Introduction
Osteoarthritis (OA) is the most common form of
musculoskeletal disease (1). It is
a complex and multifaceted disease, which is predominantly
characterized by the degradation of articular cartilage and joint
inflammation (2). Chondrocytes in
the articular cartilage regulate appropriate gene expression in
order to achieve tissue homeostasis. At present, although a number
of pathways underlying the pathogenesis of OA have been
demonstrated, it remains to be fully elucidated and current
knowledge has not provided effective approaches for prevention or
treatment.
MicroRNAs (miRNAs) are small non-coding RNAs, which
have been reported to be important regulators of gene expression in
humans and function as negative regulator of gene expression at the
post-transcriptional level via binding to complimentary sequences
in the 3′-untranslated regions (3′-UTRs) of targeting mRNAs
(3,4). Hundreds of miRNAs have been found in
various organisms, and a third of all mammalian mRNAs appear to be
under the regulation of miRNAs (5). Aberrant miRNA expression profiles
have been demonstrated to be associated with the development of OA,
and their functions are beginning to be delineated (6–8).
miR-145 has been indicated to be important in tumor
progression and metastasis, specifically in the processes of cell
proliferation and differentiation (9–11).
However, the role of miR-145 in the progression of OA and its
underlying mechanism remains to be fully elucidated. The present
study aimed to demonstrate the biological function and molecular
mechanism of miR-145 in OA. The expression of miR-145 was found to
decrease and that of tumor necrosis factor receptor superfamily,
member 11b (TNFRSF11B) was found to increase in OA cartilage
tissues, compared with normal cartilage tissues. It was also
revealed that miR-145 was important in chondrocyte proliferation
and fibrosis by directly targeting TNFRSF11B, which inhibited the
proliferation and fibrosis of OA.
Materials and methods
Cell lines and patient samples
The C-20/A4 and CH8 human articular chondrocytes
cell lines were obtained from American Type Culture Collection
(Manassas, VA, USA). All cells were cultured in Dulbecco's modified
Eagle's medium supplemented with 10% fetal bovine serum in a
humidified atmosphere of 5% CO2 at 37°C. A total of 25
paired cartilage tissues, and matched normal cartilage tissues from
traumatic amputees were obtained from the knees of patients with
OA. Each patient provided consent for the experiment, and the
present study was approved by the Ethics Committee of the
Affiliated Hospital of Jining Medical University (Jining,
China).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA from the tissues and cells were extracted
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and purified using the RNeasy Maxi kit (Qiagen
GmbH, Hilden, Germany) according to the manufacturer's protocol.
The miScript reverse transcription kit (Qiagen GmbH) and RevertAid
First Strand cDNA Synthesis kit (Qiagen GmbH) were used to obtain
the total cDNA. The miScript SYBR Green PCR kit (Qiagen GmbH) and
SYBR® Premix Ex Taq™ II (Takara Bio, Inc., Otsu, Japan)
were used for the qPCR assay. The PCR reaction mixture contained: 1
µl cDNA, 2 µl specific primer, 2 µl 10X miScript SYBR Green PCR
Master Mix (or SYBR® Premix Ex Taq™ II) and 5 µl
RNase-free water. The amplification conditions were as follows:
95°C for 10 min, followed by 45 cycles at 95°C for 30 sec, 60°C for
30 sec and 72°C for 30 sec, and a final extension step at 72°C for
5 min. Human U6 and GAPDH were used as the internal control genes.
The miR-145- and U6-specific primers were as follows: miR-145
forward 5′-GAATCCCTTAGATGCTAAGATG-3′ and reverse (miScript SYBR
Green PCR kit universal primer); U6, forward
5′-CTCGCTTCGGCAGCACA-3′ and reverse 5′-ACGCTTCACGAATTTGCGT-3′. The
TNFRSF11B- and GAPDH-specific primers were as follows: TNFRSF11B,
forward 5′-CCCCTTGCCCTGACCACTACTA-3′ and reverse
5′-CGATTGCACTCCTGCTTGACGT-3′; GAPDH, forward
5′-ACATCAAGAAGGTGGTGAAGCAGG-3′ and reverse
5′-AGCGTCAAAGGTGGAGGAGTGG-3′. The relative expression level was
determined using the delta-delta Cq method (12).
Cell transfection
The miR-145 mimics, negative control (NC) mimics,
small interfering (si)TNFRSF11B and negative control were designed
and synthesized by Invitrogen Life Technologies; Thermo Fisher
Scientific, Inc. The sequence of the miR-145 mimic was
5′-GUCCAGUUUUCCCAGGAAUCCCU-3′ and that of the NC mimic was
5′-UUCUCCGAACGUGUCACGUUU-3′. The sequence of siTNFRSF11B was
5′-CAGGCACUUGAGGCUUUCAGUGAUA-3′ and that of the NC was
5′-CAGUACUUUUGUGUAGUACAA-3′. Cell transfection was performed using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocols.
In vitro cell proliferation assay
Cell proliferation was measured using a
3-(4,5-dimethylth-iazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. The C-20/A4 and CH8 cells (~4×103) were seeded
into a 96-well plate and transfected with the miR-145 or NC mimics,
and siTNFRSF11B or the NC at 37°C. At 0, 24, 48 or 72 h-post
transfection, 25 µl of MTT reagent (5 mg/ml; Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) was added to each plate and
incubated at 37°C for 4 h. Subsequently, the MTT medium mixture was
discarded and 150 µl of dimethyl sulfoxide was added to each plate
and agitated for 30 min at 37°C to solubilize the crystals.
Absorbance was measured at a wavelength of 450 nm using an ELISA
microplate reader (Bio-Rad Laboratories, Inc., CA, USA).
Dual-luciferase reporter assay
In order to confirm whether miR-145 was able to
directly bind the 3′-UTR of the TNFRSF11B gene, the miR-145 target
sequences of TNFRSF11B were inserted between the Xhol and
NotI restriction sites of the 3′-UTR of the hRluc gene in
the psiCHeCK™-2 luciferase vector (Promega Corporation, Madison,
WI, USA). Primer sequences for the 3′-UTR of TNFRSF11B mRNA were
designed by Thermo Fisher Scientific, Inc., as follows: Forward
5′-CTCGCTTCGGCAGCACA-3′ and reverse 5′-ACGCTTCACGAATTTGCGT-3′. The
recombination plasmid of psiCHECK-2-TNFRSF11B-3′-UTR was
constructed, which contained the potential binding sites, and the
potential binding sites were manually mutated by exchanging the G
and T, A and C. Subsequently, the luciferase recombination reporter
constructs, together with the miR-145 mimics or NC mimics, were
cotransfected into the C-20/A4 and CH8 cells at 37°C. The Firefly
and Renilla luciferase activities were detected using a dual
luciferase assay system (Promega Corporation), according to the
manufacturer's protocol, 24 h following transfection. Normalized
data were calculated as the quotient of Renilla/Firefly
luciferase activities.
Western blot analysis
Total proteins of the chondrocyte tissues and cells
were extracted using RIPA buffer (Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with protease inhibitor cocktail
(EMD Millipore, Billerica, MA, USA). Protein concentration was
determined using the Bio-Rad Protein Assay Dye Reagent Concentrate
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The extracted
proteins (30 µg) were separated by 12% SDS-PAGE and transferred
onto a polyvinylidene difluoride membrane (EMD Millipore). The
membranes were blocked with 5% non-fat dried milk for 2 h at 37°C.
Subsequently, the membranes were incubated with mouse
anti-TNFRSF11B monoclonal antibody (cat. no. ab105935), mouse
anti-collagen II monoclonal antibody (cat. no. ab3092), mouse
anti-collagens V monoclonal antibody (cat. no. ab112551), mouse
anti-collagen X monoclonal antibody (cat. no. ab49945), mouse
anti-GAPDH monoclonal antibody (cat. no. ab8245), rabbit
anti-matrix metalloproteinase (MMP)1 monoclonal antibody (cat. no.
ab52631), rabbit anti-MMP8 monoclonal antibody (cat. no. ab81286)
and rabbit anti-MMP13 monoclonal antibody (cat. no. ab51072), all
from Abcam (Cambridge, MA, USA), overnight at 4°C. Following
incubation, the membranes were treated with horseradish
peroxidase-labeled secondary antibodies (cat. nos. ab6728 and
ab6721; Abcam) for 1 h at room temperature. The positive blots were
detected using a chemiluminescent substrate kit (Pierce; Thermo
Fisher Scientific, Inc.).
Statistical analysis
Statistical analysis was performed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). Data are presented as the mean ±
standard deviation. Statistical significance was determined using
Student's t-test, with the exception of the MTT data, which was
analyzed using one-way analysis of variance followed by Bonferroni
multiple comparisons test. P<0.05 was considered to indicate a
statistically significant difference. All experiments were repeated
at least three times.
Results
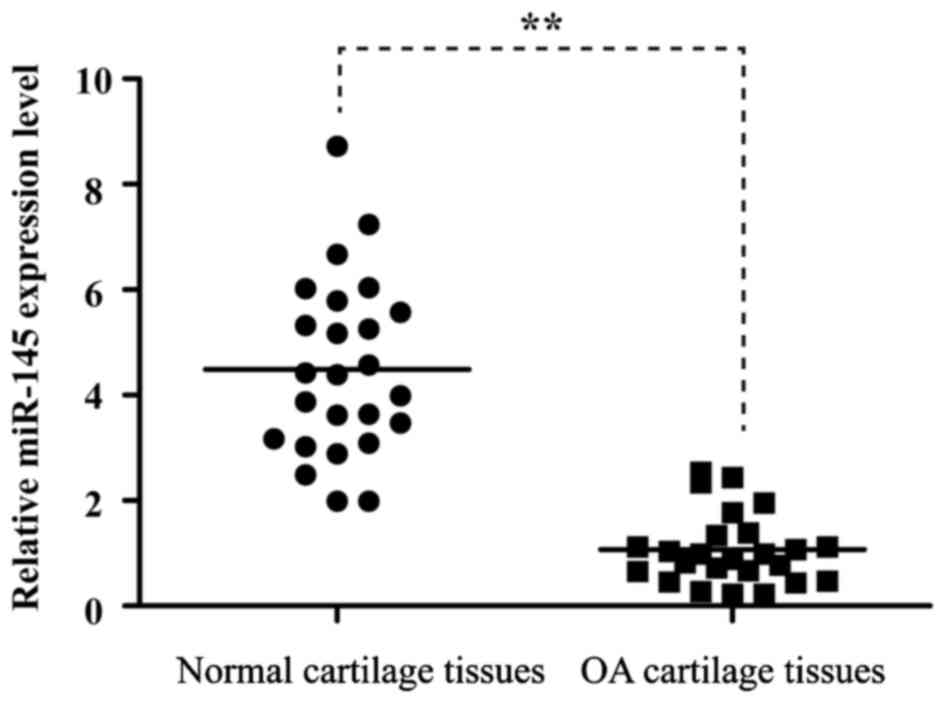
Expression of miR-145 is decreased in
OA cartilage tissues, compared with normal cartilage tissues
To determine whether the expression of miR-145 is
altered in OA cartilage tissues, the present study compared its
level of expression between knee OA and normal cartilage tissues.
As shown in Fig. 1, the expression
level of miR-145 was significantly reduced in the OA cartilage
tissues, compared with the normal cartilage tissues.
Overexpression of miR-145 induces the
arrest of chondrocyte proliferation and inhibits chondrocyte
fibrosis
To investigate the function of miR-145 in cell
proliferation, miR-145 mimics or NC mimics were transfected into
C-20/A4 and CH8 cells. The results of the MTT assay demonstrated
that the cell proliferation in the miR-145 mimic group was
significantly decreased, compared with that in the NC mimic group
(Fig. 2A). In order to examine
whether miR-145 also regulated chondrocyte fibrosis, western blot
analysis was performed to examine the human C-20/A4 and CH8 cells
transfected with miR-145 or NC mimics. It was found that the
protein expression levels of collagen II, V and X in the miR-145
mimic group were upregulated, whereas the protein expression levels
of MMP1, MMP8 and MMP13 were downregulated (Fig. 2B), compared with the levels in the
NC mimic group. These results suggested that miR-145 inhibited
chondrocyte proliferation and fibrosis.
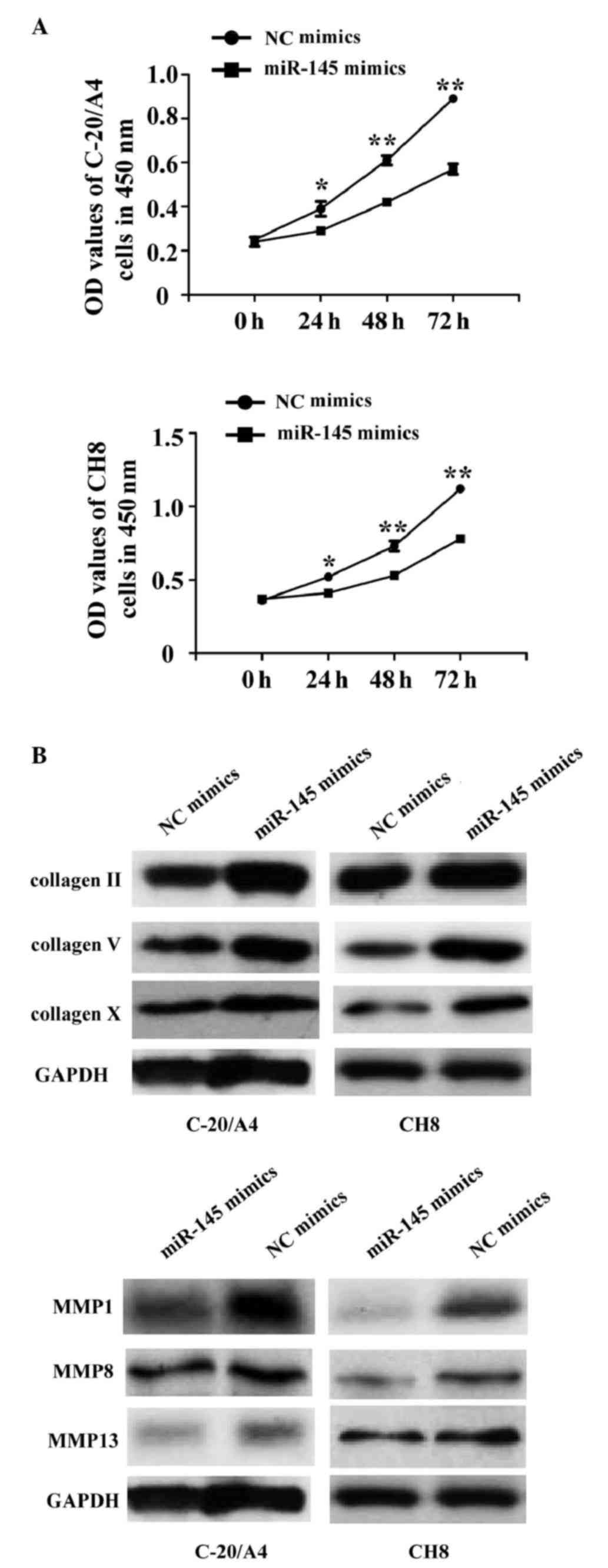 | Figure 2.Overexpression of miR-145 induces
proliferation arrest and inhibits fibrosis of chondrocytes. (A) At
24, 48, 36 and 72 h post-transfection, a
3-(4,5-dimethylth-iazol-2-yl)-2,5-diphenyltetrazolium bromide assay
was performed to detect cell proliferation. *P<0.05 and
**P<0.01. (B) Western blot analysis was performed to detect the
protein expression of collagen II, V and X, and MMP1, MMP8 and
MMP13 in C-20/A4 and CH8 cells following transfection with miR-145
or NC mimics. miR, microRNA; MMP, matrix metalloproteinase; OD,
optical density; NC, negative control. |
TNFRSF11B is a direct target of
miR-145
miRanda (http://www.microrna.org) and miRcode (http://www.mircode.org) were used to predict the
downstream target genes of miR-145. TNFRSF11B was one of the
putative genes identified, the mRNA 3′-UTR of which contained a
complementary site for the seed region of miR-145 (Fig. 3A). In order to confirm the direct
targeting of TNFRSF11B by miR-145, wild-type (WT) TNFRSF11B-3′-UTR,
containing the target sequences, or mutant (MUT) TNFRSF11B-3′-UTR
mimics were constructed and inserted into a luciferase reporter
vector to detect the effects of miR-145 on luciferase activity in
C-20/A4 and CH8 cells. As shown in Fig. 3B, miR-145 suppressed the luciferase
activity of the WT-TNFRSF11B-3′-UTR, whereas mutation of the
miR-145 binding sites inhibited this suppressive effect.
Furthermore, RT-qPCR and western blot analyses demonstrated that
transfection with miR-145 mimics inhibited the endogenous
expression of TNFRSF11B in the C-20/A4 and CH8 cells (Fig. 3C). These data suggested that
miR-145 regulated the expression of TNFRSF11B by directly targeting
its mRNA 3′-UTR in chondrocytes.
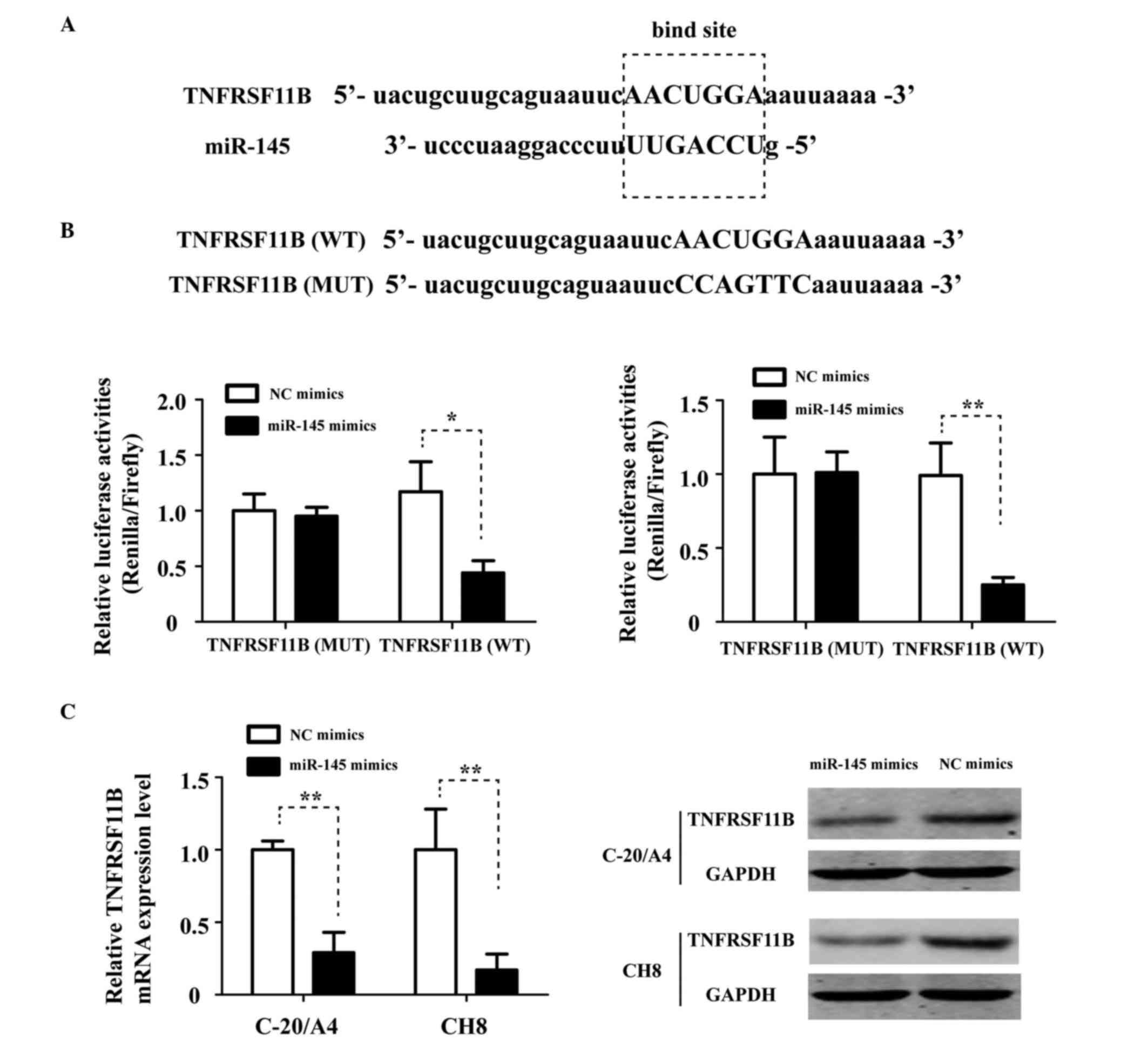 | Figure 3.TNFRSF11B is a direct target of
miR-145. (A) TNFRSF11B mRNA 3′-UTRs showing the target binding
sites for miR-145. (B) C-20/A4 and CH8 cells were cotransfected
with miR-145 mimics or NC mimics and a TNFRSF11B-3′-untranslated
region fragment containing either the WT miR-145 target sequence or
MUT sequence. (C) mRNA and protein expression of TNFRSF11B,
detected using reverse transcription-quantitative polymerase chain
reaction and western blot analyses, respectively, in C-20/A4 and
CH8 cells transfected with miR-145 mimics or NC mimics. GAPDH was
used as an internal control. All experiments were performed three
times, and a representative results are shown. *P<0.05 and
**P<0.01. miR, microRNA; TNFRSF11B, tumor necrosis factor
receptor superfamily, member 11b; WT, wild-type; MUT, mutant; NC,
negative control. |
The knock down of TNFRSF11B also inhibited
chondrocyte proliferation and fibrosis. To confirm the functional
role of TNFRSF11B in chondrocytes, TNFRSF11B was knocked down in
C-20/A4 and CH8 cells using TNFRSF11B-specific siRNAs. RT-qPCR and
western blot analyses were then used to confirm the effect of this
interference (Fig. 4A). The
knockdown of TNFRSF11B significantly suppressed cell proliferation
in the C-20/A4 and CH8 cells (Fig.
4B), compared with the NC group. The protein expression levels
of collagen II, V and X were upregulated in the siTNFRSF11B group
(Fig. 4C), whereas the protein
expression levels of MMP1, MMP8 and MMP13 were downregulated
(Fig. 4C) compared with the in the
NC group. Taken together, these results demonstrated that miR-145
regulated the proliferation and fibrosis of chondrocytes by
targeting TNFRSF11B in human OA.
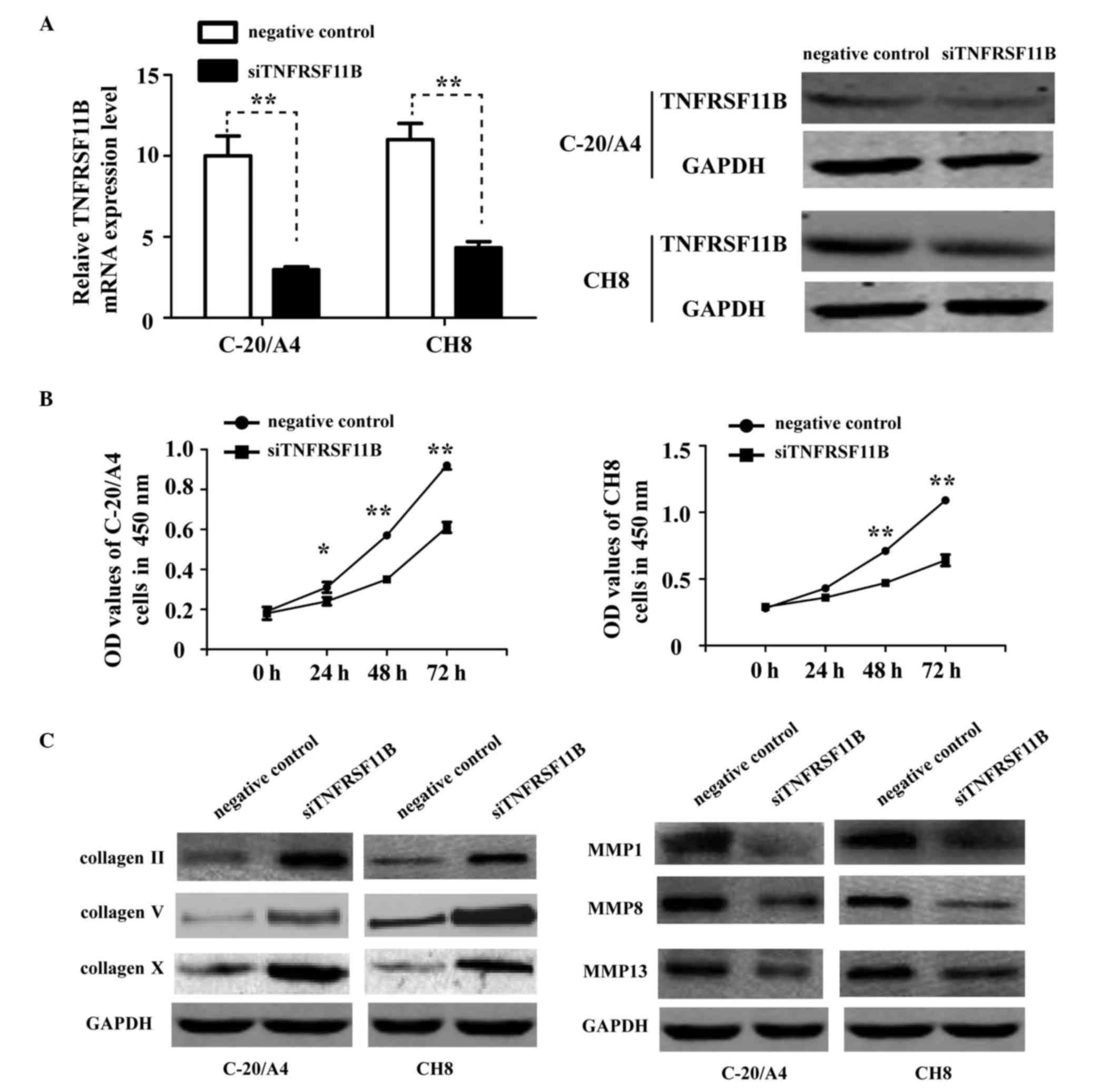 | Figure 4.Knock down of TNFRSF11B inhibits
chondrocyte proliferation and fibrosis. (A) Revesrse
tranascription-quantitative polymerase chain reaction and western
blot analyses were performed to examine the expression levels of
TNFRSF11B in C-20/A4 and CH8 cells transfected with siTNFRSF11B or
negative control. (B) A
3-(4,5-dimethylth-iazol-2-yl)-2,5-diphenyltetrazolium bromide assay
was performed to examine the proliferation of C-20/A4 and CH8 cells
transfected with siTNFRSF11B or negative control. *P<0.05 and
**P<0.01. (C) Western blot analysis was used to detect protein
expression of collagen II, V and X, and MMP1, MMP8 and MMP13 in
C-20/A4 and CH8 cells transfected with siTNFRSF11B or the negative
control. siTNFRSF11B, small interfering RNA for tumor necrosis
factor receptor superfamily, member 11b; NC, negative control; MMP,
matrix metalloproteinase; OD, optical density. |
Discussion
miRNAs are essential in maintaining and modulating
normal physiological function, however, the expression of miRNAs
are altered in response to pathological disorders (13). OA is a prevalent degenerative joint
disease, which is characterized by the progressive destruction of
articular cartilage, synovial hyperplasia and the sclerosis of
subchondral bone (14–17). Previous studies have demonstrated
that miRNAs are have a vital function in OA (6–8).
Thus, it is important to detect the aberrant expression of miRNAs
in OA. The present study indicated that miR-145 is essential in the
development of OA. The expression of miR-145 was significantly
decreased in human OA tissues. Transfection with a synthetic
miR-145 mimic in vitro led to significant inhibition of the
proliferation and fibrosis of C-20/A4 and CH8 cells.
In addition, bioinformatics analysis was used to
screen the targets of miR-145, and suggested that TNFRSF11B may be
a target of miR-145 in chondrocytes. The results of the
dual-luciferase reporter assay further suggested that TNFRSF11B was
a direct target of miR-145 in chondrocytes. TNFRSF11B, as a key
regulator of the process of chondrogenesis, has been reported to be
important in chondrocyte formation (18–20).
In the present study, the overexpression of miR-145 repressed the
expression of TNFRSF11B in C-20/A4 and CH8 cells and, as expected,
interference of the expression of TNFRSF11B decreased chondrocyte
growth and fibrosis.
To the best of our knowledge, the present study is
the first to provide evidence of the function of miR-145 in OA by
directly targeting TNFRSF11B. These results suggested that
controlling the expression of miR-145 offers potential as a novel
approach for the prevention and treatment of OA.
Acknowledgements
The study was supported by the Natural Science
Foundation of Shandong Province, China (grant no. ZR2010HQ036).
References
|
1
|
Miyaki S and Asahara H: Macro view of
microRNA function in osteoarthritis. Nat Rev Rheumatol. 8:543–552.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu C, Tian B, Qu X, Liu F, Tang T, Qin A,
Zhu Z and Dai K: MicroRNAs play a role in chondrogenesis and
osteoarthritis (review). Int J Mol Med. 34:13–23. 2014.PubMed/NCBI
|
|
3
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim VN: Small RNAs: Classification,
biogenesis, and function. Mol Cells. 19:1–15. 2005.PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dong S, Yang B, Guo H and Kang F:
MicroRNAs regulate osteogenesis and chondrogenesis. Biochem Biophys
Res Commun. 418:587–591. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dunn W, DuRaine G and Reddi AH: Profiling
microRNA expression in bovine articular cartilage and implications
for mechanotransduction. Arthritis Rheum. 60:2333–2339. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iliopoulos D, Malizos KN, Oikonomou P and
Tsezou A: Integrative microRNA and proteomic approaches identify
novel osteoarthritis genes and their collaborative metabolic and
inflammatory networks. PLoS One. 3:e37402008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Larne O, Hagman Z, Lilja H, Bjartell A,
Edsjö A and Ceder Y: miR-145 suppress the androgen receptor in
prostate cancer cells and correlates to prostate cancer prognosis.
Carcinogenesis. 36:858–866. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bai M, Yuan M, Liao H, Chen J, Xie B, Yan
D, Xi X, Xu X, Zhang Z and Feng Y: OCT4 pseudogene 5 upregulates
OCT4 expression to promote proliferation by competing with miR-145
in endometrial carcinoma. Oncol Rep. 33:1745–1752. 2015.PubMed/NCBI
|
|
11
|
Kim TH, Song JY, Park H, Jeong JY, Kwon
AY, Heo JH, Kang H, Kim G and An HJ: miR-145, targeting
high-mobility group A2, is a powerful predictor of patient outcome
in ovarian carcinoma. Cancer Lett. 356:937–945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Jia J, Yang S, Liu X, Ye S and
Tian H: MicroRNA-21 controls the development of osteoarthritis by
targeting GDF-5 in chondrocytes. Exp Mol Med. 46:e792014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iannone F and Lapadula G: The
pathophysiology of osteoarthritis. Aging Clin Exp Res. 15:364–372.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mortellaro CM: Pathophysiology of
osteoarthritis. Vet Res Commun. 27:(Suppl 1). S75–S78. 2003.
View Article : Google Scholar
|
|
16
|
Martel-Pelletier J: Pathophysiology of
osteoarthritis. Osteoarthritis Cartilage. 12:(Suppl A). S31–S33.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mandelbaum B and Waddell D: Etiology and
pathophysiology of osteoarthritis. Orthopedics. 28:(2 Suppl).
S207–S214. 2005.PubMed/NCBI
|
|
18
|
Golovchenko S, Hattori T, Hartmann C,
Gebhardt M, Gebhard S, Hess A, Pausch F, Schlund B and von der Mark
K: Deletion of beta catenin in hypertrophic growth plate
chondrocytes impairs trabecular bone formation. Bone. 55:102–112.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng ZY, He ZN, Zhang B and Chen Z:
Osteoprotegerin promotes the proliferation of chondrocytes and
affects the expression of ADAMTS-5 and TIMP-4 through MEK/ERK
signaling. Mol Med Rep. 8:1669–1679. 2013.PubMed/NCBI
|
|
20
|
Liang QQ, Li XF, Zhou Q, Xing L, Cheng SD,
Ding DF, Xu LQ, Tang DZ, Bian Q, Xi ZJ, et al: The expression of
osteoprotegerin is required for maintaining the intervertebral disc
endplate of aged mice. Bone. 48:1362–1369. 2011. View Article : Google Scholar : PubMed/NCBI
|