Introduction
Cardiovascular disease is currently the leading
cause of mortality, and the incidence continues to increase, in
spite of advancements in diagnosis and treatment of cardiovascular
disease. Thus, novel types of therapy are under development for
patients who are suffering from cardiovascular disease. Increasing
evidence suggests endothelial progenitor cells (EPCs) improve
neovascularization and endothelium regeneration, suggesting there
may be potential for cell therapy in the future to promote
endothelial repair and reendothelialization of ischemic tissue
(1–4). Resveratrol (RSV) is a natural
polyphenolic compound, modern medical research has demonstrated RSV
exerts multiple protective effects on the cardiovascular system,
including reducing platelet adhesion and aggregation, protecting
myocardial cells from ischemia-reperfusion injury, and suppression
of neointimal hyperplasia of injured vascular tissue (5–7). A
previous study indicated that RSV prevented the onset of EPC
senescence and enhanced telomerase activity via the
phosphatidylinositol-4,5-bisphosphate 3-kinase-Akt signaling
pathway (8). The present study
hypothesized that RSV protects EPCs from senescence via improving
pathological factors that induce EPC dysfunction in vivo. In
addition, they may also enhance the properties of EPCs that are
important for cell therapy. The aim of the present study was to
investigate whether RSV could prevent EPCs from senescence, and to
investigate the effects of RSV on the potential repair and
re-endothelialization capacity of EPCs in injured vessels.
Materials and methods
Cell isolation and culture
The technique to culture EPC subpopulations was
conducted as described previously (9,10).
The blood samples were obtained from healthy volunteers subsequent
to obtaining informed, written consent (n=20; age, 28±4 years; 10
male, 10 female), and were treated, observed and analyzed
individually in independent experiments. The peripheral blood
mononuclear cells (PBMNCs) were isolated by density-gradient
centrifugation with Ficoll separating solution (Cedarlane
Laboratories Ltd., Burlington, Ontario, Canada). The study was
approved by the ethics committee of Zhejiang University (Hangzhou,
China). PBMNCs were plated into fibronectin-coated six-well plates
in 1.5 ml EGM-2MV medium (Lonza Group, Basel, Switzerland) at a
density of 2×105/cm2, containing 10% fetal
bovine serum, vascular endothelial growth factor, fibroblast growth
factor-2, epidermal growth factor, insulin-like growth factor, and
ascorbic acid. Adherent MNCs cultured for 7–21 days in the above
conditions were used to derive late outgrowth EPCs and the colonies
exhibit a cobblestone morphology.
Determination of reactive oxygen
species (ROS) production
The membrane permeable indicator H2DCF-DA
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was
used to determine ROS production by EPCs. EPCs were loaded with 10
µmol/l H2DCF-DA in serum-free EGM-2MV medium at 37°C for
30 min and then washed twice with phosphate-buffered saline (PBS).
Following treatment with various concentrations of RSV (0.01, 0.1,
1, 5 and 10 µmol/l; Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) for 48 h, the cells were analyzed with a flow cytometer
(BD Biosciences, Franklin Lakes, NJ, USA) at an excitation
wavelength of 488 nm and an emission wavelength of 525 nm. ROS
production was determined by comparing the changes in fluorescence
intensity with those of the control. CellQuest Pro software,
version 3.1 (BD Biosciences) was used for analysis.
Measurement of NADPH oxidase activity. To
evaluate NADPH oxidase activity in EPCs, the lucigenin-derived
enhanced chemiluminescence assay (Beyotime Institute of
Biotechnology, Shanghai, China) was employed. EPCs were starved in
serum-free EGM-2MV medium for 24 h and treated with RSV (0.01, 0.1,
1, 5 and 10 µmol/l), and subsequently washed twice with ice-cold
PBS (pH 7.4) and centrifuged at 2,000 × g for 5 min at 4°C.
The cells were re-suspended in ice-cold buffer (pH 7.0) containing
1 mmol/l ethylene glycol tetraacetic acid, protease inhibitors, and
150 mmol/l sucrose, and lysed. A Bradford assay was used to
determine the total protein concentration, which was adjusted to 1
mg/ml. Every 100 µl of protein sample, including 2.5 µmol/l
lucigenin, was measured over 6 min in quadruplicate using NADPH
(100 µmol/l) as a substrate in a luminometer counter (Centro LB
960; Berthold Technologies GmbH & Co. KG, Bad Wildbad,
Germany).
Evaluation of nitric oxide (NO)
production
EPCs were starved in serum-free medium for 48 h and
treated with RSV (0.01, 0.1, 1, 5 and 10 µmol/l). The production of
NO was evaluated by identifying the concentration of NO in the
culture supernatant. The effect of RSV on NO generation of EPCs was
determined with the NO Assay kit (Beyotime Institute of
Biotechnology) according to the manufacturer's instructions.
Briefly, EPCs were seeded onto 96-well plates at a density of
2×104 cells/well, and pre-treated with or without
lipopolysaccharides (1 µg/ml) for 4 h. The cells were then
stimulated with or without different concentrations of RSV for 24
h. Subsequently, Griess reagent I and Griess reagent II from the
kit were added into the cell supernatants, and the optical density
was measured at 540 nm using the abovementioned luminometer
counter.
Determination of senescence-associated
β-galactosidase (SA-β-gal) activity
Following treatment with RSV (0.01, 0.1, 1, 5 and 10
µmol/l) for 48 h, EPC subpopulations were harvested and SA-β-gal
activity was measured according to a previously described method
(11). The SA-β-gal Activity Assay
kit was obtained from Cell Signaling Technology, Inc. (Danvers, MA,
USA). Briefly, EPCs were washed with PBS, fixed in fixative
solution (2% paraformaldehyde, 0.2% glutaraldehyde, PBS) for 10 min
at room temperature, and cultivated overnight at 37°C (no
CO2) with fresh SA-β-gal staining solution [1 mg/ml
5-bromo-4-chloro-3-indyl β-D-galactopyranoside (X-gal), 5 mmol/l
potassium ferrocyanide, 5 mmol/l potassium ferricyanide, 150 mmol/l
NaCl, 2 mmol/l MgCl2, 40 nmol/l citric acid/sodium
phosphate (pH 6.0)]. A light microscope was used to observe the
cells stained blue, and all the cells were stained with
4′,6-diamino-2-phenylindole for 10 min in order to count the total
cell numbers.
Western blot analysis
Following RSV treatment, equal amounts (20~30 µl) of
cellular proteins were obtained. The cellular proteins were
extracted using lysis buffer containing 1% NP-40, 150 mmol/l NaCl,
50 mmol/l Tris-HCl, pH 8.0, 0.5% sodium deoxycholate and 0.1% SDS.
The concentration of total proteins was measured using the
bicinchoninic acid assay (Pierce Biotechnology, Inc., Rockford, IL,
USA). and separated by 10% SDS-polyacrylamide gel and then
electrotransferred to a polyvinylidene difluoride membrane. Prior
to incubation of the membranes overnight at 4°C with the primary
antibodies, they were blocked in blocking solution (Tris-buffered
saline; TBS) containing 0.1% (v/v) Tween 20 and 5% (v/v) bovine
serum albumin (Sigma-Aldrich; Merck Millipore) for 1 h at room
temperature. The primary antibodies (1:1,000) used were as follows:
Anti-telomerase reverse transcriptase (TERT; BS60032; Bioworld
Technology, Inc., St. Louis Park, MN, USA), anti-peroxisome
proliferator-activated receptor-γ (PPAR-γ; #2430; Cell Signalling
Technology, Inc.), anti-heme oxygenase-1 (HO-1; #5853; Cell
Signalling Technology, Inc.), anti-sirtuin-1 (#2496; Cell
Signalling Technology, Inc.) and anti-GAPDH (#2118; Cell Signalling
Technology, Inc.). Subsequently, the membranes were washed
extensively in TBS containing 0.1% (v/v) Tween 20 3 times, and
incubated for 1 h at room temperature with mouse anti-rabbit
(#3677) and rabbit anti-mouse (#58802) secondary antibodies
conjugated to horseradish peroxidase (1:5,000; Cell Signalling
Technology, Inc.). Enhanced chemiluminescence (ECL) solution (ECL
Protein Biotinylation System; GE Healthcare Life Sciences,
Chalfont, UK) was used to visualize the protein bands on the
membranes. The western blotting results were analyzed using
Multi-gauge software, version 3.11 (Fujifilm, Tokyo, Japan).
Animal study
EPCs were isolated and cultured from the peripheral
blood of New Zealand rabbits (n=25; weight, 2.3–2.9 kg; 12 male and
13 female; 3–4 months old) as described above. The rabbits were
purchased from the Animal Center of Zhejiang University (Hangzhou,
China) and were individually housed with access to a high-fiber
pelleted laboratory diet and water, maintained at 23±1°C, with
30–70% relative humidity and a 12:12 h light:dark cycle (6 am-6
pm). All rabbits were distributed into 5 groups: i) Carotid injury
group without transplantation of EPCs; ii) carotid injury group
with transplantation of EPCs but without intervention; iii) carotid
injury group with transplantation of EPCs pretreated with RSV; v)
control group with sham procedure, which involved anesthesia with
ketamine, an anterior midline incision made to expose the left
common carotid artery and the internal and external carotid
arteries, which was then closed after ~10 min. The carotid injury
induced in the rabbits in the carotid injury groups was induced by
a balloon catheter. Rabbits were anesthetized with 35 mg/kg
ketamine, and an anterior midline incision was made to expose left
common carotid artery, internal and external carotid arteries. The
distal end of the external carotid artery was ligated and the
proximal common carotid artery and internal carotid artery were
temporarily interrupted. A 3F Fogarty balloon catheter was inserted
into the common carotid artery via the incision of the external
carotid artery (~3 cm) and then inflated with the pressure of 10
atmospheres. The balloon catheter was withdrawn then reentered 3
times to injury the artery. The EGM-2 suspension containing
1×106 EPCs cultured in vitro or alone was
injected locally into the arterial lumen and maintained there for
~5 min. The distal end of the external carotid artery was ligated
and the blood flow to the common carotid was restored by release of
the ligatures in the proximal common carotid artery and internal
carotid artery, and the wound was closed. No adverse neurological
or vascular effects were observed in any animal undergoing this
procedure. Rabbits were sacrificed after 1 week via injection of 10
ml air into the ear vein under ketamine anesthesia, and the target
carotid artery was removed for the measurement of
re-endothelialization and morphological study. Repair of the artery
was evaluated by staining with Evans Blue dye (Sigma-Aldrich; Merck
Millipore) according to a method described previously (12). Briefly, at 30 min prior to
sacrifice, 5 ml of saline containing 5% Evans blue was injected
into the ear vein. The artery was fixed with a perfusion of 4%
paraformaldehyde for 45 min. The area stained in blue indicates
endothelium injury and the ratio between the area free from stain
and the total injured carotid artery area was calculated. For
histological with hematoxylin and eosin (H&E) analyses, a
segment of each artery was perfusion fixed with 4% paraformaldehyde
at physiological pressure (110 mmHg) and subsequently
processed.
Statistical analysis
Values are expressed as the mean ± standard error in
the text and figures. Data were analyzed using independent t-tests
and one-way analysis of variance using GraphPad Prism, version 4
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
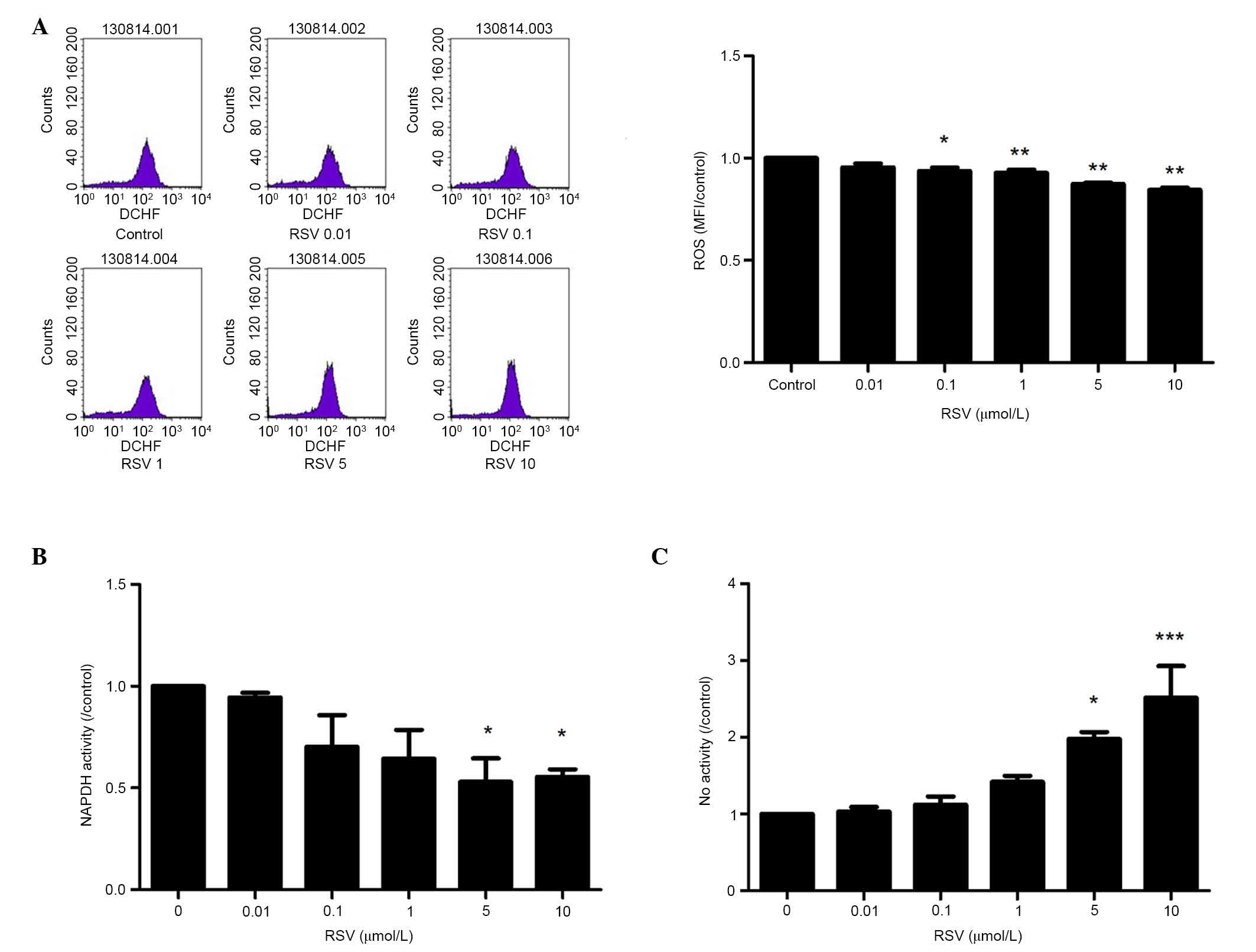
RSV reduces the oxidative reaction of
EPCs
To determine the effect of RSV on the oxidative
reaction of EPCs, the changes in ROS production, NAPDH oxidase
activity and NO production were observed. The intracellular ROS
levels were measured using a flow cytometer following staining of
EPCs with the ROS-sensitive fluorescent probe, H2DCF-DA.
As Fig. 1A demonstrates RSV
decreased DCF fluorescence in a concentration-dependent manner
(P<0.01). The NADPH oxidase activity with lucigenin-enhanced
chemiluminescence was measured. Pretreatment with RSV significantly
reduced the NADPH oxidative activity in a dose-dependent manner
(P<0.01 when the concentration of RSV was ≥1 µmol/l; Fig. 1B). Furthermore, the present study
observed the NO production of EPCs with or without RSV
pretreatment. Stimulation with RSV resulted in a
concentration-dependent increase of NO production (P<0.05;
Fig. 1C).
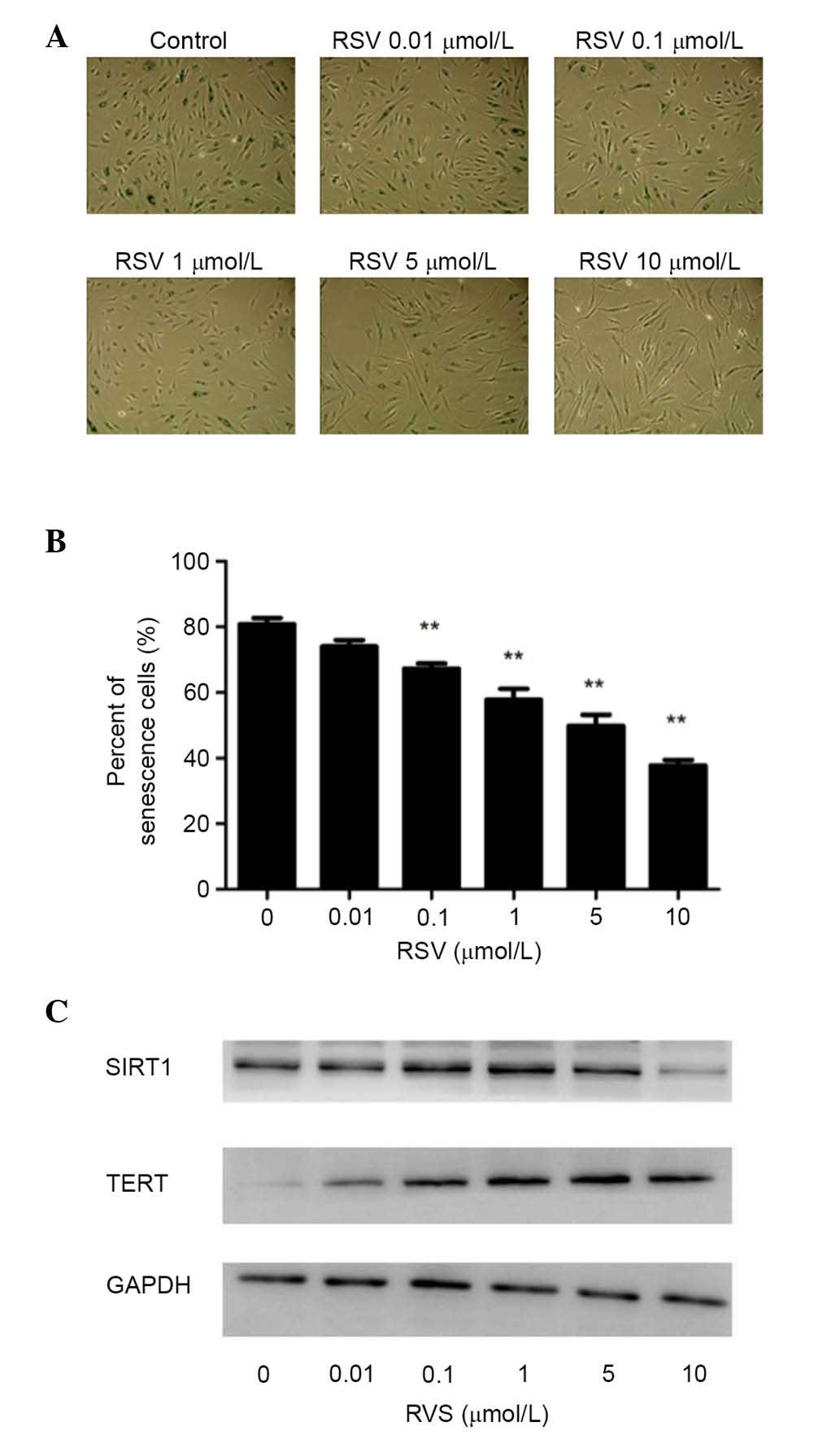
RSV inhibits premature senescence in
endothelial progenitor cell with increased expression of human
(h)-TERT
To investigate the effect of RSV on the senescent
phenotype in EPCs, cells were treated with different concentrations
of RSV for ~48 h, and it was demonstrated that treatment with RSV
significantly inhibited the senescent phenotype using the SA-β-gal
assay with concentrations of 0.1–10 µmol/l (P<0.01; Fig. 2A and B), which has been indicated
previously (13). To investigate
the mechanism by which RSV prevents premature EPC senescence, the
present study evaluated the involvement of TERT expression. As
Fig. 2C indicates, the expression
levels of h-TERT protein markedly increased by RSV in a
dose-dependent manner.
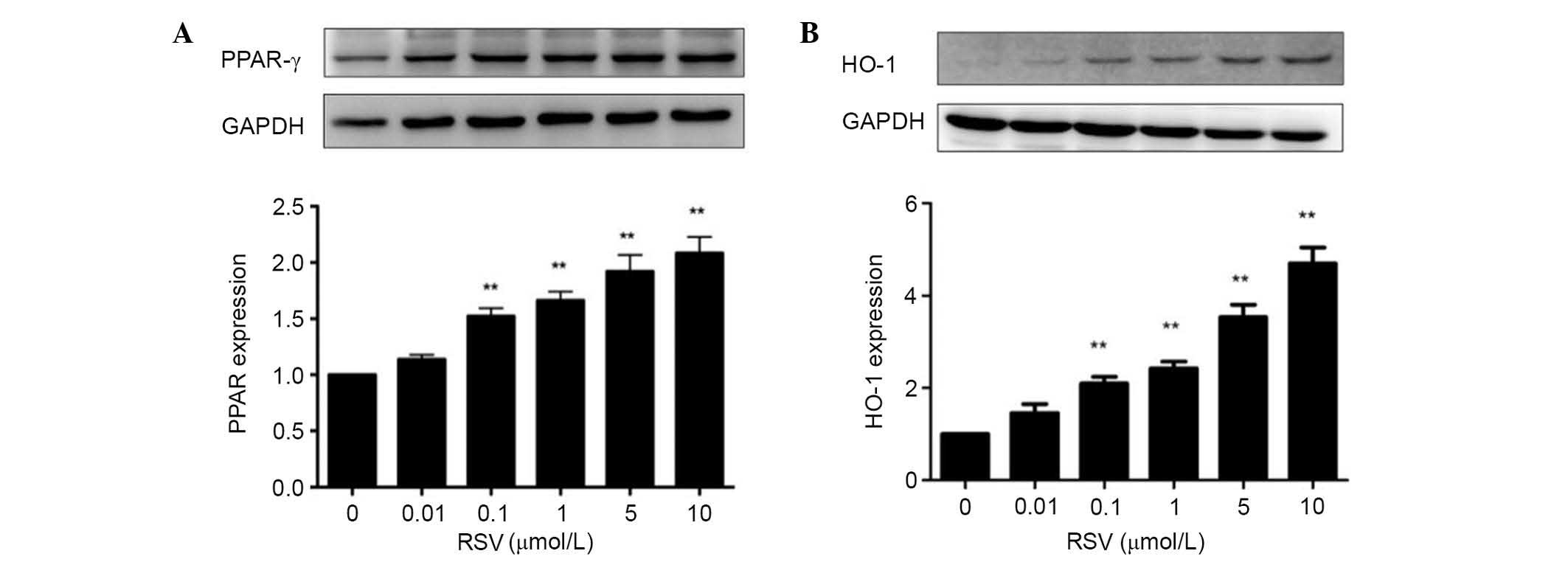
RSV activates PPAR-γ/HO-1 signaling
pathways
Treatment with RSV significantly increased the
PPAR-γ and HO-1 protein expression levels with concentrations of
0.1–10 µmol/l in a concentration-dependent manner (P<0.01;
Fig. 3).
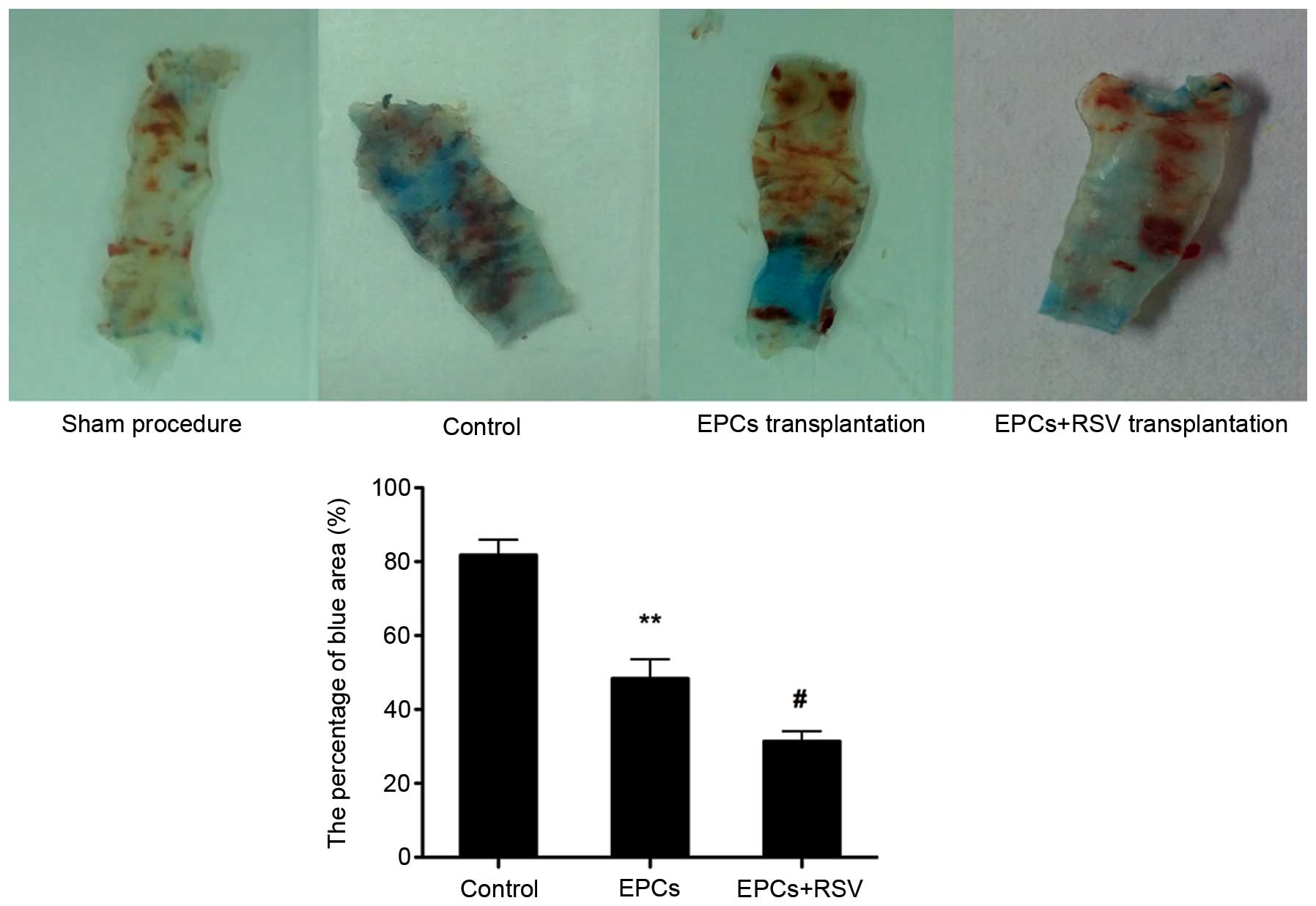
Effects of RSV on the
re-endothelialization capacity of EPCs
The H&E staining of the left common carotid
artery indicated that each layer of the normal carotid artery could
be clearly observed. The endovascular surface was covered with a
layer of flat endothelial cells. However, in the animal model with
the balloon-injured artery, the intima of the carotid artery was
injured and endothelial cells had disappeared (data not shown). As
presented in Fig. 4, the EPCs
repaired the injured artery. However, the high concentration of RVS
enhanced the re-endothelialization capacity of EPCs. EPCs treated
with RSV were demonstrated to improve re-endothelialization ability
compared to EPCs without RSV treatment (Fig. 4).
Discussion
In the present study, it was demonstrated that RSV
could prevent EPCs from senescence, and reduce the expression of
NAPDH oxidase and the oxidative reaction of EPCs, likely via the
PPAR-γ/HO-1 signaling pathways. In animal studies, the
re-endothelialization capacity of EPCs was significantly enhanced
by RSV.
RSV (3,5,4′-trihydroxystilbene) is a stilbenoid, a
type of natural phenol, and a phytoalexin produced naturally by a
number of plants in response to injury or when the plant is under
attack by pathogens, such as bacteria or fungi (14). RSV is found in numerous foods,
including the skin of grapes, blueberries, raspberries, and
mulberries (15). Extensive
studies on its activity, predominantly on cellular models,
demonstrates that RSV promotes cell proliferation, enhances
differentiation, and induces apoptosis and autophagy (16–20).
The compound also generates angiogenesis and inflammation. The
potential chemotherapeutical capacity of RSV was confirmed by
investigations into the effect of RSV on implanted cancers and
chemically induced tumors. Similarly, a study indicated that RSV
may positively influence the progression and generation of chronic
diseases including type 2 diabetes, obesity, coronary heart
disease, metabolic syndrome, and neurodegenerative pathologies in
animal models (21).
Previous studies have demonstrated that RSV enhanced
proliferation, migration and angiogenesis of EPCs, and prevent
TNF-α-induced EPC apoptosis (14,22).
Our previous study suggested that RSV inhibited the onset of EPCs
senescence may via telomerase (23). Cellular senescence is the most
important cause of EPCs dysfunction and death. In populations with
cardiovascular disease, EPC senescence is observed, and oxidized
low density lipoprotein (ox-LDL), homocysteine and angiotension II
may inhibit the function of EPCs via inducing cellular senescence
(11,24,25).
Oxidative stress may be the one of the most important mechanisms of
EPC aging, diabetes can increase the reactive oxygen species level
in EPCs, resulting in impaired vascular-healing capacity by EPCs
(26). Ox-LDL and angiotensin II
(AngII) increase the expression of NADPH oxidase subunit gp91phox
in EPCs, and induced peroxynitrite formation via inhibition of
telomerase activity, which may accelerate EPC senescence (11,24,25).
Antioxidation is an important mechanism by which RSV exerts its
biological activity. It is reported that RSV attenuated the
expression of NADPH oxidase subunit gp91phox, Ras-related C3
botulinum toxin substrate 1 and p47phox, and inhibited endothelial
cells NADPH oxidase activation and the increase of ROS levels,
which were induced by AngII, ox-LDL or homocysteine (26,27).
In addition, RSV suppresses the production of
O2−, promotes the expression of NO, and
improves endothelium-dependent vasodilation via inhibition NADPH
oxidase activation of thoracic aorta of diabetes mellitus mice
(28). A previous study
demonstrated that PPAR-γ agonist rosiglitazone can decrease the
activity of NADPH oxidase of EPCs in patients with diabetes, reduce
the production of peroxides, improve the biological activity of NO,
and improve the EPCs endothelial repair capacity (26), suggesting that PPAR-γ has an
important influence on the activity of EPCs and oxidative damage.
In a randomized, double-blind clinical trial, pioglitazone can
enhance the number and function of EPCs in patients with coronary
artery disease and normal glucose tolerance, and reduce NADPH
oxidase, suggesting the effect of on EPCs may be independent from
the hypoglycemic effect (29).
Imanishi et al (30)
demonstrated that pioglitazone inhibits AngII-induced expression of
NADPH oxidase gp91phox, prevent telomerase inactivation and EPC
senescence. Another previous study demonstrated that the PPAR-γ
agonist telmisartan may also induce proliferation of human EPCs
(31), indicating the effect of
the PPAR-γ agonist on EPCs is not specific to thiazolidinediones.
These results suggest that PPAR-γ is important in regulating the
anti-oxidative effect of EPCs and maintaining cytoativity. In the
present study, RSV activated the expression of PPAR-γ in a
concentration-dependent manner, suggesting RSV prevents EPC
senescence and enhances re-endothelialization of EPCs possibly via
the activation of PPAR-γ.
It has been demonstrated that PPAR-α and PPAR-γ
transcriptionally regulated the expression of HO-1, suggesting a
mechanism for the anti-inflammatory and anti-proliferative effects
of PPAR ligands via upregulation of HO-1 (32). HO-1 catalyzes hemoglobin into
biliverdin and carbon monoxide, and it is a key line of defence
against oxidative stress in the cardiovascular system (33). Previous studies have demonstrated
that HO-1 inhibits the activity of NADPH oxidase, decreases the
level of ROS, and improves endothelial function in endothelial
cells of diabetic rats (34). A
previous study demonstrated that RSV upregulated the expression of
HO-1 in coronary artery endothelial cells and myocardial cells of
rats, reduced the myocardial infarction area following anterior
descending coronary artery ligation, and promoted the recovery of
cardiac function; however, HO-1 antagonist tin protoporphyrin IX
blocks these effects, suggesting HO-1 is important in the
anti-oxidative effect of RSV (35,36).
Deshane et al (37)
reported that HO-1 mediated EPCs migration and angiogenesis induced
by stromal cell-derived factor-1. Wu et al (38) demonstrated that HO-1 could increase
the number of circulating EPCs, enhance the colony formation
ability of EPCs, and promote re-endothelialization (38). All of these results indicated that
HO-1 may be the downstream element of the regulation of EPCs
oxidative reaction and cytoactivity by PPAR-γ. The present study
also demonstrated that RSV increases the expression levels of
PPAR-γ and HO-1, and the re-endothelialization of EPCs, which may
indicate that RSV restores the cardiovascular repair capacity of
EPCs via the PPAR-γ and HO-1 signaling pathways.
However, the current study has certain limitations.
Firstly, the mechanism by which RSV activates the PPAR-γ and HO-1
signaling pathway was not illustrated clearly, further studies are
required to elucidate the detailed underlying mechanisms. Secondly,
type II diabetes and gene knockdown animal models are required to
further confirm the effect of RSV. Animal models of type II
diabetes will be used in future study by our laboratory to further
investigate the effect of RSV.
In conclusion, RSV prevents EPCs from senescence and
reduces the oxidative reaction of EPCs. PPAR-γ and HO-1 signaling
pathways are activated by RSV and RSV also promotes the
re-endothelialization capacity of EPCs.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81001435) and the
Medical Science and Technology Foundation of Zhejiang Province
(grant no. 2012RCA034).
Glossary
Abbreviations
Abbreviations:
|
RSV
|
resveratrol
|
|
EPC
|
endothelial progenitor cell
|
|
PBMNCs
|
peripheral blood mononuclear cells
|
|
ECFCs
|
late outgrownth EPCs
|
|
SA-β-gal
|
senescence-associated
β-galactosidase
|
References
|
1
|
Shi Q, Rafli S, Wu MH, Wijelath ES, Yu C,
Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, et al: Evidence
for circulating bone marrow-derived endothelial cells. Blood.
92:362–367. 1998.PubMed/NCBI
|
|
2
|
Asahara T, Masuda H, Takahashi T, Kalka C,
Pastore C, Silver M, Kearne M, Magner M and Isner JM: ‘Bone marrow
origin of endothelial progenitor cells responsible for postnatal
vasculogenesis in physiological and pathological
neovascularization’. Circ Res. 85:221–228. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Werner N and Nickenig G: Influence of
cardiovascular risk factors on endothelial progenitor cells:
Limitations for therapy? Arterioscler Thromb Vasc Biol. 26:257–266.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thum T and Bauersachs J: Endothelial
progenitor cells as potential drug targets. Curr Drug Targets
Cardiovasc Haematol Disord. 5:277–286. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Agarwal B and Baur JA: Resveratrol and
life extension. Ann N Y Acad Sci. 1215:138–143. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Borriello A, Bencivenga D, Caldarelli I,
Tramontano A, Borgia A, Zappia V and Ragione F Della: Resveratrol:
From basic studies to bedside. Cancer Treat Res. 159:167–184. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marques FZ, Markus MA and Morris BJ:
Resveratrol: Cellular actions of a potent natural chemical that
confers a diversity of health benefits. Int J Biochem Cell Biol.
41:2125–2128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xia L, Wang XX, Hu XS, Guo XG, Shang YP,
Chen HJ, Zeng CL, Zhang FR and Chen JZ: Resveratrol reduces
endothelial progenitor cells senescence through augmentation of
telomerase activity by Akt-dependent mechanisms. Br J Pharmacol.
155:387–394. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng H, Fu G, Dai T and Huang H:
Migration of endothelial progenitor cells mediated by stromal
cell-derived factor-1alpha/CXCR4 via PI3K/Akt/eNOS signal
transduction pathway. J Cardiovasc Pharmacol. 50:274–280. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng H, Dai T, Zhou B, Zhu J, Huang H,
Wang M and Fu G: SDF-1alpha/CXCR4 decreases endothelial progenitor
cells apoptosis under serum deprivation by PI3K/Akt/eNOS pathway.
Atherosclerosis. 201:36–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Assmus B, Urbich C, Aicher A, Hofmann WK,
Haendeler J, Rössig L, Spyridopoulos I, Zeiher AM and Dimmeler S:
HMG-CoA reductase inhibitors reduce senescence and increase
proliferation of endothelial progenitor cells via regulation of
cell cycle regulatory genes. Circ Res. 92:1049–1055. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lindner V, Fingerle J and Reidy MA: Mouse
model of arterial injury. Circ Res. 73:792–796. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Opie LH and Lecour S: The red wine
hypothesis: From concepts to protective signalling molecules. Eur
Heart J. 28:1683–1693. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang XB, Huang J, Zou JG, Su EB, Shan QJ,
Yang ZJ and Cao KJ: Effects of resveratrol on number and activity
of endothelial progenitor cells from human peripheral blood. Clin
Exp Pharmacol Physiol. 34:1109–1115. 2007.PubMed/NCBI
|
|
15
|
Aggarwal BB, Bhardwaj A, Aggarwal RS,
Seeram NP, Shishodia S and Takada Y: Role of resveratrol in
prevention and therapy of cancer: Preclinical and clinical studies.
Anticancer Res. 24:2783–2840. 2004.PubMed/NCBI
|
|
16
|
Wang Q, Li H, Wang XW, Wu DC, Chen XY and
Liu J: Resveratrol promotes differentiation and induces
Fas-independent apoptosis of human medulloblastoma cells. Neurosci
Lett. 351:83–86. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Delmas D, Rébé C, Lacour S, Filomenko R,
Athias A, Gambert P, Cherkaoui-Malki M, Jannin B, Dubrez-Daloz L,
Latruffe N and Solary E: Resveratrol-induced apoptosis isassociated
with Fas redistribution in the rafts and the formation of a
death-inducing signaling complex in colon cancer cells. J Biol
Chem. 278:41482–41490. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bernhard D, Tinhofer I, Tonko M, Hübl H,
Ausserlechner MJ, Greil R, Kofler R and Csordas A: Resveratrol
causes arrest in the S-phase prior to Fas-independent apoptosis in
CEM-C7H2 acute leukemia cells. Cell Death Differ. 7:834–842. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dörrie J, Gerauer H, Wachter Y and Zunino
SJ: Resveratrol induces extensive apoptosis by depolarizing
mitochondrial membranes and activating caspase-9 in acute
lymphoblastic leukemia cells. Cancer Res. 61:4731–4739.
2001.PubMed/NCBI
|
|
20
|
Borriello A, Bencivenga D, Caldarelli I,
Tramontano A, Borgia A, Zappia V and Ragione F Della: Resveratrol:
From basic studies to bedside. Cancer Treat Res. 159:167–184. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Balestrieri ML, Schiano C, Felice F,
Casamassimi A, Balestrieri A, Milone L, Servillo L and Napoli C:
Effect of low doses of red wine and pure resveratrol on circulating
endothelial progenitor cells. J Biochem. 143:179–186. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xia L, Wang XX, Hu XS, Guo XG, Shang YP,
Chen HJ, Zeng CL, Zhang FR and Chen JZ: Resveratrol reduces
endothelial progenitor cells senescence through augmentation of
telomerase activity by Akt-dependent mechanisms. Br J Pharmacol.
155:387–394. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Imanishi T, Hano T, Sawamura T and Nishio
I: Oxidized low-density lipoprotein induces endothelial progenitor
cell senescence, leading to cellular dysfunction. Clin Exp
Pharmacol Physiol. 31:407–413. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Imanishi T, Hano T and Nishio I:
Angiotensin II accelerates endothelial progenitor cell senescence
through induction of oxidative stress. J Hypertens. 23:97–104.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bao XM, Wu CF and Lu GP: Atorvastatin
inhibits homocysteine-induced oxidative stress and apoptosis in
endothelial progenitor cells involving Nox4 and p38MAPK.
Atherosclerosis. 210:114–121. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sorrentino SA, Bahlmann FH, Besler C,
Müller M, Schulz S, Kirchhoff N, Doerries C, Horváth T, Limbourg A,
Limbourg F, et al: Oxidant stress impairs in vivo
reendothelialization capacity of endothelial progenitor cells from
patients with type 2 diabetes mellitus: Restoration by the
peroxisome proliferator-activated receptor-gamma agonist
rosiglitazone. Circulation. 116:163–173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carluccio MA, Ancora MA, Massaro M,
Carluccio M, Scoditti E, Distante A, Storelli C and De Caterina R:
Homocysteine induces VCAM-1 gene expression through NF-kappaB and
NAD(P)H oxidase activation: protective role of Mediterranean diet
polyphenolic antioxidants. Am J Physiol Heart Circ Physiol.
293:H2344–H2354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang H, Zhang J, Ungvari Z and Zhang C:
Resveratrol improves endothelial function: Role of TNF{alpha} and
vascular oxidative stress. Arterioscler Thromb Vasc Biol.
29:1164–171. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Werner C, Kamani CH, Gensch C, Böhm M and
Laufs U: The peroxisome proliferator-activated receptor-gamma
agonist pioglitazone increases number and function of endothelial
progenitor cells in patients with coronary artery disease and
normal glucose tolerance. Diabetes. 56:2609–2615. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Imanishi T, Kobayashi K, Kuroi A, Ikejima
H and Akasaka T: Pioglitazone inhibits angiotensin II-induced
senescence of endothelial progenitor cell. Hypertens Res.
31:757–765. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Honda A, Matsuura K, Fukushima N, Tsurumi
Y, Kasanuki H and Hagiwara N: Telmisartan induces proliferation of
human endothelial progenitor cells via PPARgamma-dependent PI3K/Akt
pathway. Atherosclerosis. 205:376–384. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Krönke G, Kadl A, Ikonomu E, Blüml S,
Fürnkranz A, Sarembock IJ, Bochkov VN, Exner M, Binder BR and
Leitinger N: Expression of heme oxygenase-1 in human vascular cells
is regulated by peroxisome proliferator-activated receptors.
Arterioscler Thromb Vasc Biol. 27:1276–1282. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Abraham NG and Kappas A: Pharmacological
and clinical aspects of heme oxygenase. Pharmacol Rev. 60:79–127.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kruger AL, Peterson SJ, Schwartzman ML,
Fusco H, McClung JA, Weiss M, Shenouda S, Goodman AI, Goligorsky
MS, Kappas A and Abraham NG: Up-regulation of heme oxygenase
provides vascular protection in an animal model of diabetes through
its antioxidant and antiapoptotic effects. J Pharmacol Exp Ther.
319:1144–1152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thirunavukkarasu M, Penumathsa SV, Koneru
S, Juhasz B, Zhan L, Otani H, Bagchi D, Das DK and Maulik N:
Resveratrol alleviates cardiac dysfunction in
streptozotocin-induced diabetes: Role of nitric oxide, thioredoxin,
and heme oxygenase. Free Radic Biol Med. 43:720–729. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Das S, Fraga CG and Das DK:
Cardioprotective effect of resveratrol via HO-1 expression involves
p38 map kinase and PI-3-kinase signaling, but does not involve
NFkappaB. Free Radic Res. 40:1066–1075. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Deshane J, Chen S, Caballero S,
Grochot-Przeczek A, Was H, Li Calzi S, Lach R, Hock TD, Chen B,
Hill-Kapturczak N, et al: Stromal cell-derived factor 1 promotes
angiogenesis via a heme oxygenase 1-dependent mechanism. J Exp Med.
204:605–618. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu BJ, Midwinter RG, Cassano C, Beck K,
Wang Y, Changsiri D, Gamble JR and Stocker R: Heme oxygenase-1
increases endothelial progenitor cells. Arterioscler Thromb Vasc
Biol. 29:1537–1542. 2009. View Article : Google Scholar : PubMed/NCBI
|