Introduction
Sterilization of medical devices is an important
means of reducing the incidence of iatrogenic diseases (1). Recent developments in sterilization
methods are aimed at improving the effectiveness, applicability and
cost-effectiveness of the process. One such sterilization technique
involves treatment with gas plasma, which is generated by removing
electrons from gases to produce a highly excited mixture of charged
nuclei and free electrons. Gas plasma treatment is particularly
suitable for the sterilization of thermolabile medical devices
(2–4).
Recently, a nitrogen gas plasma instrument
(BLP-TES), which generates nitrogen gas plasma using a fast
high-voltage pulse from a static induction (SI) thyristor power
supply was developed (5–11). Our previous studies (5–11)
have demonstrated that nitrogen gas plasma can be utilized to
inactivate various microorganisms. However, the mechanisms of
action of gas plasma on microorganisms remain to be elucidated.
Previous studies have demonstrated that treatment of microorganisms
with nitrogen gas plasma may induce changes in their lipids
(5), proteins (7) and carbohydrates (8). These findings suggest that the
changes induced by sterilizing factors produced during the
generation of nitrogen gas plasma may contribute to the
inactivation of microorganisms.
Previous studies have demonstrated that nitrogen gas
plasma treatment using BLP-TES efficiently inactivates bacteria and
viruses as well as endotoxins (5–11).
Furthermore, Geobacillus stearothermophilus, which is often
used as a biological indicator for gas plasma and is one of the
most resistant microbes against physical and chemical treatments,
was inactivated by treatment with BLP-TES (6,12).
However, it remains unclear how the nitrogen gas plasma generated
by BLP-TES inactivates this bacterial spore. In the present study,
G. stearothermophilus spores on a stainless steel (SUS) disk
and filter paper were treated with nitrogen gas plasma using
BLP-TES and any changes to the genomic DNA were analyzed.
Furthermore, the effect of various sterilizing factors generated
during operation of the BLP-TES instrument were also
investigated.
Materials and methods
Nitrogen gas plasma measurement and
treatment
BLP-TES produces nitrogen gas plasma by means of a
fast high-voltage pulse applied using a SI thyristor power supply
as described in our previous studies (5–7).
Cathode electrodes (earth electrodes) were placed between the anode
electrodes (high voltage electrodes). All bacterial spore samples
and chemical indicators were placed on a plastic net on the earth
electrodes during nitrogen gas plasma treatment. First, the chamber
box containing the sample was decompressed and degassed, and then
nitrogen gas (99.9995%, Okano, Co., Ltd., Kadena, Japan) was
introduced. The pressure in the box was maintained at ~0.5
atmospheres during the discharge at 1.5 kilo pulse/sec (kpps).
Bacterial spores and culture
A filter paper strip and SUS disk coated with G.
stearothermophilus ATCC7359 (SGM Biotech Inc., Bozeman, MT,
USA) were used for treatment. In certain experiments, a tablet
containing G. stearothermophilus EZ-SPORE (Microbiologics,
Inc., St. Cloud, MN, USA) was used. Each tablet was suspended in 1
ml of hydration buffer (Microbiologics, Inc.) in accordance with
the manufacturer's protocols and then spotted and dried onto a
cover glass. The SUS disk, filter paper, or cover glass were
treated with nitrogen gas plasma. In all cases, the treated and
untreated G. stearothermophilus was mixed with tryptic soy
broth (TSB) pH 7.3±0.2 (Raven Japan Co., Ltd., Koshigaya, Japan)
and pH indicator bromocresol purple (BCP) prior to incubation at
56°C. A color change in the medium as a result of proliferation of
the bacteria served as an index of viability.
Polymerase chain reaction (PCR)
Plasma-treated or untreated G.
stearothermophilus samples on paper filters or SUS disks were
resuspended in distilled water and genomic DNA was then eluted by
heat treatment at 100°C for 15 min. The liberated DNA samples were
subjected to PCR amplification comprising 40 cycles of 94°C for 1
min, 55°C for 1 min, and 72°C for 1 min, followed by a final
incubation at 72°C for 10 min. GEOBAC primers (GEOBAC-F and
GEOBAC-R) were used in the amplification and based on the 16S-23S
rRNA gene internal transcribed spacer (ITS) region sequences.
GEOBAC-F (5′-TAAGCGTGAGGTCGGTGGTTC-3′) targeted the gene of
tRNAIle, and GEOBAC-R (5′-GCGCTCTCGGCTTCTTCCTT-3′)
targeted the 3′ end region of Geobacillus ITS (13). Gstearo-16S primers were targeted to
16S rRNA of G. stearothermophilus and designed to the DNA
sequence with Genbank Accession no. EU484358 (www.ncbi.nlm.nih.gov/genbank). The primer
sequences were as follows: Forward, 5′-CTTCGGGTCGTAAAGCTCTG-3′
termed F1′ and reverse, 5′-CCTTTGAGTTTCAGCCTTGC-3′ termed R1 and
5′-GAATTCCGCTCTCCTCTCCT-3′ termed R2. All the amplifications were
performed using a PC320 model thermocycler (Astec Co. Ltd., Kasuya,
Japan). The PCR products were initially analyzed by agarose gel
electrophoresis and then subsequently verified by DNA sequencing.
The PCR products obtained by standard PCR and qPCR were subcloned
into pT7Blue T-vector (EMD Millipore, Billerica, MA, USA) and
subjected to DNA sequencing (ABI PRISM 3100 Genetic Analyzer;
Applied Biosystems; Thermo Fisher Scientific, Inc.) to verify the
identity of the amplified product.
Quantitative PCR (qPCR)
The extracted genomic DNA samples were subjected to
quantitative PCR using SYBR Premix Ex TaqII (Tli RNase H Plus;
Takara Bio Inc., Otsu, Japan) and the following primers for 16S
rRNA of G. stearothermophilus (Genbank Accession no.
EU484358): Forward, 5′-CACACTGGGACTGAGACACG-3′ and reverse,
5′-CATTGCGGATTCCCTAC-3′ for region 1; forward,
5′-ACGGTACCTCACGAGAAAGC-3′ and reverse, 5′-TCGCCCCCTACGTATTACC-3′
for region 2; and forward, 5′-CATTCGGTTGGGCACTCTA-3′ and reverse,
5′-AAGGGGCATGATGATTTGAC-3′ for region 3. The thermocycling
conditions used for the quantitative PCR were as follows: 95°C for
30 sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 1 min.
Relative quantification was performed using the 2−∆∆Cq
method (14).
Scanning electron microscopy
(SEM)
The G. stearothermophilus-contaminated paper
strips were treated with nitrogen gas plasma (1.5 kpps) for 0 and
15 min, and then fixed with 2% glutaraldehyde/0.1 M phosphate
buffer (pH 7.4) overnight at 4°C. The cover glasses were
subsequently treated with 2% osmium tetroxide at 4°C for 3 h.
Samples were dehydrated through a graded ethanol series (50–100%
ethanol) at room temperature. Finally, the samples were subjected
to critical point drying and evaporation coating by osmium plasma
ions. SEM was performed using a JSM-6320F (JEOL Ltd., Tokyo, Japan)
instrument at 5 kV using a magnification of ×20,000.
Temperature measurements
The temperature in the box during operation of the
instrument was measured using THERMO LABEL 5E (NiGK Corporation,
Kawagoe, Japan), which was placed on the earth electrode. The
ambient temperature in the nitrogen gas plasma instrument box was
measured using a fiber optic thermometer (FT1420A; Takaoka Electric
MFG. Co. Ltd., Tokyo, Japan) during nitrogen gas plasma
treatment.
Measurement of ultraviolet (UV)
radiation
Indicator label-H (NiGK Corporation) was placed on
the earth electrode and treated with nitrogen gas plasma (1.5 kpps)
for 0–30 min. Analysis of emission during operation of the nitrogen
gas plasma instrument was performed using a USB multichannel
spectrophotometer (S-2431; Soma Optics, Ltd., Tokyo, Japan).
Oxidative stress measurements
Chemical indicators were used to estimate the
respective concentration of 2–80 mg/l NO2−
(nitrite; Kyoritsu Chemical-Check Lab., Corporation, Tokyo, Japan),
10–500 mg/l NO3− (nitrate; Kyoritsu
Chemical-Check Lab. Corporation) and 0.5–25 mg/l
H2O2 (Merck Ltd. Tokyo, Japan). The following
indicators were used: Quantofix Nitrite (Macherey-Nagel, GmbH &
Co. KG, Düren, Germany), Quantofix Nitrite 3000, Quantofix Peroxide
25, Quantofix Peroxide 100, Nitrite, Quantofix Nitrate and
Quantofix Active oxygen, all obtained from Macherey-Nagel GmbH,
Duren, Germany), which were placed on a plastic net on the earth
electrodes prior to treatment with nitrogen gas plasma using a
BLP-TES device at 1.5 kpps for 0–30 min.
Treatment with reagents, heating or
UV
For heat treatment, a suspension of G.
stearothermophilus at 3.1×104 colony-forming unit
(CFU)/ml was incubated at temperatures ranging from 40–100°C for 30
min using a block incubator (BI-516S; Astec Co. Ltd). For UV
treatment, filter papers contaminated with G.
stearothermophilus (2.1×106 CFU) were exposed to
long wavelength UV-A and short wavelength UV-C radiation from a
handheld UV transilluminator (UVGL-58; UVP, Inc., Upland, CA) for
30 min on each side of the paper strip (distance from lamp to paper
strip, 1.3 cm). The energy (mJ/cm2) of UV-A and UV-C was
estimated on the basis of a color change of UV indicator (UV
label-H). Samples of G. stearothermophilus suspension
(3.1×104 CFU/ml) were subjected to oxidative stress by
incubation in the presence of hydrogen peroxide (0–3%; Wako Pure
Chemical Industries, Ltd., Osaka, Japan), peroxynitrite (0–4.92 mM;
Dojindo Molecular Technologies, Inc., Kumamoto, Japan) or
3-(4-morpholinyl)sydnonimine hydrochloride (SIN-1; 1.0
mM-1.0×10−5 mM; Dojindo Molecular Technologies, Inc.),
which produces superoxide anions and nitric oxide resulting in the
generation of peroxynitrite.
Analysis of DNA oxidation by
enzyme-linked immunosorbent assay (ELISA)
G. stearothermophilus samples on filter
papers were frozen at −80°C overnight and dipped into 500 µl of
High Molecular Weight (HMW) buffer containing 10 mM Tris-HCl (pH
8.0), 0.1 M ethylenediaminetetraacetic acid (pH 8.0), and 0.5%
sodium dodecyl sulfate prior to boiling for 10 min. Following
centrifugation at 10,000 × g for 15 min, an aliquot (50 µl)
of the supernatant was subjected to the ELISA. To detect
8-hydroxy-2′-deoxyguanosine (8-OHdG), Highly Sensitive 8-OHdG Check
ELISA kit (Japan Institute for the Control of Aging, NIKKEN SEIL
Co., Ltd., Fukuroi, Japan) and 8-OHdG Assay Preparation reagent set
(Wako Pure Chemical Industries, Ltd.) was used in accordance with
the manufacturer's protocols. In this kit, a competitive ELISA
utilizing monoclonal antibody (clone N45.1), which is highly
specific for 8-OHdG, was employed. The concentrations of 8-OHdG was
quantified by comparison of absorbance at a wavelength of 450 nm
with standards of 8-OHdG diluted with HMW buffer.
Statistical analysis
The results are presented as the mean ± standard
deviation of replicate experiments (n=6). The statistical analysis
of significant difference between plasma-treated and untreated
samples was performed by Mann-Whitney U test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Nitrogen gas plasma treatment
decreased the number of viable bacteria spores
The inactivation efficiency of the nitrogen gas
plasma was investigated (Fig. 1).
The bacterial spores of G. stearothermophilus were spotted
and dried onto a SUS disk or filter paper and treated with nitrogen
gas plasma at 1.5 kpps for 0, 5, 15 or 30 min. The spores were then
incubated in TSB medium containing BCP at 56°C for 6, 24 and 48 h
and the change in pH of the culture broth was used as the index of
bacterial proliferation. The results after 6 h incubation of the
spores taken from the SUS disk (initial population,
1.9×106 CFU) and filter paper (initial population,
1.4×106 CFU) indicated that nitrogen gas plasma
treatment decreased the number of viable bacterial spores on the
surface of the two materials. However, longer incubation of the
samples (24 and 48 h) demonstrated that the inactivation efficiency
was greater for the spores recovered from the filter paper compared
with those from the SUS disk.
 | Figure 1.Inactivation of Geobacillus
stearothermophilus by nitrogen gas plasma treatment. A total of
ten filter papers (strip) and ten SUS disks (disk) contaminated
with spores of G. stearothermophilus at 1.4×106
CFU and 1.9×106 CFU, were treated with nitrogen gas
plasma at 1.5 kpps for 0, 5, 15 and 30 min using a BLP-TES device.
The BLP-TES instrument generates gas plasma by a short high-voltage
pulse generated from a static induction thyristor power supply. The
treated bacterial spores were incubated in tryptic soy broth
medium, which included the pH indicator bromocresol purple, at 56°C
for 6, 24 and 48 h. Proliferation of G. stearothermophilus
in the medium was monitored by the development of a yellow color
due to acidification. The proliferation of G.
stearothermophilus following nitrogen gas plasma treatment (1.5
kpps) was then carefully monitored. The growth of the bacteria
following a 6 h incubation was 100% for the untreated samples and
0% for samples treated with nitrogen gas plasma (5–30 min treatment
period) recovered from filter papers and SUS disks. However,
prolonged incubation (24 and 48 h) of the spores recovered from
filter papers gave 100% growth for the untreated sample and sample
treated for 5 min, 10% after 15 min treatment and 0% after 30 min
treatment. By contrast, all G. stearothermophilus samples
recovered from SUS disks, even those subjected to 30 min nitrogen
gas plasma treatment, proliferated to 100% after 24 and 48 h
incubation. SUS, stainless steel; CFU, colony-forming units. |
The fraction negative method, calculated using the
Halvorson-Ziegler formula (15)
and Stumbo-Murphy-Cochran procedure (16), indicated the decimal reduction time
(D-value) for the filter paper samples was 2.48 min at 15
min of treatment, while the D value for the SUS disk could
not be calculated, suggesting lower inactivation efficiency in the
SUS disk.
No major changes to spores were
observed following nitrogen gas plasma treatment
SEM analysis was performed to investigate the effect
of nitrogen gas plasma treatment on the structure of the bacterial
spore surface (Fig. 2). Careful
examination indicated no evidence of shrunken or aggregated spores
after nitrogen gas plasma treatment. However, spores subjected to
nitrogen gas plasma treatment for 15 min exhibited a slight
increase in surface roughness compared with untreated spores.
DNA oxidation was induced by nitrogen
gas plasma treatment
Potential changes to the bacterial genomic DNA
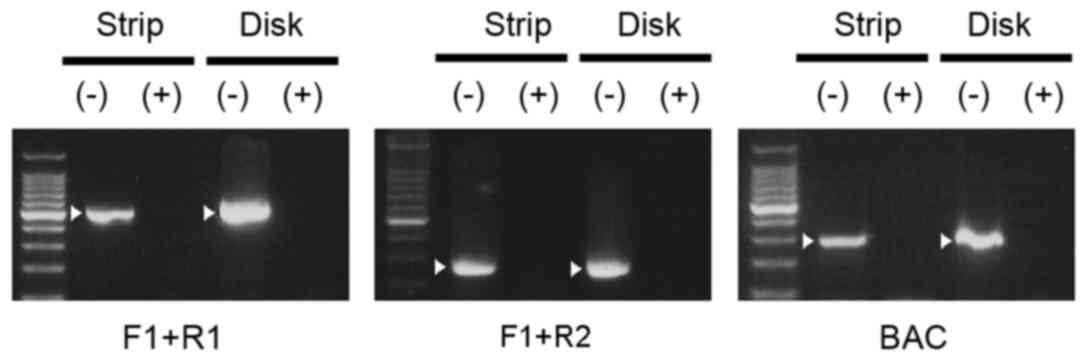
induced by nitrogen gas plasma treatment were observed (Figs. 3 and 4). SUS disks (disk) and filter papers
(strip) were treated with nitrogen gas plasma for 0 and 15 min and
then subjected to PCR analysis using the three pairs of primers
designed against the sequence of G. stearothermophilus 16S
rRNA (F1+R1, F1+R2, and GEOBAC-F+GEOBAC-R; Fig. 3). Agarose gel electrophoresis
indicated that no PCR products were amplified from genomic DNA
samples of G. stearothermophilus after 15 min treatement
with nitrogen gas plasma on a SUS disk or filter paper. By
contrast, PCR products of the anticipated size were generated from
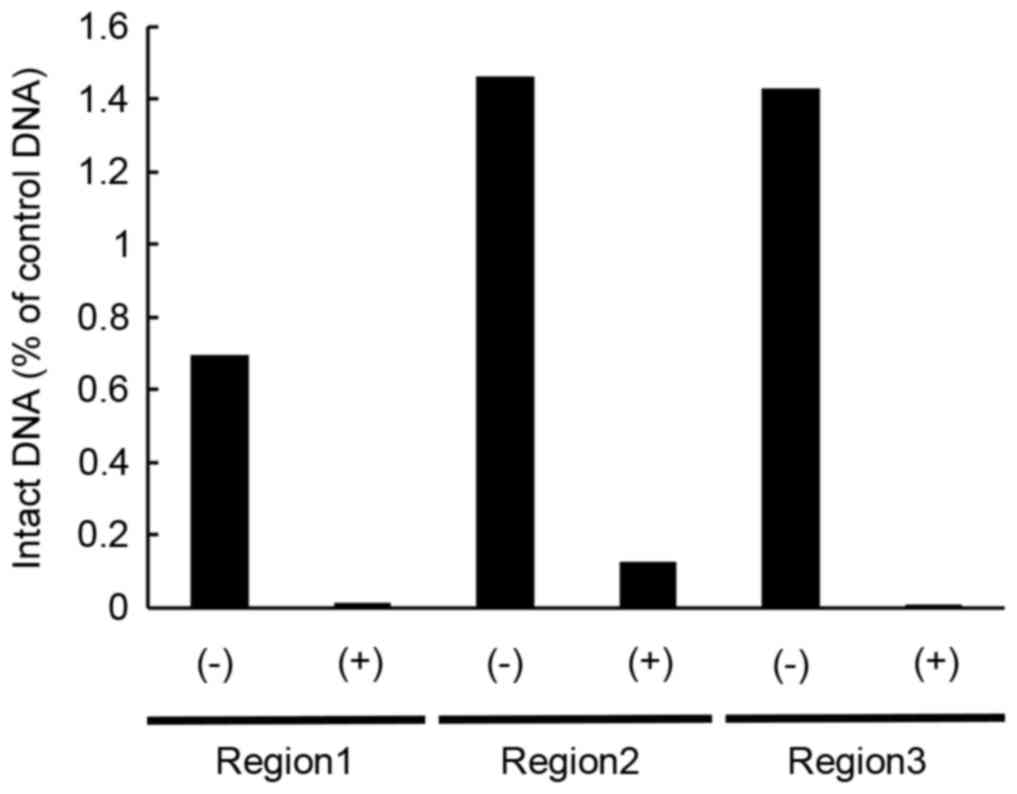
the untreated samples. Quantitative analysis using qPCR, which
employed three pairs of primers designed against three different
regions of 16S rRNA (region 1–3), demonstrated a decrease of intact
genomic DNA in the nitrogen gas plasma treated bacterial spores of
G. stearothermophilus compared with untreated samples
(Fig. 4). The quantity of intact
DNA detected in the nitrogen gas plasma treated samples was ~1/10
that of the untreated samples. DNA sequence analysis demonstrated
that in each case the anticipated product had been amplified.
Specifically, 99–87% (F1+R1), 99–96% (F1+R2) for Genbank Accession
no. EU484358, and 100% (GEOBAC-F+GEOBAC-R) for Genbank Accession
no. EU157949, 99% (region 1), 100% (region 2) and 100% (region 3)
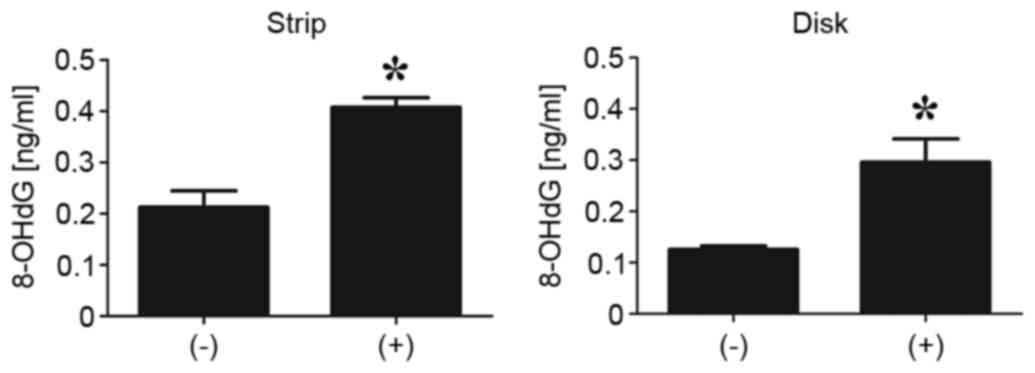
for Genbank Accession no. KJ722527. Furthermore, the ELISA
demonstrated a significant increase in the level of 8-OHdG in G.
stearothermophilus containing filter paper and SUS disk
following nitrogen gas plasma treatment (P<0.01; Fig. 5). 8-OHdG is a product of oxidative
damage to DNA formed by hydroxyl radicals, singlet oxygen and
direct photodynamic action (17).
Thus, the results indicate nitrogen gas plasma treatment of G.
stearothermophilus spores induces oxidation of the DNA.
Generation of heat, UV-A and oxidative
stress in the BLP-TES instrument
Previous studies have confirmed that at least three
variables are generated during operation of the nitrogen gas plasma
instrument BLP-TES that may be responsible for its sterilizing
action, namely heat, UV-A and oxidative stress (10). This is similar to other gas plasma
instruments (18,19). Temperature measurement of the
sample box in the BLP-TES instrument using a fiber thermometer gave
readings of 65°C at 7.5 min, 75°C at 15 min, and 85°C at 30 min
during operation of the device. UV measurements using a paper
indicator indicated a color change in a time-dependent manner
(equivalent to 0 mJ/cm2 at 0 min, 25 mJ/cm2
at 7.5 min, and 50 mJ/cm2 at 30 min). Consistent with
these findings, UV-A was detected by analysis of the emission using
a spectrophotometer (S-2431; data not shown). In addition, the
levels of chemicals were further estimated by the index of color
change. The increase of hydrogen peroxide depended on treatment
time and was estimated to be 7.5±0.9 mg/l at 30 min. Production of
nitrite was also observed during operation of the instrument and
was estimated to be 5.5±0.7 mg/l at 30 min, while that of nitrate
was 92.7±19.0 mg/l at 30 min (data not shown).
The individual contribution of these three variables
(heat, UV, and oxidative stress) to inactivation efficiency was
examined. Following exposure to each of these variables, at a level
equivalent to that observed during operation of the gas plasma
device, spores of G. stearothermophilus (3.1×104
CFU) were incubated for 48 h. The change in color of the medium was
then checked against an index to determine the bacterial
proliferation. The results indicated that heating at 40–100°C for
30 min did not affect the viability of the bacterial spores
(Table I). UV-C treatment for 30
min (>300 mJ/cm2) using a transilluminator (UVGL-58)
completely inactivated bacterial spores (100%), while UV-A
treatment (75–142 mJ/cm2) for the same length of time
did not (Table II). In the case
of oxidative stress, treatment with peroxynitrite
(4.92–4.92×10−5 mM), SIN-1 (1.0–1.0×10−5 mM)
or hydrogen peroxide (3×2−5-3×2−9%) for 30
min did not inactivate the bacterial spores (Tables III–V). However, exposure of the spores to an
elevated level of hydrogen peroxide (3–3×2−4%) resulted
in their complete inactivation (Table III).
 | Table I.Effect of heat treatment for 30 min on
the viability of G. stearothermophilus. |
Table I.
Effect of heat treatment for 30 min on
the viability of G. stearothermophilus.
|
|
Temperature
(°C) |
|---|
|
|
|
|---|
| Viability | 40 | 50 | 60 | 65 | 70 | 75 | 80 | 85 | 90 | 95 | 100 |
|---|
| Cell growth | + | + | + | + | + | + | + | + | + | + | + |
 | Table II.Effect of UV treatment for 30 min on
the viability of G. stearothermophilus. |
Table II.
Effect of UV treatment for 30 min on
the viability of G. stearothermophilus.
| Viability | UV-A | UV-C |
|---|
| Cell growth
(%) | 100 (3/3) | 0 (0/3) |
 | Table III.Effect of hydrogen peroxide treatment
for 30 min on the viability of G. stearothermophilus. |
Table III.
Effect of hydrogen peroxide treatment
for 30 min on the viability of G. stearothermophilus.
|
|
H2O2 (%) |
|---|
|
|
|
|---|
| Viability | 3 |
3×2−1 |
3×2−2 |
3×2−3 |
3×2−4 |
3×2−5 |
3×2−6 |
3×2−7 |
3×2−8 |
3×2−9 |
|---|
| Cell growth | − | − | − | − | − | + | + | + | + | + |
 | Table V.Effect of SIN-1 treatment for 30 min
on the viabilty of G. stearothermophilus. |
Table V.
Effect of SIN-1 treatment for 30 min
on the viabilty of G. stearothermophilus.
|
| SIN-1 [mM] |
|---|
|
|
|
|---|
| Viability | 1.0 |
1.0×10−1 |
1.0×10−2 |
1.0×10−3 |
1.0×10−4 |
1.0×10−5 |
|---|
| Cell growth | + | + | + | + | + | + |
Discussion
The inactivation rates of bacterial spores on the
SUS disks were low compared to those on the filter papers following
nitrogen gas plasma treatment at a frequency of 1.5 kpps,
indicating that different surfaces elicit a different effect on
spore viability. These findings are consistent with a previous
study, which reported the inactivating efficiency of G.
stearothermophilus spores on SUS disks was low compared to
spores placed on filter papers (12).
In the present study, the D value determined
by nitrogen gas plasma treatment of G. stearothermophilus on
filter papers (D-value=2.48 min) was similar to that of
hydrogen peroxide vapor (2 mg/l; D-value=2.1 min; G.
stearothermophilus SUS disk) or steam sterilization (121°C;
D-value=2.2 min; G. stearothermophilus filter strip).
Thus, the nitrogen gas plasma instrument enables efficient
inactivation of bacterial spores, equivalent to that observed by
exposure to hydrogen peroxide vapor or steam sterilization in the
case of samples on filter paper. Hydrogen peroxide vapor is one of
the major sterilization methods for thermolabile medical devices
(20), while steam sterilization
is the conventional sterilization method. However, hydrogen
peroxide vapor treatment is not compatible with numerous materials
due to its powerful oxidizing activity (21). By contrast, as gas plasma
penetration at the surface of the material is slight at ~10–20 nm
(6), the sterilization procedure
does not reduce the functional integrity of the medical instrument.
However, as certain surface materials, such as SUS, may reduce
inactivation efficiency, compatibility of materials should be
checked prior to employing nitrogen gas plasma treatment.
One of the sterilization factors is UV-A radiation
emitted during the generation of nitrogen gas plasma (10). Thus, it was assumed that gas plasma
treatment may elicit its sterilizing action by damaging the genomic
DNA of microorganisms. The present study assessed potential damage
to the genomic DNA by analyzing the 16S rRNA gene of gas plasma
treated and untreated spores by PCR and qPCR. The results
demonstrated clear evidence that DNA in the bacterial spores is
damaged by nitrogen gas plasma treatment. Furthermore, increased
levels of 8-OHdG following nitrogen gas plasma suggested that the
DNA damage is the result of oxidation. The present study also
assessed whether UV-A emitted during the operation of nitrogen gas
plasma contributes, at least in part, to the sterilization process.
However, UV-A exposure did not efficiently inactivate the bacterial
spores. Thus, other factors appear to be predominantly responsible
for the microbial inactivation. SEM images demonstrated an
irregular surface structure of bacterial spores following treatment
with nitrogen gas plasma. The observed changes in cell surface
structures are possibly due to oxidative stress produced during gas
plasma generation. Therefore, inactivation of bacterial spores
during nitrogen gas plasma treatment may the result of changes in
the cell surface components, as well as damage to genomic DNA as a
result of oxidative stress. In summary, the nitrogen gas plasma
instrument produces at least three variables, heat, UV-A and
oxidative stress, during its operation, which may be responsible
for bacterial inactivation. Among these factors, hydrogen peroxide
is the most effective for inactivation of G.
stearothermophilus. However, there may be a synergistic effect
involving the other factors that contribute to the bactericidal
activity of nitrogen gas plasma treatment.
Hydrogen peroxide, similarly to formaldehyde or
chlorine, exhibits good cell permeability, and may act to reduce
the water content of the spores by hydrating dipicolinic acid in
the core of the spore (22–24).
Thus, hydrogen peroxide generated from the nitrogen gas plasma
instrument, in addition to oxidizing the bacterial spore surface,
may exert its effect by reducing the water content in the
cytoplasm. In addition, other reactive chemical species may be
produced during operation of the BLP-TES device. Indeed, the
preliminary data indicated a change in the color of the chemical
indicator Quantofix Active oxygen which reacts with a broad range
of oxidants, including potassium monopersulfate triple salt
(KHSO5, KHSO4 and
K2SO4) and hydrogen peroxide, bromine
(Br2), hypochlorite (OCl−), peracetic acid
(CH3COOOH) and chlorine (Cl2) (25). Thus, other reactive chemical
species in addition to hydrogen peroxide may contribute to the
observed bacterial inactivation and may indicate a synergistic
inactivating effect. Thus, further studies are required in order to
identify other reactive chemical species that contribute to the
bactericidal action of the BLP-TES instrument.
In conclusion, nitrogen gas plasma treatment is an
effective means of sterilization and disinfection, suggesting this
methodology has practical potential for sterilization of medical
devices in the fields of medicine and dentistry, as well as
disinfection in the food industry.
Acknowledgements
The authors of the present study would like to thank
Ms. Haruna Ohshiro and Ms. Rika Imamura (Laboratory of Biometabolic
Chemistry, School of Health Sciences, University of the Ryukyus,
Nishihara, Japan) for their technical assistance and Dr. Naohiro
Shimizu (NGK Insulators, Mizuho-ku, Japan) for their technical
support. The current study was supported by a Grant-in-Aid for the
Promotion of Basic Research Activities for Innovative Biosciences
from the Bio-oriented Technology Research Advancement Institution,
the Science and Technology Research Promotion Program for
Agriculture, Forestry, Fisheries and Food Industry (grant no.
26015A), and a Grant-in-Aid from the Amano Institute of Technology,
as well as Grants-in-Aid for Scientific Research on Innovative
Areas (grant nos. 22110514, 24110717 and 16K04997) from the Japan
Society for the Promotion of Science.
References
|
1
|
McDonnell GE: Antisepsis, Disinfection,
and Sterilization: Types, Action, and Resistance. ASM Press;
Washington, D.C: 2007
|
|
2
|
Sakudo A and Shintani H: Sterilization and
disinfection by plasma: Sterilization mechanisms, biological and
medical applications. Nova Science Publishers, Inc.; New York:
2011
|
|
3
|
Laroussi M: Low temperature plasma-based
sterilization: Overview and state-of-the-art. Plasma Process Polym.
2:391–400. 2005. View Article : Google Scholar
|
|
4
|
Moisan M, Barbeau J, Crevier MC, Pelletier
J, Philip N and Saoudi B: Plasma sterilization: Methods and
mechanisms. Pure Appl Chem. 74:349–358. 2002.
|
|
5
|
Sakudo A, Shimizu N, Imanishi Y and Ikuta
K: N2 gas plasma inactivates influenza virus by inducing
changes in viral surface morphology, protein, and genomic RNA.
Biomed Res Int. 2013:6942692013.PubMed/NCBI
|
|
6
|
Shintani H, Shimizu N, Imanishi Y, Sekiya
T, Tamazawa K, Taniguchi A and Kido N: Inactivation of
microorganisms and endotoxins by low temperature nitrogen gas
plasma exposure. Biocontrol Sci. 12:131–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakudo A, Higa M, Maeda K, Shimizu N,
Imanishi Y and Shintani H: Sterilization mechanism of nitrogen gas
plasma: Induction of secondary structural change in protein.
Microbiol Immunol. 57:536–542. 2013.PubMed/NCBI
|
|
8
|
Sakudo A, Misawa T, Shimizu N and Imanishi
Y: N2 gas plasma inactivates influenza virus mediated by
oxidative stress. Front Biosci (Elite Ed). 6:69–79. 2014.PubMed/NCBI
|
|
9
|
Shintani H, Sakudo A, Burke P and
McDonnell G: Gas plasma sterilization of microorganisms and
mechanisms of action. Exp Ther Med. 1:731–738. 2010.PubMed/NCBI
|
|
10
|
Maeda K, Toyokawa Y, Shimizu N, Imanishi Y
and Sakudo A: Inactivation of Salmonella by nitrogen gas plasma
generated by a static induction thyristor as a pulsed power supply.
Food Control. 52:54–59. 2015. View Article : Google Scholar
|
|
11
|
Sakudo A, Toyokawa Y and Imanishi Y:
Nitrogen Gas Plasma Generated by a Static Induction Thyristor as a
Pulsed Power Supply Inactivates Adenovirus. PLoS One.
11:e01579222016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawamura K, Sakuma A, Nakamura Y, Oguri T,
Sato N and Kido N: Evaluation of bactericidal effects of
low-temperature nitrogen gas plasma towards application to
short-time sterilization. Microbiol Immunol. 56:431–440. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuisiene N, Raugalas J, Stuknyte M and
Chitavichius D: Identification of the genus Geobacillus using
genus-specific primers, based on the 16S-23S Rrna gene internal
transcribed spacer. FEMS Microbiol Lett. 277:165–172. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Halvorson HO and Ziegler NR: Application
of Statistics to Problems in Bacteriology: I. A Means of
Determining Bacterial Population by the Dilution Method. J
Bacteriol. 25:101–121. 1933.PubMed/NCBI
|
|
16
|
Stumbo CR, Murphy JR and Cochran J: Nature
of Thermal Death Time Curves for P.A. 3679 and Clostridium
botulinum [1950]. Food Technol. 4:321–326. 1950.
|
|
17
|
Valavanidis A, Vlachogianni T and Fiotakis
C: 8-hydroxy-2′- deoxyguanosine (8-OhdG): A critical biomarker of
oxidative stress and carcinogenesis. J Environ Sci Health C Environ
Carcinog Ecotoxicol Rev. 27:120–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Laroussi M and Leipold F: Evaluation of
the Roles of Reactive Species, Heat and UV radiation in the
Inactivation of bacterial Cells by Air Plasmas at Atmospheric
Pressure. Int J Mass Spectrom. 233:81–86. 2004. View Article : Google Scholar
|
|
19
|
Laroussi M, Mendis DA and Rosenberg M:
Plasma Interaction with Microbes. New J Phys. 5:1–41. 2003.
|
|
20
|
Shintani H: Application of vapor phase
hydrogen peroxide sterilization to endoscope. Biocontrol Sci.
14:39–45. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shintani H: Simultaneous Achievement of
Sterility Assurance Level (SAL) of 10−6 and Material and
Functional Compatibility in Gas Plasma Sterilization Running Title:
Simultaneous SAL and Compatibility. Pharm Regul Aff. 4:1312015.
|
|
22
|
Eskenazi S, Bychkowski OE, Smith M and
MacMillan JD: Evaluation of glutaraldehyde and hydrogen peroxide
for sanitizing packaging materials of medical devices in sterility
testing. J Assoc Off Anal Chem. 65:1155–1161. 1982.PubMed/NCBI
|
|
23
|
Kenar L, Ortatatli M, Yaren H,
Karayilanoglu T and Aydogan H: Comparative sporicidal effects of
disinfectants after release of a biological agent. Mil Med.
172:616–621. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Setlow B, Yu J, Li YQ and Setlow P:
Analysis of the germination kinetics of individual Bacillus
subtilis spores treated with hydrogen peroxide or sodium
hypochlorite. Lett Appl Microbiol. 57:259–265. 2013.PubMed/NCBI
|
|
25
|
MACHEREY-NAGEL GmbH & Co. KG, .
QUANTOFIX® Active oxygen. simpleftp://ftp.mn-net.com/english/Instruction_leaflets/QUANTOFIX/91349en.pdf#search=%27Quantofix+Active+oxygen%27Accessed
25 August 2015.
|