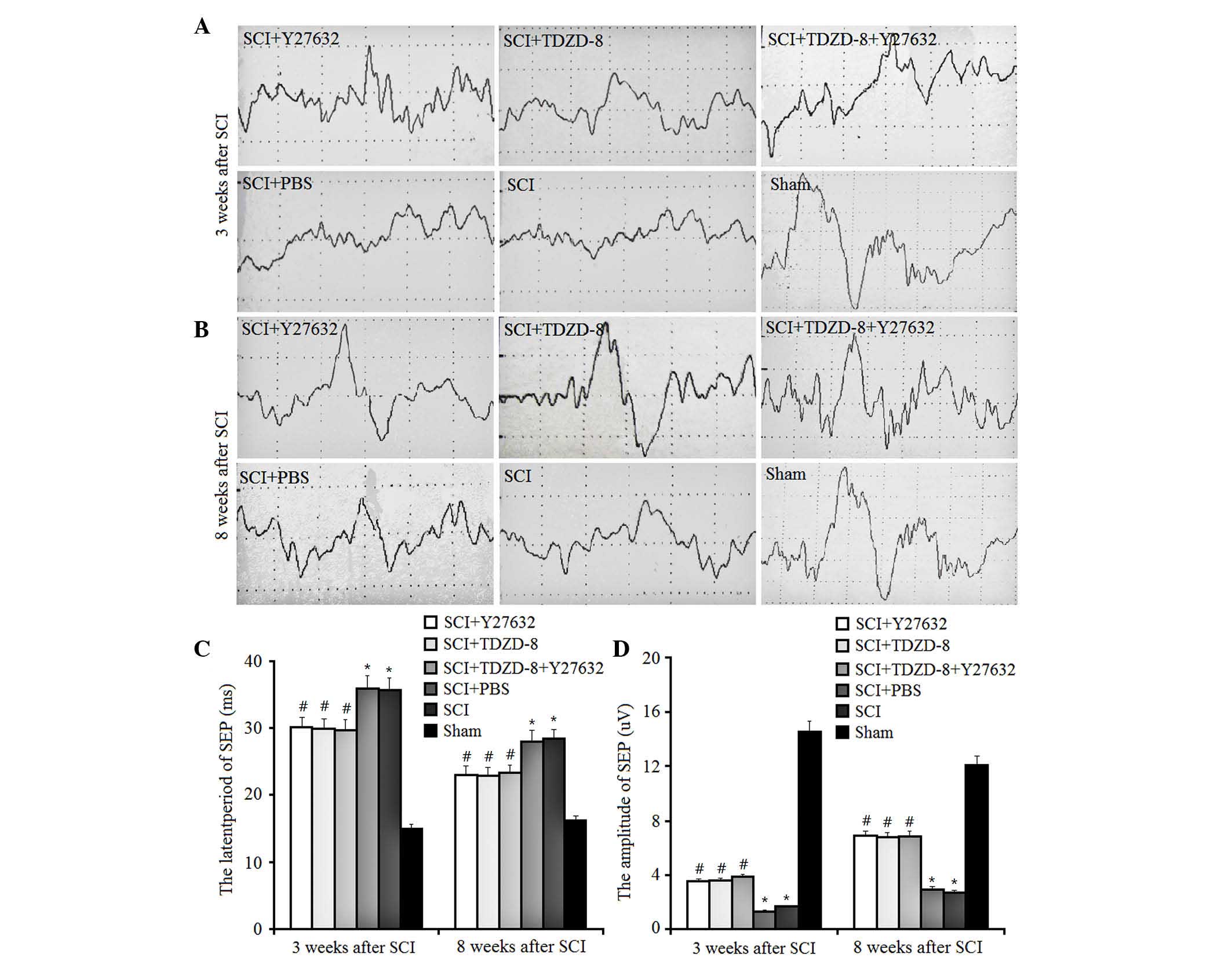
The present study also examined the latent periods
and amplitudes of SEP in SCI rats (Fig. 5). SEP is generated by
physiologically or electrically stimulating the afferent peripheral
nerve fibers. In SCI rats, downregulated latent periods and
upregulated amplitudes of SEP indicate improved functional recovery
of spinal cord tissues. The results of the present study indicated
that injuries to the spinal cord increased the latent periods of
SEP and decreased the amplitudes. In addition, treatment with
Y27632 or TDZD-8 inhibited these changes, resulting in a decrease
in latent periods and an increase in amplitudes of SEP; however,
there were no significant differences between these treatment
groups. These findings suggest that treatment with Y27632, TDZD-8
or their combined application exert the same effect on the motor
function of the lower limbs of SCI rats.
Discussion
Effects of Rho-ROCKII inhibitors on
axonal regeneration
In the present study, a TUNEL assay and
immunohistochemical staining revealed that the number of apoptotic
cells was significantly decreased, and the expression of GAP-43 was
increased in SCI rats treated with the Rho-ROCKII inhibitor Y27632,
as compared with SCI rats treated with or without PBS. In addition,
an anterograde tracer analysis indicated that Y27632 improved
axonal regeneration. Furthermore, Y27632 treatment increased BBB
scores and SEP amplitudes, and decreased SEP latent periods, thus
suggesting that Y27632 protects the motor function of lower limbs
in SCI rats. These results indicated that inhibition of the
Rho-ROCKII signaling pathway may reduce injuries to the spinal cord
and induce marked axonal regeneration in SCI rats.
The protective effects of Rho-ROCKII signaling
inhibitors on axonal regeneration reported in the present study are
supported by the results of a previous study. Kubo et al
demonstrated that suppressing the Rho-ROCKII signaling pathway
protects neurons, inhibits apoptosis, reduces glial scars and
promotes axonal regeneration (23). Another study by Chan et al
also reported that a Rho-ROCKII signaling inhibitor is able to
protect axonal sprouting and promote functional recovery after SCI
(16). In addition, ROCK
inhibition may modulate neurite growth and protect neurons from
excitotoxicity-induced cell death (24). These findings suggested that the
induction of axonal regeneration by inhibiting Rho-ROCKII signaling
is promising.
It is well known that CNS axons do not regenerate as
easily as peripheral axons following injury (25), predominantly due to inhibitors
present in the CNS myelin and in the glial scar. Myelin-associated
inhibitors (MAIs), such as myelin-associated glycoprotein and
oligodendrocyte-myelin glycoprotein (4,26),
are predominantly expressed at the surface of the cytomembrane,
which is adjacent to the axon and myelin sheath. Following SCI,
upregulated MAIs activate Rho GTPase and phosphorylate ROCKII
(27). The activation of ROCK
further leads to phosphorylation of various target proteins,
including myosin light chain, CRMP2, and microtubule-associated
protein 2 (13). This
phosphorylation generates cascading signals, resulting in the
breakdown of the growth cone cytoskeleton and inhibition of axonal
regeneration. The inhibition of ROCK activity following treatment
with Y27632 is able to reduce the injuries to the growth cone
cytoskeleton to a certain extent and restore axonal
regeneration.
Although Rho-ROCKII signaling inhibitors markedly
improved axonal regeneration in the present study, larger in
vivo studies are required to identify the appropriate doses of
these inhibitors. Chan et al reported that a high dose of
Y27632 exerts beneficial effects; however, low doses may be
detrimental to rats following SCI (16). In addition, a previous study
demonstrated that systemic treatment with high doses of Y27632
significantly enhanced the regeneration of motor axons over short
distances, whereas the regeneration of sensory fibers remained
largely unchanged (28).
Therefore, more studies are required before these inhibitors can be
used in clinical practice.
Effects of GSK-3β inhibitors on axonal
regeneration
In addition to Rho-ROCKII signaling inhibitors,
GSK-3β signaling inhibitors have been reported to protect axonal
regeneration following SCI (17,29).
The present study demonstrated that the GSK-3β signaling inhibitor
TDZD-8 significantly decreased the number of apoptotic cells,
increased GAP-43 expression and induced marked axonal regeneration
in SCI rats. In addition, TDZD-8 administration protected the motor
function of the lower limbs of SCI rats, as detected by increased
BBB scores and SEP amplitudes, and decreased SEP latent periods.
These results indicated that inhibition of GSK-3β signaling may
alleviate SCI and promote axonal regeneration.
Previous studies have reported that axon guidance
molecules are more abundant following SCI, and
neuropilin-1/plexin-A serves to activate phosphatidylinositol-3
kinase and produce phosphatidylinositol 3,4,5-triphosphate. These
activated factors further activate GSK-3β and produce a signaling
cascade that collapses the growth cone. Kim et al reported
that a stronger knockdown of GSK-3β markedly reduced axonal growth
in dissociated cultures and slice preparations, whereas a moderate
reduction of GSK-3 activity, via the use of pharmacological
inhibitors, induced axon branching (14). GSK-3 is a downstream convergent
point for several axon growth regulatory pathways. As the first
non-competitive inhibitor of ATP, TDZD-8 is more selective than
other inhibitors of GSK-3β. In addition to the effects of the
Rho-ROCKII inhibitor Y27632, the results of the present study
demonstrated that treatment with TDZD-8 reduced SCI and induced
axonal regeneration in SCI rats. However, the effects of TDZD-8 and
Y27632 on axonal regeneration and functional recovery of the lower
limbs were not significantly different. It may be hypothesized that
this lack of difference is due to differences in sensitivity. In
addition, the dose used in the present study may have affected the
results; therefore, the appropriate in vivo dosage requires
further exploration.
Effects of combined application of
Rho-ROCKII and GSK-3β inhibitors on axonal regeneration
Several inhibitors appear in the in vivo
microenvironment following SCI; therefore, the effect of
suppressing only one of these inhibitors in order to promote axonal
regeneration is limited. Gopalakrishnan et al suggested that
a ROCK inhibitor alone could not completely abolish the inhibitory
effects of chondroitin sulfate proteoglycans (30). TDZD-8 has been shown to inhibit
activation of GSK-3β, leading to CRMP-2 dephosphorylation; however,
it was unable to inhibit ROCK-induced phosphorylation (15). Therefore, TDZD-8 could not
completely inhibit the collapse of the growth cone. Simultaneous
inhibition of ROCKII and GSK-3β signaling may abolish the
inhibitory effects of endogenous inhibitors and effectively induce
axonal regeneration following SCI. The results of the present study
further supported the hypothesis that combined administration of
Rho-ROCKII and GSK-3β inhibitors could improve axonal
regeneration.
The protective effects of combined application of
Rho-ROCKII and GSK-3β inhibitors on axonal regeneration are
associated with the reduction of cellular apoptosis. A previous
study by Dubreuil et al suggested that Rho is activated
after SCI and upregulates the expression of p75 neurotrophin
receptor (p75NTR), thus resulting in apoptosis (31). Conversely, suppressing the
overactivation of Rho after SCI protects cells from
p75NTR-dependent apoptosis. Furthermore, Cuzzocrea et al
reported that TDZD-8 was able to inhibit apoptosis by upregulating
B-cell lymphoma 2 (Bcl-2) expression and downregulating
Bcl-2-associated X protein expression (17). In addition, TDZD-8 could inhibit
apoptosis in the hippocampus following ischemia-reperfusion injury
by reducing the release of cytochrome c and the activation
of caspase-9, which promotes mitochondria-mediated apoptosis
(32). These findings indicated
that the Rho-ROCKII and GSK-3β signaling pathways are involved in
apoptosis following SCI, and the protective effects of suppressing
one signaling pathway alone may be limited. The combined
application of Rho-ROCKII and GSK-3β inhibitors may yield better
results. The present study demonstrated that the number of
apoptotic cells was decreased in SCI rats following treatment with
Y27632 or TDZD-8. However, the reduction in the number of apoptotic
cells during the early stage of SCI was more pronounced after the
combined administration of Y27632 and TDZD-8, as compared with
Y27632 or TDZD-8 alone. Therefore, the combined administration of
Rho-ROCKII and GSK-3β signaling inhibitors may more effectively
protect against cellular apoptosis, which may serve an important
role in axonal regeneration.
In addition, the functional recovery of lower limbs
in rats is an important indicator of axonal regeneration after SCI.
However, the BBB and SEP results were not significantly altered in
the treatment groups compared with the SCI group. In rats, the SCI
operation results in neuronal loss and axonal injury. Treatment
with Y27632 or TDZD-8, or their combined administration, could only
reduce these injuries in axons and promote axonal regeneration, but
could not restore lost neurons. Therefore, the functional recovery
of the lower limbs in SCI rats was limited and incomplete, although
drug-induced axonal regeneration relieves SCI to a certain extent.
Future pharmacological studies should investigate the protection of
neuronal loss, in addition to the induction of axonal
regeneration.
In conclusion, in the present study, an SCI rat
model was administered daily doses of the ROCKII inhibitor Y27632
and/or the GSK-3β inhibitor TDZD-8. Subsequently, the degree of
injury in the spinal cord, axonal regeneration and the functional
recovery of lower limbs were investigated. The results demonstrated
that treatment with Y27632 and TDZD-8 significantly inhibited
cellular apoptosis, enhanced GAP-43 expression and promoted neurite
outgrowth. In addition, the combined application of Y27632 and
TDZD-8 more effectively protected the spinal cords of SCI rats from
secondary injuries, when compared with Y27632 or TDZD-8 alone.
Acknowledgements
The present study was sponsored by the National
Natural Science Foundation of China (grant no. Y20110028) and the
Project of Sichuan Department of Science and Technology (grant no.
2015JY0224).
References
|
1
|
Horner PJ and Gage FH: Regenerating the
damaged central nervous system. Nature. 407:963–970. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen MS, Huber AB, van der Haar ME, Frank
M, Schnell L, Spillmann AA, Christ F and Schwab ME: Nogo-A is a
myelin-associated neurite outgrowth inhibitor and an antigen for
monoclonal antibody IN-1. Nature. 403:434–439. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Domeniconi M, Cao Z, Spencer T,
Sivasankaran R, Wang K, Nikulina E, Kimura N, Cai H, Deng K, Gao Y,
et al: Myelin-associated glycoprotein interacts with the Nogo66
receptor to inhibit neurite outgrowth. Neuron. 35:283–290. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang KC, Koprivica V, Kim JA, Sivasankaran
R, Guo Y, Neve RL and He Z: Oligodendrocyte-myelin glycoprotein is
a nogo receptor ligand that inhibits neurite outgrowth. Nature.
417:941–944. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Madura T, Yamashita T, Kubo T, Fujitani M,
Hosokawa K and Tohyama M: Activation of Rho in the injured axons
following spinal cord injury. EMBO Rep. 5:412–417. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alabed YZ, Pool M, Ong Tone S, Sutherland
C and Fournier AE: GSK3 beta regulates myelin-dependent axon
outgrowth inhibition through CRMP4. J Neurosci. 30:5635–5643. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmandke A and Strittmatter SM and
Strittmatter SM: ROCK and Rho: Biochemistry and neuronal functions
of Rho-associated protein kinases. Neuroscientist. 13:454–469.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fournier AE, Takizawa BT and Strittmatter
SM: Rho kinase inhibition enhances axonal regeneration in the
injured CNS. J Neurosci. 23:1416–1423. 2003.PubMed/NCBI
|
|
9
|
Shao Z, Browning JL, Lee X, Scott ML,
Shulga-Morskaya S, Allaire N, Thill G, Levesque M, Sah D, McCoy JM,
et al: TAJ/TROY, an orphan TNF receptor family member, binds
Nogo-66 receptor 1 and regulates axonal regeneration. Neuron.
45:353–359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arimura N, Inagaki N, Chihara K, Ménager
C, Nakamura N, Amano M, Iwamatsu A, Goshima Y and Kaibuchi K:
Phosphorylation of collapsin response mediator protein-2 by
Rho-kinase. evidence for two separate signaling pathways for growth
cone collapse. J Biol Chem. 275:23973–23980. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoshimura T, Kawano Y, Arimura N, Kawabata
S, Kikuchi A and Kaibuchi K: GSK-3beta regulates phosphorylation of
CRMP-2 and neuronal polarity. Cell. 120:137–149. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou FQ and Snider WD: Cell biology.
GSK-3beta and microtubule assembly in axons. Science. 308:211–214.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mueller BK, Mack H and Teusch N: Rho
kinase, a promising drug target for neurological disorders. Nat Rev
Drug Discov. 4:387–398. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim WY, Zhou FQ, Zhou J, Yokota Y, Wang
YM, Yoshimura T, Kaibuchi K, Woodgett JR, Anton ES and Snider WD:
Essential roles for GSK-3s and GSK-3-primed substrates in
neurotrophin-induced and hippocampal axon growth. Neuron.
52:981–996. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lingor P, Teusch N, Schwarz K, Mueller R,
Mack H, Bähr M and Mueller BK: Inhibition of Rho kinase (ROCK)
increases neurite outgrowth on chondroitin sulphate proteoglycan in
vitro and axonal regeneration in the adult optic nerve in vivo. J
Neurochem. 103:181–189. 2007.PubMed/NCBI
|
|
16
|
Chan CC, Khodarahmi K, Liu J, Sutherland
D, Oschipok LW, Steeves JD and Tetzlaff W: Dose-dependent
beneficial and detrimental effects of ROCK inhibitor Y27632 on
axonal sprouting and functional recovery after rat spinal cord
injury. Exp Neurol. 196:352–364. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cuzzocrea S, Genovese T, Mazzon E,
Crisafulli C, Di Paola R, Muià C, Collin M, Esposito E, Bramanti P
and Thiemermann C: Glycogen synthase kinase-3 beta inhibition
reduces secondary damage in experimental spinal cord trauma. J
Pharmacol Exp Ther. 318:79–89. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kawasaki T, Nishio T, Kawaguchi S and
Kurosawa H: Spatiotemporal distribution of GAP-43 in the developing
rat spinal cord: A histological and quantitative immunofluorescence
study. Neurosci Res. 39:347–358. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park DY, Mayle RE, Smith RL,
Corcoran-Schwartz I, Kharazi AI and Cheng I: Combined
transplantation of human neuronal and mesenchymal stem cells
following spinal cord injury. Global Spine J. 3:1–6. 2014.
View Article : Google Scholar
|
|
20
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Caizhong X, Chunlei S, Beibei L, Zhiqing
D, Qinneng D and Tong W: The application of somatosensory evoked
potentials in spinal cord injury rehabilitation.
NeuroRehabilitation. 35:835–840. 2014.PubMed/NCBI
|
|
22
|
Kim DH and Jahng TA: Continuous
brain-derived neurotrophic factor (BDNF) infusion after
methylprednisolone treatment in severe spinal cord injury. J Korean
Med Sci. 19:113–122. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kubo T, Hata K, Yamaguchi A and Yamashita
T: Rho-ROCK inhibitors as emerging strategies to promote nerve
regeneration. Curr Pharm Des. 13:2493–2499. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jeon BT, Jeong EA, Park SY, Son H, Shin
HJ, Lee DH, Kim HJ, Kang SS, Cho GJ, Choi WS and Roh GS: The
Rho-kinase (ROCK) inhibitor Y-27632 protects against
excitotoxicity-induced neuronal death in vivo and in vitro.
Neurotox Res. 23:238–248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fournier AE and Strittmatter SM: Repulsive
factors and axon regeneration in the CNS. Curr Opin Neurobiol.
11:89–94. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu BP, Fournier A, GrandPre T and
Strittmatter SM: Myelin-associated glycoprotein as a functional
ligand for the Nogo-66 receptor. Science. 297:1190–1193. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Buchsbaum RJ: Rho activation at a glance.
J Cell Sci. 120:1149–1152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Joshi AR, Bobylev I, Zhang G, Sheikh KA
and Lehmann HC: Inhibition of Rho-kinase differentially affects
axon regeneration of peripheral motor and sensory nerves. Exp
Neurol. 263:28–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dill J, Wang H, Zhou F and Li S:
Inactivation of glycogen synthase kinase 3 promotes axonal growth
and recovery in the CNS. J Neurosci. 28:8914–8928. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gopalakrishnan SM, Teusch N, Imhof C,
Bakker MH, Schurdak M, Burns DJ and Warrior U: Role of Rho kinase
pathway in chondroitin sulfate proteoglycan-mediated inhibition of
neurite outgrowth in PC12 cells. J Neurosci Res. 86:2214–2226.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dubreuil CI, Winton MJ and McKerracher L:
Rho activation patterns after spinal cord injury and the role of
activated Rho in apoptosis in the central nervous system. J Cell
Biol. 162:233–243. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Collino M, Thiemermann C, Mastrocola R,
Gallicchio M, Benetti E, Miglio G, Castiglia S, Danni O, Murch O,
et al: Treatment with the glycogen synthase kinase-3beta inhibitor,
TDZD-8, affects transient cerebral ischemia/reperfusion injury in
the rat hippocampus. Shock. 30:299–307. 2008. View Article : Google Scholar : PubMed/NCBI
|